Using dynamic dewpoint isotherms to determine the optimal storage conditions of inert dust-treated hard red winter wheat
2020-03-10KoumeYoJenniferAnthonyRonloMghirngDviHgstrumKunynZhuSurmnymBhrirju
Koume Yo,Jennifer Anthony,Ronlo Mghirng,Dvi Hgstrum,Kunyn Zhu,Surmnym Bhrirju
a Department of Grain Science and Industry,Kansas State University,Manhattan 66506,USA
b Department of Chemical Engineering,Kansas State University,Manhattan 66506,USA
c Department of Biological and Agricultural Engineering,Kansas State University,Manhattan 66506,USA
d Department of Entomology,Kansas State University,Manhattan 66506,USA
ABSTRACT Water-solid interactions play a key role in determining the efficacy of inert dusts.The critical water activity(Awc)for phase transition in amorphous materials is an important characteristic of amorphous inert dusts used as grain protectants.As water activity(Aw)rises above Awc,amorphous dusts undergo a transition from glassy or vitreous state to rubbery state.Such a transition induces dramatic changes in material properties,texture and structure,and hence impact their performance as grain protectants.Full Dynamic Dewpoint Isotherms(DDI)of a synthetic amorphous zeolite intended for grain protection were generated using the Vapor Sorption Analyzer(VSA)to determine Awc by investigating the relationship between moisture content and Aw at constant temperatures.Sorption experimental data was fitted using three sorption isotherm models:Guggenheim-Anderson-de Boer(GAB),Double Log Polynomial(DLP),and Brunauer-Emmet-Teller(BET).DLP model was the best model to estimate zeolite and wheat sorption isotherms.Full sorption isotherms of zeolite and wheat obtained at 25,35,and 45°C clearly showed the hysteresis phenomenon.The hysteresis loops were of type H3 for wheat,and of type H4 for zeolite powder.The intensity of hysteresis remained unchanged for wheat.However,the intensity of hysteresis decreased with increasing temperatures during water adsorption by porous zeolite powder.Monolayer moisture content values for each sorption direction were provided only by GAB and BET models and indicated a decrease in monolayer moisture content with an increase in temperature.The net isosteric heats of sorption and the differential enthalpy of zeolite estimated by the Clausius-Clapeyron equation and determined graphically decreased with increasing moisture content.Conversely,differential entropy of zeolite decreased with increasing zeolite moisture content.The optimal moisture content of inert dust for grain treatment was dependent on wheat moisture content and wheat storage temperature.This is the first time that a synthetic amorphous dust is being investigated for grain protection.Our results recommend the application of inert dusts at the optimal moisture content to mitigate moisture migration within the system“wheat-dust”,thus ensuring dust maximal efficacy.
Keywords: Dynamic dewpoint isotherm Critical water activity GAB BET DLP Sorption kinetics Inert dust Wheat
1.Introduction
Inert dusts encompass various dry and chemically unreactive powders applied onto grain at the time of storage into silos or bins, on farm or off-farm.Prior to grain treatment,inert dusts are commonly stored in plastic sacks or bucket containers.Inert dusts are well-known for their hygroscopic nature and their tendency to quickly absorb ambient humidity.If not stored in tightly sealed containers,they can undergo a severe caking process as a result of particle agglomeration due to moisture migration.Natural or time consolidation of hygroscopic dust may also occur as a result of compaction created by the weight of the dust on itself.Consolidation of the dust also takes place as the dust is subjected to vibration during transport from the manufacturing plant to the farm, the elevator facility, or the production line.Caking is worsened by high ambient relative humidity,increased consolidation pressure and longterm powder storage[1].Often times,by the time the inert dust reaches the farm,the elevator,or the laboratory,the moisture content has increased 5-10 times, and by the time the pest prevention program is implemented,the moisture content would have increased 15-20 folds.The dust becomes very hard and difficult to remove from the container,delaying grain treatment,making necessary additional expenses,significantly reducing dust efficacy,and increasing the risk for greater deterioration of grain quality.Consolidation from gradual settling activity is not the only concern to reckon with while handling inert dusts.Even after a successful storage of an amorphous dust prior to application onto grain,consolidation can resurface while the treated grain is sitting inside the bin or silo.Caking occurring inside bins or silos is amplified as a result of the system “grain-dust”interacting with the ambient moisture and temperature.Caking inside a silo can amplify ratholing,especially if the silo is operating in funnel flow mode.In either case,before or after grain treatment,inert dust caking is due to moisture migration.The main mechanism responsible for the caking of amorphous powders is sintering[2].
The efficacy of inert dusts against insects derives from their ability to kill insects through desiccation[3-7].Consequently,the insecticidal activity that can be expected from a dust increases as the water activity(Aw)of the dust and/or the ambient relative humidity decreases.The moisture and temperature at which the grain is stored determine its susceptibility to stored-product insects[8].Moisture uptake during dust storage prior to application onto grain negatively impacts the overall effectiveness of the dust.In fact,insects could better resist desiccation by taking advantage of the moisture already present within the moist dust to replenish the body water content loss induced by desiccation.Using dry powders with moisture as low as 1% is not enough to maximize the insecticidal efficacy,though.The limiting parameter is the Awof the dust interacting with the grain.Optimal storage conditions can be defined as a combination of environmental parameters(storage temperature,water activity of dust and grain)that foster a maximum stability of the treated grain by considerably depressing the likelihood of water migration within the system“dust-grain”.At a given temperature,high differences in grain water activity relative to dust water activity will cause the dust to adsorb or desorb water.Moisture equilibration between grain and the moist dust results in a too moist grain that is easily prone to mold and fungi infestation,especially when grain moisture reaches 15%and beyond.On the other hand,a too dry dust can have a drying action on the stored grain.Stored grain is primarily sold based on weight and selling a too dry grain equates with a significant decrease in profitability.Hence,understanding the underlying mechanisms leading to the dramatic changes in the physical integrity of the dust or how moisture migrates within the system“dust-grain”is crucial.A practical approach in determining moisture migration is the construction of sorption isotherms which help predict the moisture or water activity under specific storage conditions.
A moisture sorption isotherm defines the relationship between Awand moisture content at a constant temperature[9,10].A full isotherm comprises both an adsorption and a desorption curves.Adsorption isotherms are obtained by wetting a sample from a dry state,whereas,desorption isotherms are obtained by drying a sample from a wet state.Graphically, these two curves mismatch when they are superimposed.This phenomenon is called hysteresis and simply means that the moisture content at each water activity is higher during desorption than adsorption.Besides allowing a rapid moisture content determination from water activity analysis through an isotherm curve,sorption isotherms can help decide on a safe water activity that maximizes the dust shelf-life and storage stability while avoiding over drying or over wetting.To preserve their intrinsic properties,inert dusts should be stored under specific environment conditions that would prevent them from reaching a specific water activity content known as the critical water activity.It's vital to have a clear understanding of the implications of the critical water activity with respect to phase transitions.A rigorous investigation of kinetics of sorption properties of an inert dust, for instance, provides insight into proper packaging requirements.The differential enthalpy or isosteric heat of sorption (ΔHd) indicates the state of absorbed water by the solid material and measures the energy changes that occur during the sorption process.In other words, the isosteric heat of sorption is the energy required for evaporating the adsorbed water from liquid to gas status at determined moisture content.The net isosteric heat of sorption(Δhd)represents the quantity of energy exceeding the heat of vaporization of water(ΔHvap)associated with the sorption process.The net isosteric heat of sorption gives a measure of the water-solid binding strength.The net isosteric heat of sorption is obtained by subtracting the latent heat of vaporization of pure water from the differential enthalpy.The latent heat of vaporization of pure water is the energy required to evaporate pure water from liquid to gas state and is equal to 2,257 kJ/kg (40,626 J/mol).The latent heat of vaporization of pure water is the energy required to change a gram of a liquid into the gaseous state at the boiling point.
Several methods are available for the construction of sorption isotherms.Recent technologies tend to emphasize automation and speed.The dynamic dew point isotherm(DDI) provides a greater resolution and is the least timeconsuming method as there is no need for equilibrium to establish.DDI generates dynamic isotherms as opposed to equilibrium isotherms.The DDI is a water activity and gravimetric analysis method that controls neither water content nor water activity, but dries or wets the sample and measures water activity and water content during the wetting or drying process.The DDI approach keeps the temperature of the sample constant,while scanning relative humidity or water activity,resulting in the determination of the critical water activity of the material at the experimental temperature(Application note,Decagon devices,Pullman, Washington, USA).The working principle of the vapor sorption analyzer (VSA) during a DDI test is that water content is determined using a high precision magnetic force balance and water activity is measured using a chilled-mirror dew point sensor.During desorption, dry air flows over the sample while during adsorption wet air passes over the sample.After a short period of time, the VSA halts air flow and takes a snapshot of the sorption process by directly measuring the water activity and weight.The high resolution of dynamic isotherms makes them valuable for observing sudden changes in sorption properties associated with matrix changes such as glass transition.
The objectives of this study were:to construct full boundary sorption isotherms of Hard Red Winter(HRW)wheat and Odor-Z-Way (Inert dust) and determine the critical water activity for phase transition;to determine the relationship between the critical Awand storage temperature for wheat and zeolite;to assess the fitting of sorption experimental data with three isotherms models:Guggenheim-Anderson-de Boer (GAB), Double Log Polynomial (DLP),Brunauer-Emmet-Teller(BET);to determine the sorption kinetics of the synthetic amorphous zeolite;and to determine the optimum dust moisture content for wheat protection,at given storage temperature and initial wheat moisture content.
2.Material&methods
2.1.Wheat and zeolite preparation
It is necessary that the grain and the powder sub-samples used for analysis are representative of the initial lots or batches for valid and reproducible data.HRW wheat was procured from Heartland Mills(Marienthal,Kansas,USA)and zeolite powder was supplied by Odor-Z-Way(Phillipsburg, Kansas, USA).Wheat samples sizes were reduced by pouring one 50-lb bag of wheat into a Boerner Divider(Seedburo Boerner Divider)and into a chute splitter device(Gilson Co,Ohio,USA)and repeatedly halved until a sample of desired size was obtained.Zeolite samples sizes were reduced by pouring one 50-lb plastic bucket of dust into a chute splitter device(Gilson Co,Ohio,USA)and repeatedly halved until a sample of desired size was obtained.A total of ninety samples(250 g)were made and nine samples were systematically selected,of which,three samples were randomly assigned to each of three temperature levels(25,35,and 45°C)considered for sorption isotherms.The initial grain moisture content was determined using a Moisture Analyzer Model 930(Shore Sales Co.,Rantoul,Illinois,USA).Zeolite powder was dried overnight at 25 °C and moisture content was determined by thermogravimetric analysis(TGA)(Pyris 1 TGA,Perkin Elmer).All subsequent tests were performed with wheat at 11.5%±0.5%moisture content (wet basis) and zeolite powder at 2.0% ±0.1%moisture content(wet basis).
2.2.Sorption isotherms
Separate sorption isotherms were constructed for the zeolite powder and for the samples of HRW wheat using VSA.The VSA is capable of generating both dynamic and equilibrium isotherms.Dynamic isotherms,however,are required to investigate phase transitions and to determine critical water activities.The performance characteristics and the operating specifications of the VSA are presented in Table 1.An isotherm standard test was run using a sample of microcrystalline provided by Decagon (data not shown).The kinetic and moisture sorption isotherm curves generated were compared to a preloaded standard microcrystalline curve and less than 1%variation was the criterion to validate the working performance of the VSA.
Full dynamic dewpoint adsorption and desorption isotherms were obtained at 25,35,and 45°C to fully investigate hysteresis phenomenon, to determine the critical water activity for phase transition, and to find optimal moisture content of zeolite needed for application ontoHRW wheat.Critical water activity provides useful information for maintaining textural properties and preventing caking and clumping.Three replications were done for each isotherm temperature and(10.1±0.1)g zeolite powder or grain was used for each run.Isotherms generated using the VSA typically yielded unique sets of data;hence it was not possible to average the data.
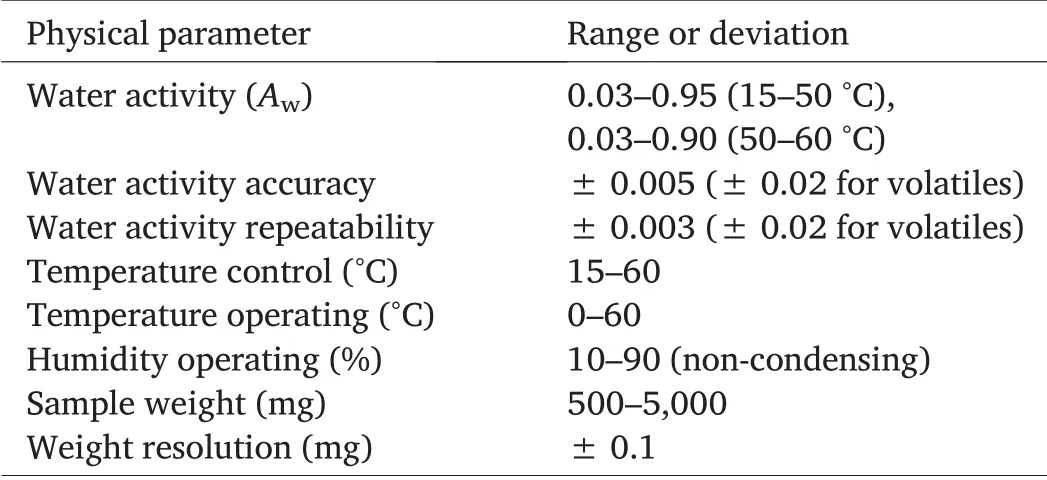
Table 1 Specifications of the Vapor Sorption Analyzer.
2.3.Water activity linear offset and weight calibration
To ensure the accuracy of the isotherms generated,water activity capacitance and chilled mirror sensors and the instrument balance were verified for correct performance.Before running a new isotherm,water activity verification was conducted using the 0.76 Aw(6 mol/kg NaCl) and the 0.25 Aw(13.4 mol/kg LiCl) standards.Weight calibration was conducted against a NIST twogram standard weight (National Institute of Standards and Technology, Maryland, USA) provided by Meter Group(formerly,Decagon).
2.4.Using DDI to investigate phase transitions
To investigate phase transition events using dynamic isotherms,a DDI test was performed with the following settings: initial water activity of 0.1, final water activity of 0.90, flow rate of 40 mL/min, resolution of 0.01, and no timeout.These settings enabled the generation of isotherms with resolutions high enough to allow the detection of inflection points.Sharp inflection points in the isotherm indicate critical water activity for phase transition.The critical water activity for wheat or zeolite powder at constant temperature was determined by second derivative curve smoothing strategies using the Excel spreadsheet developed by Meter Group,which is based on the Savitzky and Golay[11]smoothing and differentiation method.
2.5.Isotherms models
Three isotherm models were evaluated for their ability to fit the experimental data for HRW kernels and zeolite powder isotherms:Guggenheim-Anderson-de Boer(GAB),Double Log Polynomial (DLP), and Brunauer-Emmet-Teller(BET).The model equations are shown below:
DLP(Double Log Polynomial)

where m is the moisture content(%);x=ln(-ln(Aw));b0,b1,b2,and b3are empirical constants.
GAB(Guggenheim-Anderson-de Boer)
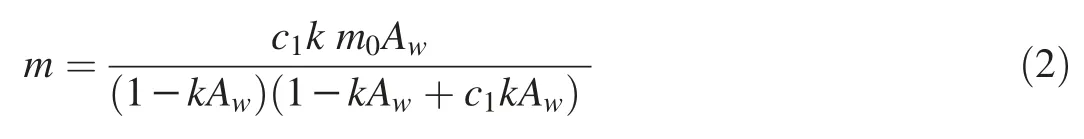
where m is the moisture content(%);m0is the monolayer moisture content(%);Awis the water activity at moisture content m;c1,k,and m0are empirical constants.
BET(Brunauer-Emmet-Teller)
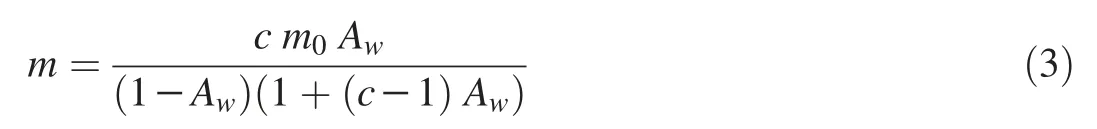
where m is the moisture content(%);m0is the monolayer moisture content(%);Awis the water activity at moisture content m;c and m0are empirical constants.
2.6.Error functions and assessment of goodness-of-fit
Five error functions were used to evaluate the goodnessof-fit of each isotherm model.Vpredis the moisture content calculated from the model,Vobsis the experimental moisture content,is the average experimental moisture content,and n is the number of data points in the experimental sorption isotherm.
Coefficient of determination(R2)
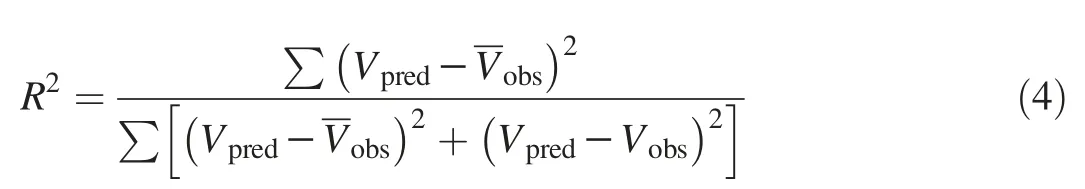
The sum of the squares of the errors(SSE)

The sum of the absolute errors(SAE)

The mean relative deviation(MRD)

The standard error of prediction(SE)
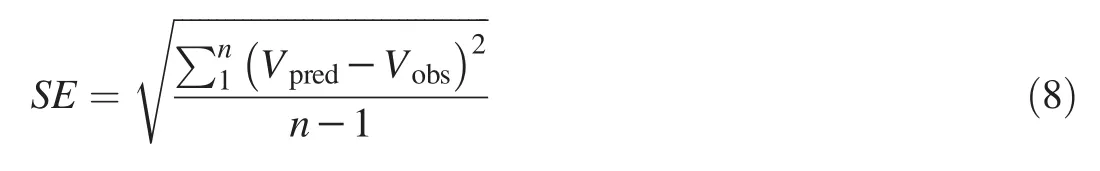
2.7.Optimal water activity and equilibrium moisture content(EMC)
When grain is admixed with an amorphous dust,the direction of moisture sorption for each entity in the mixture is dictated by the water activity until an equilibrium condition is achieved.To prevent moisture migration during storage,the water activity of the dust must be equal to that of HRW wheat,at constant storage temperature.The water activity values and moisture content of HRW wheat,at constant temperature,can either be calculated from the bestfitting adsorption or desorption equations or determined graphically from the sorption isotherms.The corresponding moisture content of the inert dust to meet this requirement is then obtained graphically or through the best-fitting adsorption equations of the zeolite powder.Once the water activity of the system wheat-inert dust at equilibrium is determined,it is essential to verify that this value does not exceed the critical water activity for each component of the system.
2.8.Sorption kinetics:Net isosteric heat of sorption,differential enthalpy,and differential entropy
The relationship between the net isosteric heat of sorption(Δhd)and the differential entropy(ΔSd)is given by:
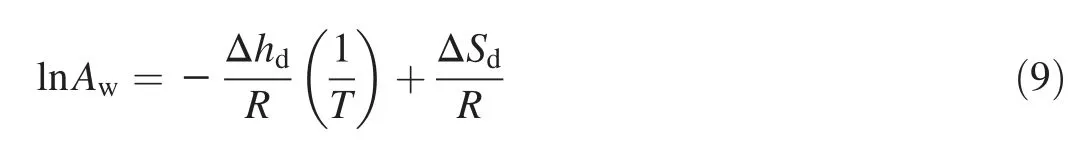
The differential entropy of sorption was obtained from the Y-axis intercept by plotting ln(Aw)vs.1/T,while the net isosteric heat of sorption was obtained from the slope of the line resulting from plotting ln(Aw)versus 1/T at constant moisture content[12].
The relationship between the net isosteric heat of sorption(Δhd)and the differential enthalpy(ΔHd)is given by:

where,ΔHvapis the latent heat of water vaporization.
2.9.Water activity prediction:The Clausius-Clapeyron equation
The relationship between water activity and temperature is given by the Clausius-Clapeyron equation:
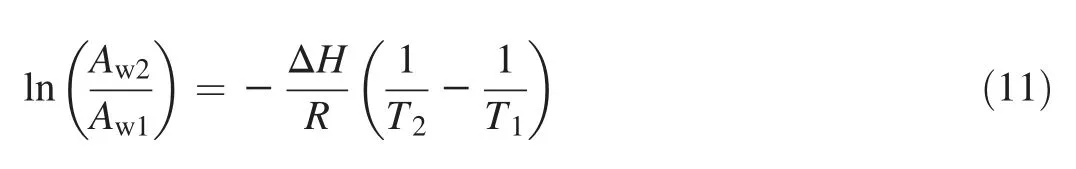
Knowing the heat of sorption,water activity at a given temperature can be derived from the Clausius-Clapeyron equation:

where Aw1is the water activity at temperature T1(K),Aw2is the water activity at temperature T2(K),ΔH=heat of sorption (J/mol), and R is the universal gas constant(8.31 J/(mol·K)).
The heat of sorption(ΔH)is moisture-dependent and is calculated by dividing by R the slope of the line obtained by plotting ln(Aw) vs 1,000/T.The heat of sorption (ΔH)in our study was automatically determined by using the Temperature Effect Tool included in the Moisture Analysis Toolkit 1.0.1328 (Meter group, Pullman, Washington,USA).Briefly,at least three isotherm files and three specific temperatures(°C)are uploaded in the software and one specific value of moisture(%wet basis)is specified as well as the direction of sorption.The heat of sorption generated is specific to that moisture content.
2.10.Statistical analysis
The coefficients for the different models were obtained using Moisture Analysis Toolkit 1.0.1328 (Meter Group).The coefficient of determination(R2),the sum of squares of the errors(SSE),the sum of the absolute errors(SAE),the mean relative deviation(MRD),and the standard error of estimate(SE)were used to assess and compare the goodness of fit of each model.Critical water values at different storage temperatures were compared using SAS One-way ANOVA.
3.Results&discussion
3.1.Full sorption isotherms
Water activities at the extremities of the sorption curve(below 0.1 and above 0.8) usually require much longer time for equilibrium to establish.Even though DDI do not require equilibrium conditions to proceed,the amount of time required to complete the sorption analysis was significantly decreased by limiting the water activity range between 0.2 and 0.8.The full sorption isotherms of zeolite powder and wheat samples were shown in Figs.1 and 2.According to BET classification[13]and the IUPAC classification[14],HRW wheat had typical type II sigmoid shape isotherm,whereas,zeolite powder had a sorption isotherm close to resembling a type IV sigmoid shape isotherm.Type II isotherms are exhibited by nonporous or macroporous solids (pore size >50 nm) and represents unrestricted monolayer-multilayer adsorption,while Type IV isotherms are mostly characteristic of mesoporous solids (pore size 2-50 nm) and are indicative of multilayer adsorption followed by capillary condensation [15].Although the Brunauer and the IUPAC classifications of gas-solid adsorption are commonly used to determine the nature of adsorption, Donohue and Aranovich [16] have found some limitations to these classifications because they rely on the absolute adsorption rather than the Gibbs adsorption,hence giving the incorrect impression that adsorption isotherms are always monotonically increasing functions of pressure.Full sorption isotherms of zeolite and wheat obtained at 25,35,and 45°C clearly showed the hysteresis phenomenon (Figs.1 & 2), resulting from lower equilibrium moisture contents during adsorption than during desorption.In fact, the hysteresis phenomenon does not occur in pores smaller than 2 nm.Hysteresis is rather observed when the pores are large enough for the adsorbing molecules to condense to a liquid[17,18].Moreover,the type of hysteresis indicates the shape of the pores of the material being analyzed.Considering the IUPAC classification of isotherms, the hysteresis loops were of type H3 for HRW wheat,and of type H4 for zeolite powder.Type H3 hysteresis are aggregates of plate-like particles forming slit-like pores, while type H4 hysteresis are narrow slitlike pores,particles with internal voids of irregular shape and broad size distribution, mostly hollow spheres with walls composed of ordered mesoporous silica[19,20].Adsorption hysteresis in porous materials such as inert dusts is characterized both by the shapes of the hysteresis loops and by the way in which they depend on temperature[21].The intensity of hysteresis remained unchanged for HRW wheat, as hysteresis loop height and width did not change with increased temperatures(Fig.2).Conversely,the intensity of hysteresis decreased with increased temperatures during water adsorption by porous zeolite powder (Fig.1).Similar observations were made by Dubinin[22] during adsorption of CO2on porous silica gel.The explanation provided by Dubinnin is that the hysteresis loops develop somewhat below the triple point temperature of the adsorptive, shrink as the temperature is raised and disappear some distance below the bulk critical temperature.
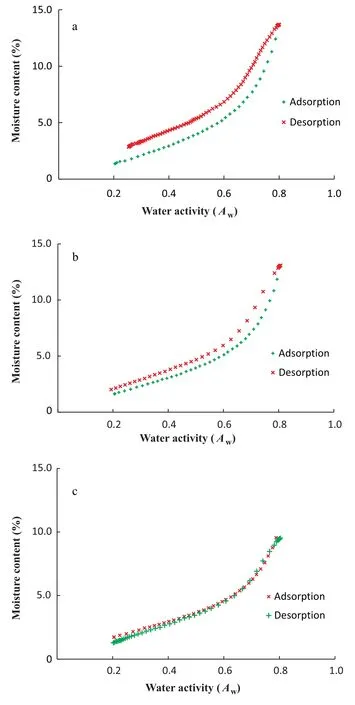
Fig.1.Full sorption isotherms of zeolite powder at 25(a),35(b),and 45°C(c).
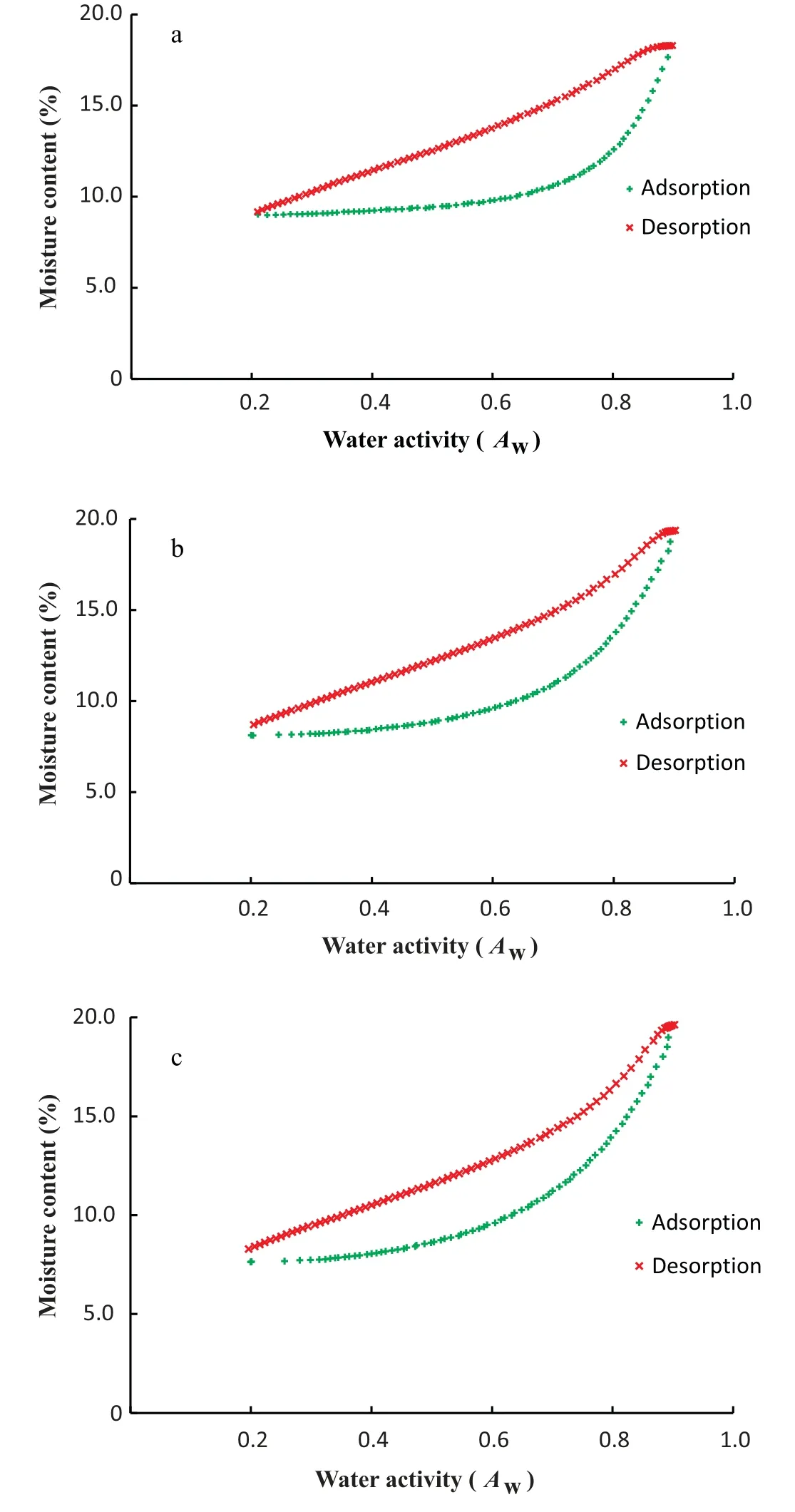
Fig.2.Full sorption isotherms of HRW wheat at 25 (a), 35 (b), and 45°C(c).
3.2.Isotherm models
Experimental data were fitted by means of GAB, DLP,and BET models.All three models are single-temperature models,meaning they do not involve temperature as a parameter.Estimated parameters of models for the sorption isotherms of HRW wheat and zeolite powder are presented in Tables 2 and 3.The coefficient of determination is often the first error function considered in isotherm sorption data fitting.The coefficient of determination (R2) is the square of the correlation(r)between predicted and experimental values and it ranges from 0 to 1.While achieving R2=1 is most preferred,this is virtually impossible,and common values of R2are comprised between 0 and 1.The best fitting model should feature an R2close to 1, which means the dependent variable can be predicted with minimal error from the independent variable.Other error functions reported in sorption data fitting include the standard error (SE), the sum of the absolute errors (SAE), the sum of the squares of the errors (SSE), and the mean square error (MSE).Contrary to R2, these four error functions must be kept as minimal as possible for the model to be meaningful.Error function values associated to adsorption and desorption data of zeolite powder and HRW wheat are featured in Tables 2 and 3.Irrespective of sorption direction,DLP model was the best model to estimate zeolite and HRW wheat sorption isotherms,followed by GAB and BET models.As expected,BET model provided almost perfect fitting to sorption data, only in the water activity range 0-0.5[23].Monolayer moisture content values(m0)for each sorption direction were provided only by GAB and BET models and indicated a decrease in monolayer moisture content with an increase in temperature(Tables 2 and 3).The monolayer moisture content value is the value at which a food product is most stable.Duckworth and Smith [24] demonstrated that solute movement was not detectable below the monolayer value but was detectable above it.Because reactant mobility is a prerequisite for reactivity,lower mobility at the monolayer translates into greater product stability.A faster determination ofthe moisture content of any sample of wheat or zeolite with known initial water activity can be achieved by using the DLP fitting equations generated for each sorption direction(Table 4).
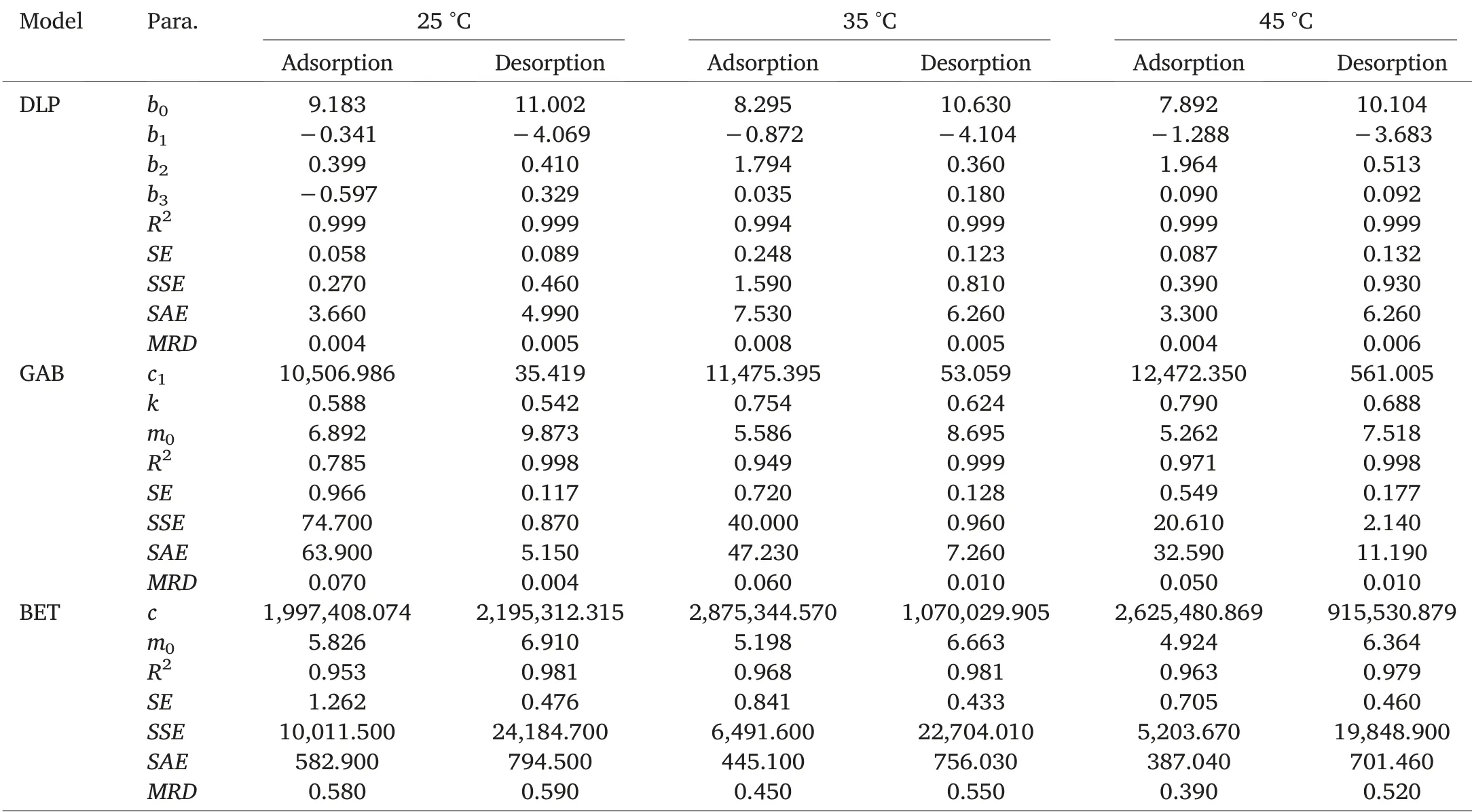
Table 2 Estimated parameters of models for the sorption isotherms of HRW wheat.
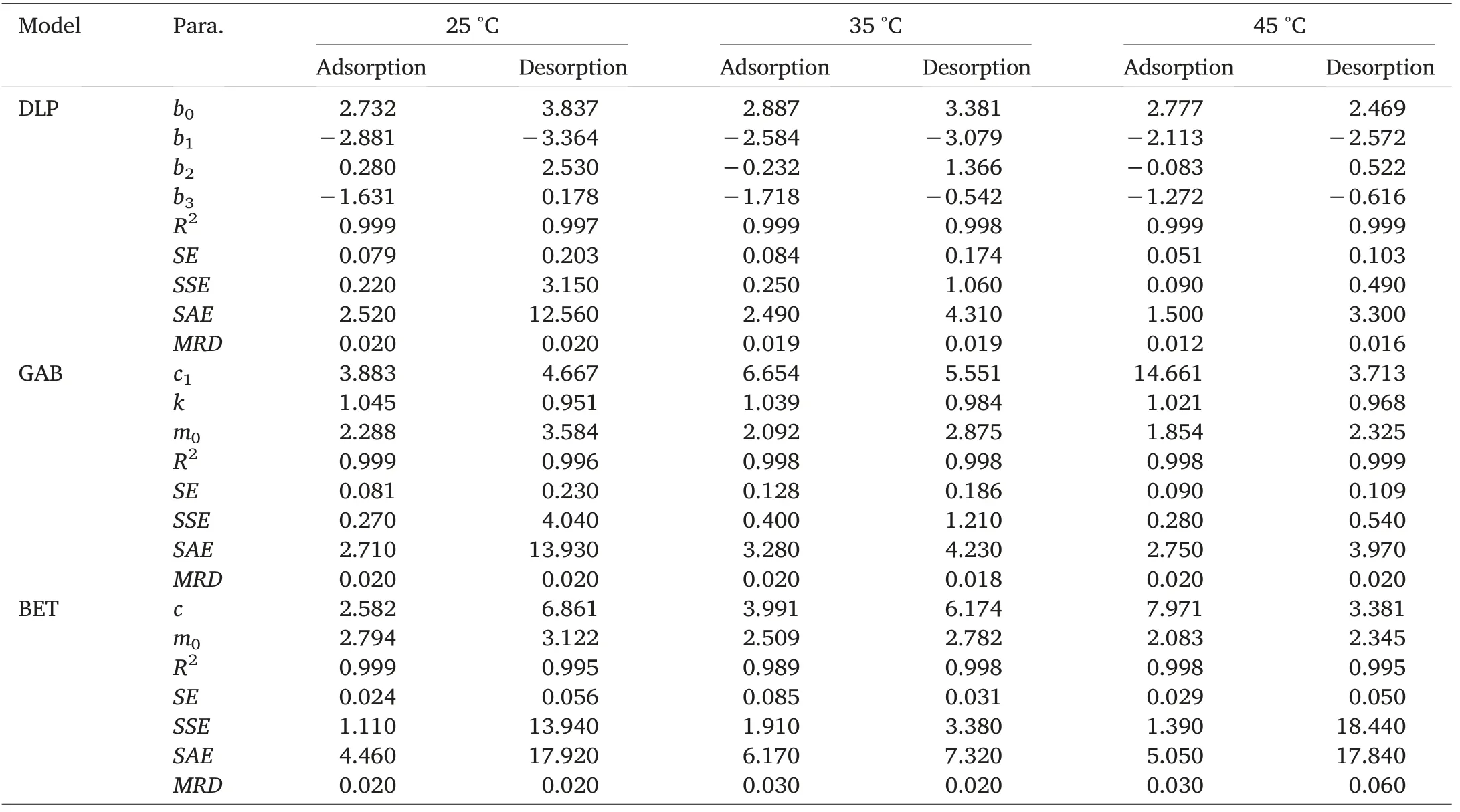
Table 3 Estimated parameters of models for the sorption isotherms of zeolite powder.
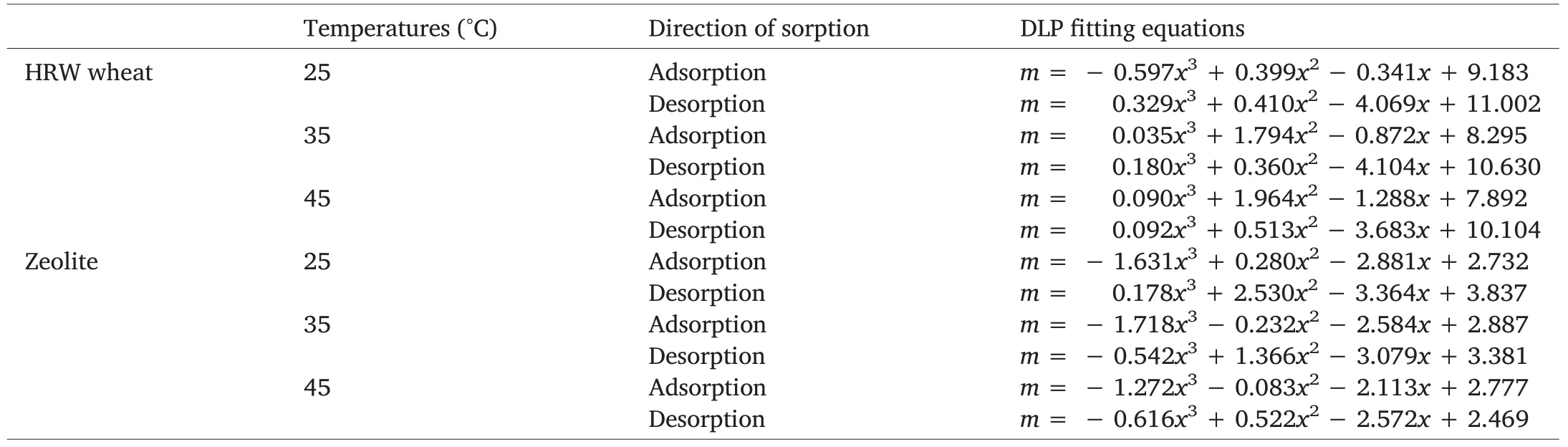
Table 4 Best-fitting equations to sorption experimental data of zeolite powder and HRW wheat.
3.3.Critical Aw for phase transitions
The critical water activity(Awc)for phase transition is the water activity of HRW wheat or zeolite powder above which the rate of phase transition reactions is accelerated.Critical water activities are necessary for maintaining textural properties and preventing caking and clumping.If the water activity of the inert dust or the grain rises above the critical water activity for phase transition,the stability will decrease as time dependent processes such as stickiness,structure collapse,and crystallization speed up significantly [25].Critical water activity values for zeolite powder significantly increased from 0.639 to 0.657 as temperatures increased from 25 to 45°C.The same trend was observed with HRW wheat as water activity valuesincreased from 0.700 to 0.790 for the same temperature range(Table 5).The corresponding equilibrium moisture content for each critical water activity indicates the maximum storage moisture content at a specific temperature.For instance,moisture of zeolite should not exceed 6.07%at 25 °C and wheat moisture content should not exceed 15.24%at 25°C(Table 5).Plotting the second derivative of moisture content versus water activity yields the first matrix transition or glass transition,the second matrix transition (onset of crystallization), and the third matrix transition(the point of dissolution or deliquescence).Depending on the type of material being analyzed (amorphous,or crystalline),all or part of the transition matrices are present on the second derivative plot.
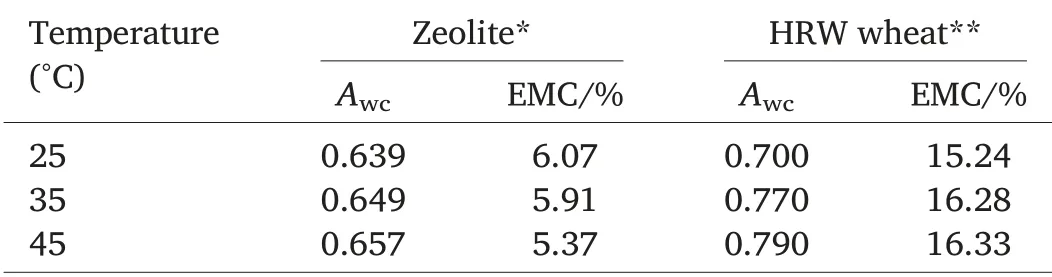
Table 5 Critical water activity(Awc)and corresponding equilibrium moisture content(EMC)(%wet basis)for phase transition in zeolite powder and HRW wheat.
3.4.Optimal storage Aw and EMC
Wheat desorption isotherm at a specific temperature was used to find the corresponding water activity if we intended to store wheat at a specific moisture content.The corresponding water activity value was introduced into zeolite adsorption curve to determine the corresponding moisture content by linear extrapolation.We generated a table to recommend storage moisture contents for inert dust and wheat at different temperatures(Table 6).For example,if wheat at 12%moisture content is intended to be stored at 25°C,it should be treated with zeolite dust at 3.53%moisture content and the water activity at equilibrium is expected to be around 0.46.
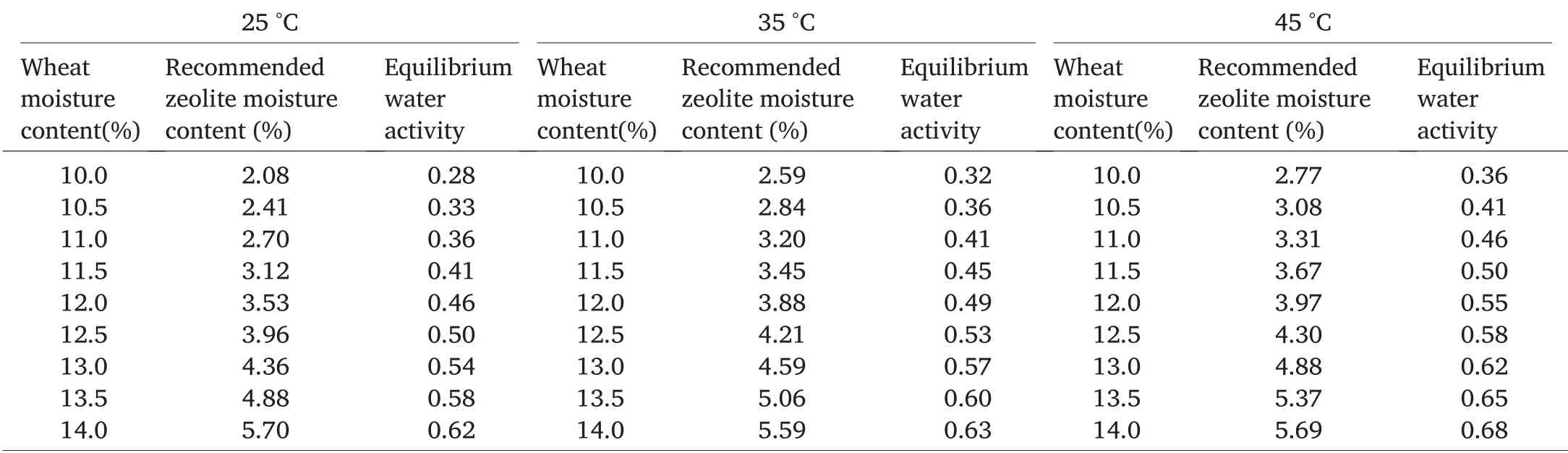
Table 6 Recommended zeolite moisture content and equilibrium water activity for grain(HRW wheat)protection at different grain moisture contents and storage temperatures.
3.5.Temperature effect:Water activity prediction
The“Temperature-effect”tool of the VSA software can help with the prediction of water activity of wheat and zeolite at different storage temperatures(Table 7).Water activity was strongly linearly correlated(0.995 <R2<0.999)with temperature at constant zeolite or wheat moisture contents(Table 8).The slopes of the lines obtained by plotting water activity as a function of temperature are higher during desorption than adsorption, which is a mathematical corroboration of the hysteresis phenomenon taking place in both wheat and zeolite samples.The equations presented in Table 8 allow an easy and quick determination of the water activity of zeolite or wheat samples of known moisture content stored under a specific temperature.
The linear relationship between zeolite moisture content(Z)and wheat moisture content(W)is temperature dependent and expressed as:
Z=0.868W-6.782(R2=0.98,25°C).
Z=0.740W-4.946(R2=0.99,35°C).
Z=0.744W-4.812(R2=0.98,45°C).
These linear equations can help determine faster and with at least 98%accuracy the moisture content of a synthetic amorphous dust required to protect grain of known moisture content under a specific storage temperature.For instance, at 25 °C if wheat is 12% moisture content (wet basis),the optimal moisture content for zeolite to be applied on wheat is computed as follows:
Z =0.868×12-6.782 =3.634.
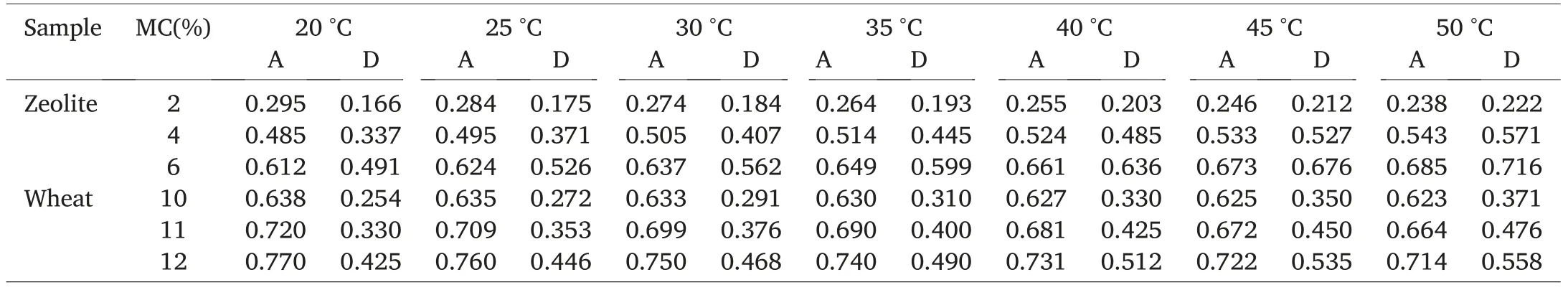
Table 7 Predicted water activity during adsorption and desorption of HRW wheat and zeolite powder at different moisture contents and storage temperatures.
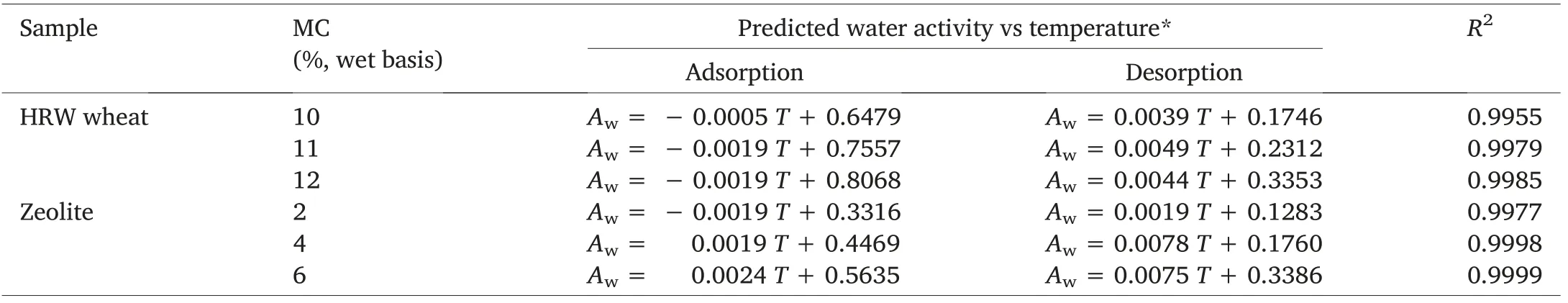
Table 8 Temperature effect:Prediction of water activity of wheat and zeolite at different moisture contents.
3.6.Sorption kinetics
Sorption kinetics(isosteric heat of sorption,net isosteric heat of sorption,and differential entropy)were determined for zeolite powder at different moisture contents(Fig.3).The net isosteric heats of sorption and the differential enthalpy of zeolite estimated by the Clausius-Clapeyron equation and determined graphically decreased with increasing moisture content of zeolite.Conversely,differential entropy of zeolite decreased with increasing zeolite moisture content.Similar results were found by Bonner and Kenney [26], Heras et al.[27] and Bennaceur et al.[28],while investigating the net isosteric heat of sorption of dry persimmon leaves,Henna leaves,and energy sorghum,respectively,at various moisture contents.The explanation to that observation is that,at lower moisture content,water molecules are tightly bound to the active polar sites by primarily the hydrogen bond.
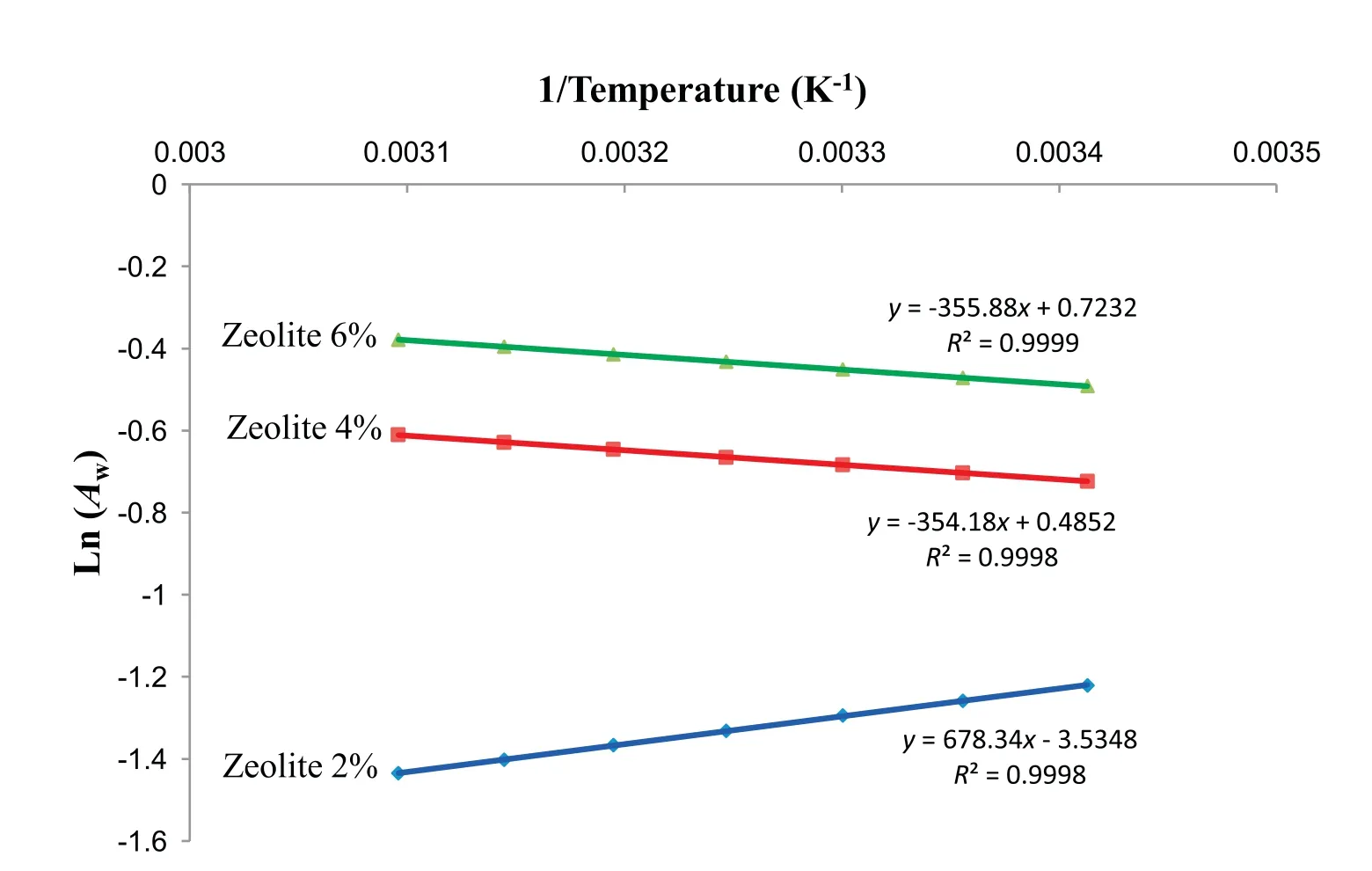
Fig.3.Graphical determinations of the net isosteric heat of sorption,the differential enthalpy,and the differential entropy of zeolite powder at different moisture contents.
4.Conclusions
The optimal moisture content of the synthetic amorphous zeolite intended for grain protection is temperature and wheat moisture dependent and it increases with increasing storage temperature and wheat moisture content.The application of a dust at the optimal moisture content helps minimize moisture migration within the system“wheat-dust”, thus ensuring inert dust maximal efficacy.Further studies will investigate the effects of moisture content and application rates of inert dusts on dust and wheat physical properties.
Conflicts of interest
The authors declare that there are no conflicts of interests.
Acknowledgements
This research was funded by the Plant Biosecurity Cooperative Research Center(Department of Industry and Science, Australian Government.Grant No.63058).We would like to express our heartfelt gratitude to Dr.Michael Robinson,CEO and currently Managing Director of the Australian Plant Biosecurity Science Foundation(APBSF).
杂志排行
Grain & Oil Science and Technology的其它文章
- Studies of the quality parameters of blended oils and sensory evaluation of gram flour products
- Optimization for quantification of sorghum tannins by Ferric ammonium citrate assay
- Recent advances in quality deterioration and improvement of starch in frozen dough
- The efficacy of sorghum flour addition on dough rheological properties and bread quality:A short review
- Retraction notice to"Health benefits of black rice-A review".[GAOST 2(2019)109-113]
