Colonization and Probiotic Effect of Metschnikowia sp. C14 in the Intestine of Juvenile Sea Cucumber, Apostichopus japonicus
2020-03-09LIMingBAOPengyunSONGJianDINGJianfengLIUYubinandMAYuexin
LI Ming, BAO Pengyun, SONG Jian, DING Jianfeng, LIU Yubin, and MA Yuexin
Colonization and Probiotic Effect ofsp. C14 in the Intestine of Juvenile Sea Cucumber,
LI Ming, BAO Pengyun, SONG Jian, DING Jianfeng, LIU Yubin, and MA Yuexin*
,,116023,
Viable cell count was used to determine whethersp. C14 can colonize the intestine of juvenile sea cucumber. Sea cucumber individuals were divided into two groups, which were fed the control diet for 38 days or the C14-supplemented diet at 105cellsg−1diet for 28 days, then the control diet from day 29 to day 38. The number of C14 cells in the intestine of sea cucumber fed the C14-supplemented diet significantly increased from day 7 to day 28, and decreased from day 29 to day 38. Sea cucumber fed with the diet containing C14showed a significant increase in trypsin activity and lipase activity from day 21 to day 33 compared with the control. Feeding C14 significantly improved the phagocytic activity and respiratory burst in coelomocytes from day 21 to day 35 and from day 14 to day 38, respectively. In addition, there was an obvious enhancement in lysozyme activity (from day 21 to day 38 or day 33), phenoloxidase activity (from day 21 to day 28) and total nitric oxide synthase activity (from day 14 to day 38) in coelomic fluid supernatant and/or coelomocyte cell lysate supernatant compared with the control. There were significant positive correlations between the number of C14 cells colonizing the intestine and trypsin activity of the intestine, lysozyme activity of the coelomic fluid supernatant and coelomocyte lysate supernatant from sea cucumber. These data suggested that the number of C14 cells should be maintainedat 105cfu(colony-forming units)g−1intestinematerial for the maximum benefit.
; feeding duration; colonization;sp. C14; digestive enzyme activity; immune parameter
1 Introduction
Probiotics are live microorganisms that can benefit the health of the host (FAO/WHO, 2001). For the aquatic ani- mals, the beneficial effect may be reached by various me- chanisms including, for example, nutritional complement, improvement of the digestibility of feed, enhancement of innate immunity, antibacterial activity, stimulation of bio- logical processes, improvement of the quality of the water among others (Verschuere., 2000; Prado., 2010; Newaj-Fyzul., 2014). In recent years, bacterial and yeast probiotics such as(Zhao, 2012),sp. BC26 (Liu., 2013),(Yan, 2014a),(Yang., 2015; Zhao., 2016), the mixture ofand(Li., 2015),(Ma, 2013, 2014), lactic acid bacteria (Li., 2018),sp. (Liu., 2012; Yang, 2014),(Yan, 2014b),(Chi.,2014),(Wang., 2015),(Zhang., 2017),sp. (Yang., 2015a, 2015b),(Chi, 2014),sp. (Liu.,2017) and(Chi., 2014) have been demonstrated to improve growth, digestive enzyme activity and immune response of sea cucumber, and enhance their resistance to pathogen attack. In addition, dietary ad- ministration of probiotics do not affect significantly the intestinal microbiota of sea cucumber (Ma., 2018, 2019). Analysis of gut microbiota revealed a significant difference in the relative abundance ofbetween diseased and healthy sea cucumbers (Zhang, 2018). The intestinal microbiota homeostasis of sea cucumber can be improved by probiotics (Yang., 2017).
Several studies have applied different feeding durations varying between 7 and 45 days to improve the immune res- ponse in sea cucumber (Liu., 2012; Ma, 2013; Chi., 2014)though the reason for choosing different durations is not clear. Three strains of marine yeast have been confirmed to be capable of colonizing insea cucum- ber intestine for at least 31 days after cessation of feedingfollowing 15 days of administration (Ma, 2014; Yang, 2014; Yang., 2015a). In the South African abalone, there is a positive correlation between yeast/bacterium quantity and enzyme activity (Macey and Coyne, 2006). The yeassp. C14 was ori- ginally isolated from the intestine of healthy adult sea cucumber(Liu., 2012). It can effectively colonize the juvenile sea cucumber intestinedietary supplementation and improve their specific growth rate and digestive enzyme activity (Yang., 2014) and immune response and resistance to pathogen infection (Liu., 2012). Nevertheless, no information is available for the correlation between the number of probiotic cells in sea cucumber intestine and the probiotic effect. Therefore, the aim of this study was to determine the effect of C14 with different feeding durations on digestive enzyme activity and immune response of sea cucumber.
2 Materials and Methods
2.1 Yeast and Diet Preparation
The yeast C14 was cultured overnight in yeast-peptone- dextrose (YPD) broth at 25℃ with constant shaking. The cellular suspension was centrifuged at 1000×and 4℃ for 10min with the pellet washed and re-suspended in 0.9% NaCl. The concentration of the yeast suspensionwas ad- justed to about 106cellsmL−1using a haemocytometer slide. The formulation and proximate composition of the control diet were prepared following the method described by Liu. (2012). Suspension was added to the control diet and mixed thoroughly to achieve 1×105cellsg−1, which was prepared each day to guarantee the vitality of C14. Selection of the C14 dose inthe diet was based on the data documented previously (Liu., 2012).
2.2 Feeding Trail
Sea cucumber individuals purchased from a commercial farm were acclimated to the rearing condition for two weeks. Then selected sea cucumber individuals with similar size (0.92±0.01g) were randomly distributed into six plastic tanks (100L), 100 each. Sea cucumber in three tanks was fed the C14-supplemented diet for 28 days, then the control diet from day 29 to day 38 while the animal in other three tanks was fed the control diet for 38 days. Sea cucumber was fed once a day at 16:00. Water temperature ranged from 17℃ to 22℃, salinity from 33 to 34 and acidity from pH 7.8 to 8.2. Dissolve oxygen was maintained at or near saturation by aerationair stones. Fifty liters of water each tank was replaced with fresh seawater every day.
2.3 Sample Collection
After the start of feeding, ten sea cucumber individuals each tank were randomly sampled for enumeration of live yeast, digestive enzyme activity and immune parameter assays on days 7, 14, 21, 28, 29, 31, 33, 35 and 38. Before sampling, sea cucumber was starved for 16h (Liu., 2013). Fifty microliters of coelomic fluid from each of ten sea cucumber individuals was pooled and mixed with an equal volume of isotonic aqueous anticoagulant solution (Xing, 1998). Four hundred microliters of the coe- lomic fluid was taken for phagocytic activity and respiratory burst tests while the remaining was centrifuged at 800×and 4℃ for 10min with the supernatant collected and used directly for lysozyme (LSZ), phenoloxidase (PO) and total nitric oxide synthase (T-NOS) activities assays. The coelomocyte lysate supernatant (CLS) was prepared following the method described by Ma. (2018) and used for LSZ, PO and T-NOS activities assays.
The intestines from ten sea cucumber individuals were pooled and homogenized in nine volumes of sterile 0.9% NaCl. A portion of the intestinal homogenate was taken for enumeration of live yeasts, and the remaining was cen- trifuged with the supernatant used for digestive enzyme activity analysis.
2.4 Protein Content Assay
The soluble protein content of the coelomic fluid, CLS and the supernatant of intestine homogenates were meas- ured following the method described by Bradford (1976).
2.5 Determination of the Number of C14 in Intestine
Intestinal homogenates were serially diluted using 0.9% NaCl, and 0.1mL volume was spread onto the surface of duplicate plates of yeast-peptone-dextrose agar and incu- bated at about 25℃ for 7 days. The yeast cell number is recorded as colony-forming units (cfu) per gram of fresh intestinematerial (cfug−1).
2.6 Digestive Enzyme Activity Measurement
Trypsin, amylase and lipase activities were measured according to the methods described by Ma. (2018).
2.7 Immune Parameter Assay
Phagocytic activity of coelomocytes was measured using the uptake of neutral red stained zymosan particles based on the method of Ma. (2013). The respiratory burst or superoxide anion generation of coelomocytes was measured spectrophotmetrically using a nitroblue tetrazolium (NBT) assay (Song and Hsieh, 1994) except that the assaying temperature was changed from 37℃ to room temperature (about 20℃). The absorbance of the dissolved cytoplasmic formazan was read at 630nm and expressed as NBT activity per 100μL coelomic fluid (Sajeevan, 2006). LSZ activity in coelomic fluid supernatant (CF) and CLS was estimated following the method of Ma. (2018). PO activity was determined spectrophotometrically using L-3,4-dihydroxyphenylalanine as a substrate and trypsin as an elicitor as previously described by Ma. (2013). T-NOS activity was determined by colorimetric analysis using a commercial test kit (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s instructions except that the temperature for the reaction was changed to room temperature (about 20℃).
2.8 Statistical Analysis
Statistical analysis was conducted using the SPSS 19.0 for windows. Data of the digestive enzyme activity and immune parameter between two groups were analyzed using an independent samples-test. The number of live yeast was analyzed using one-way analysis of variance. If significance was detected, Tukey’s multiple range test was used to compare the means between sampling dates. Pearson’s product moment correlation coefficient was used to determine the strengths of the association between di- gestive enzyme activity/innate immune parameter and yeast cell numbers. Prior to statistical analysis, the yeast cell num- ber was logarithm-transformed to alleviate heteroscedasti- city. Differences were considered significant if<0.05.
3 Results
3.1 Colonization and Persistence of C14 in the Intestine of Sea Cucumber
C14 was only isolated from the intestine of sea cucum- ber fed the probiotic-supplemented diet. Moreover, the num- ber of C14 cells was significantly increased from day 7 to day 28 (<0.05) with the highest 4.43×105cfug−1on day 28. However, the C14 cell number in the intestines decreased significantly within 10 daysfollowing cessation of feeding with the C14-supplemented diet (<0.05) (Fig.1). There was no significant difference in C14 cell number be- tween day 21 and day 33.
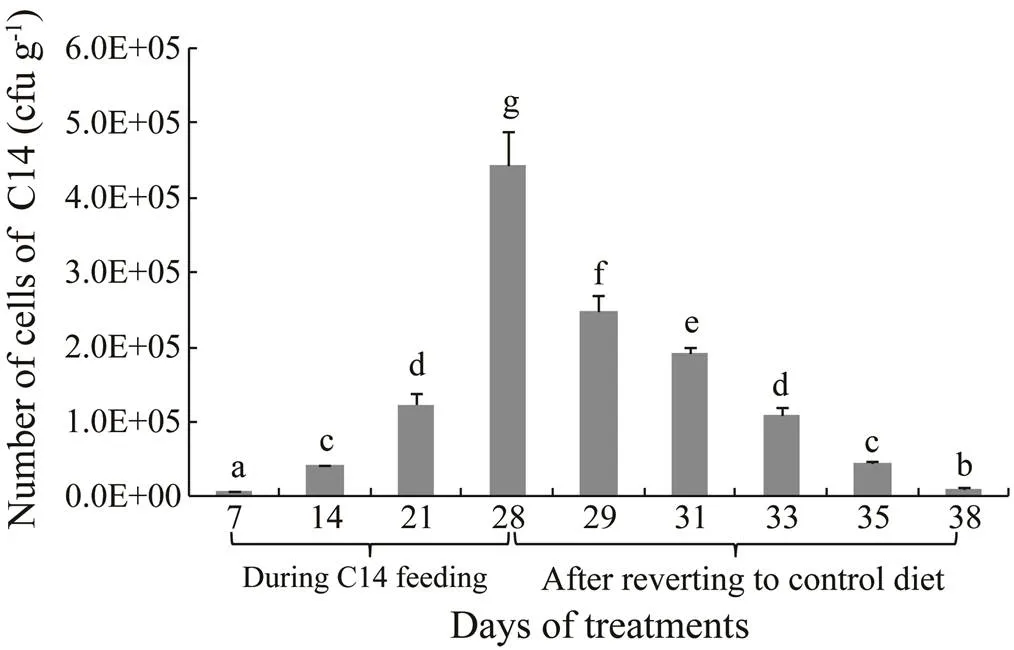
Fig.1 The number of C14 cells in the intestines of sea cucumber during 28 days of feeding with C14-supplemented diet and after reverting to control diet for another 10 days.Means without a same letter differed significantly (P<0.05) between the feeding regimes.
3.2 Digestive Enzyme Activity
The intestinal trypsin and lipase activities in sea cucumber fed with C14-supplemented diet for 21 and 28 days were significantly higher than those in the animal fed the control diet (<0.05) (Figs.2–3). Furthermore, there was significant difference in trypsin and lipase activitiesbetween two groups from day 29 to day 33 after the cessation of feeding (<0.05). However, no significant difference in amylase activity was observed between the control and the C14 feeding groups.
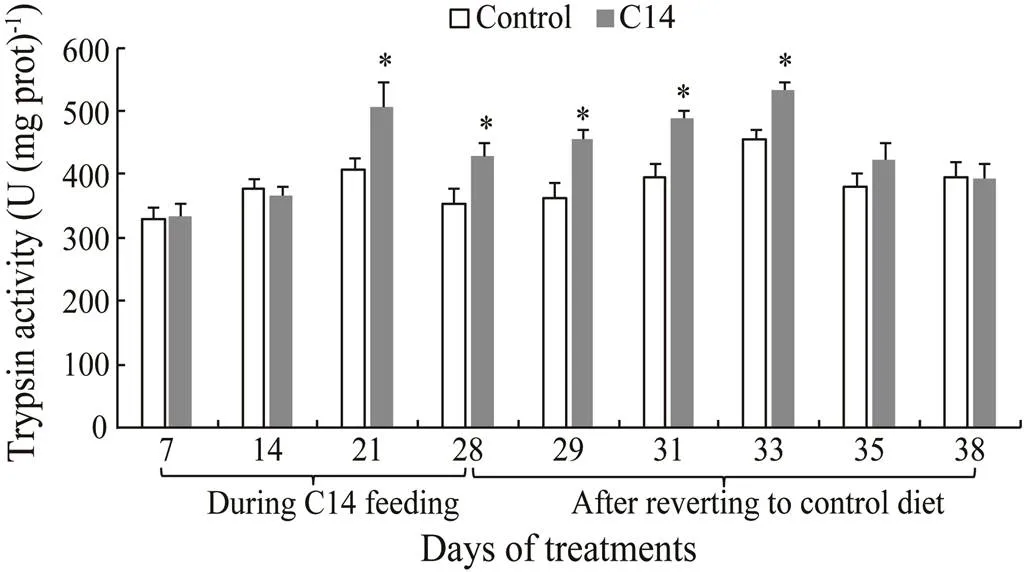
Fig.2 Intestinal trypsin activity of sea cucumber cross 38 days of feeding; Data represent means±SD (n=3). * Significant difference (P<0.05) from the control group at the same sampling date.
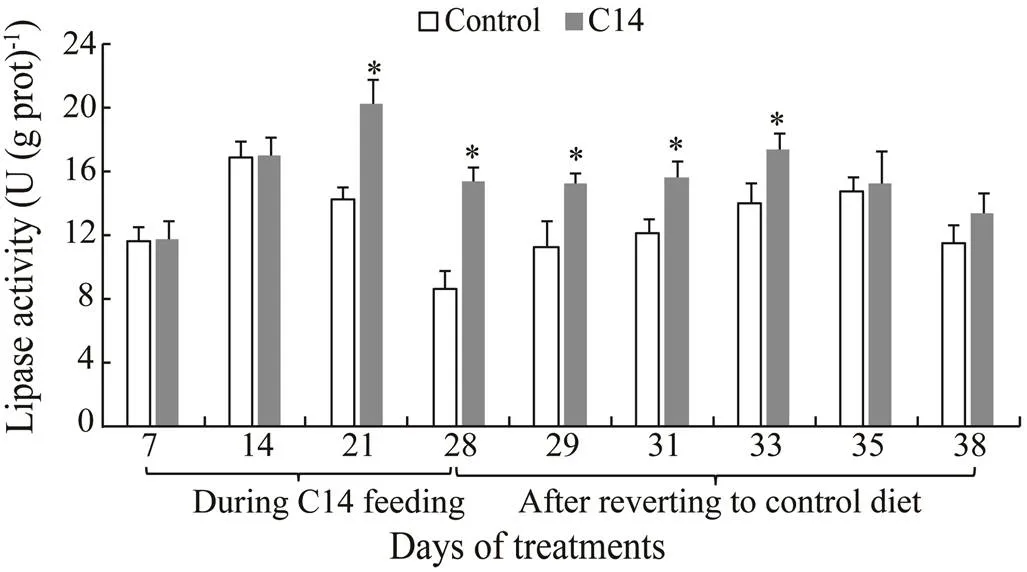
Fig.3 Intestinal lipase activity of sea cucumber cross 38 days of feeding; Data represent means±SD (n=3). * Significantly difference (P<0.05) from the control group at the same sampling date.
A correlation analysis was conducted to determine the strength of the association between digestive enzyme activity and the number of C14 cells in intestine in sampling period. The Pearson’s product moment correlation between trypsin activity and the C14 cell number was positive (=0.68;<0.05). Nevertheless, there was no correlation between lipase or amylase activity and the C14 cell number (Table 1).
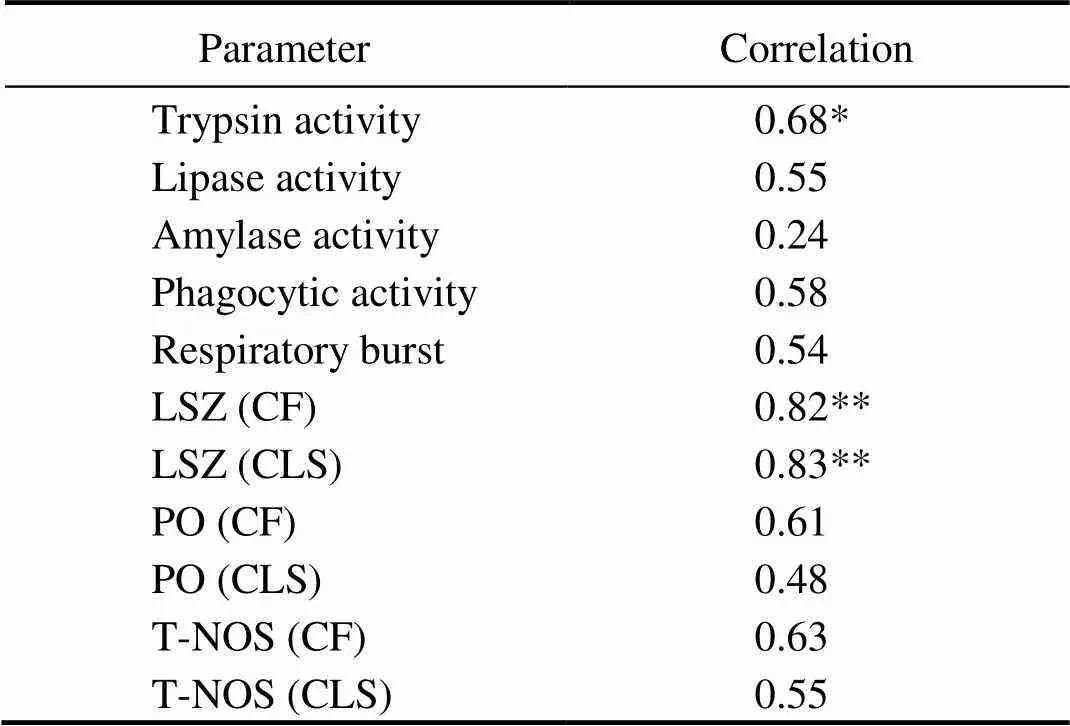
Table 1 Pearson’s correlations between parameters and logarithm-transformed numbers of C14 cells
Notes: * Significantly different at<0.05; ** Significantly different at<0.01 (=9).
3.3 Immune Parameter
The phagocytic activity of sea cucumber fed C14-supple- mented diet showed a significant increase compared to the control after 21 and 28 days of feeding (<0.05) (Fig.4). Moreover, there was significant difference in phagocytic activitybetween two groups from day 29 to day 35 after the cessation of feeding (<0.05) (Fig.4).

Fig.4 Phagocytic activity in coelomocytes of sea cucumber over 38 days of feeding. Data represent means±SD (n=3). * Significantly difference (P<0.05) from the control group at the same sampling date.
Sea cucumber fed with C14-supplemented diet for 14, 21 and 28 days had a significant higher respiratory burst than those fed the control diet (<0.05). When the animal was shifted to the control diet, significant differences were still observed between two groups from day 29 to day 38 (<0.05) (Fig.5).
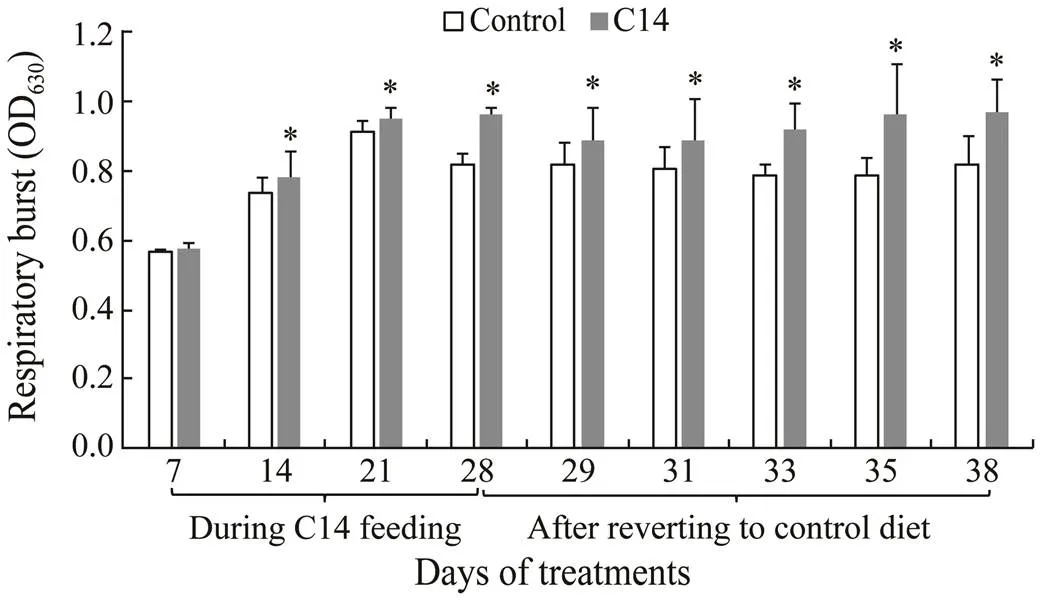
Fig.5 Respiratory burst in coelomocytes of sea cucumber over 38 days of feeding. Data represent means±SD (n=3). * Significantly difference (P<0.05) from the control group at the same sampling date.
There was a significant increase in the LSZ activity of CF and CLS of sea cucumber fed C14-supplemented diet on days 21 and 28 compared with the level observed in the control (<0.05) (Figs.6–7). Furthermore, the LSZ activity of CF and CLS from the experimental sea cucumber was statistically higher than those of control animal from day 29 to day 38, and from day 29 to day 33, respectively, after the cessation of feeding (<0.05).
Sea cucumbers fed with C14-supplemented diet had significantly higher PO activity in the CF on days 21 and 28, and in the CLS on day 14 compared with those fed the control diet (<0.05) (Figs.8–9). However, no significant difference in PO activity was observed between two groupson the other days.
The T-NOS activity in the CF and CLS of sea cucumber fed C14-supplemented diet was statistically different fromthat of control from day 14 to day 38 (<0.05) (Figs.10–11).
Different digestive enzyme activities and immune parameters were observed among the 38 days from the control group. A correlation analysis was conducted to deter- mine the strength of the association between innate immune parameters and the number of C14 cells in the intestines over the 38-day sampling period. The Pearson’s product moment correlation between LSZ activity in CF/ CLS and the C14 cell numbers revealed a positive correlation (=0.82/0.83;<0.01). Conversely, no correlation was found between the other innate immune parameters and C14 cell numbers (Table 1).
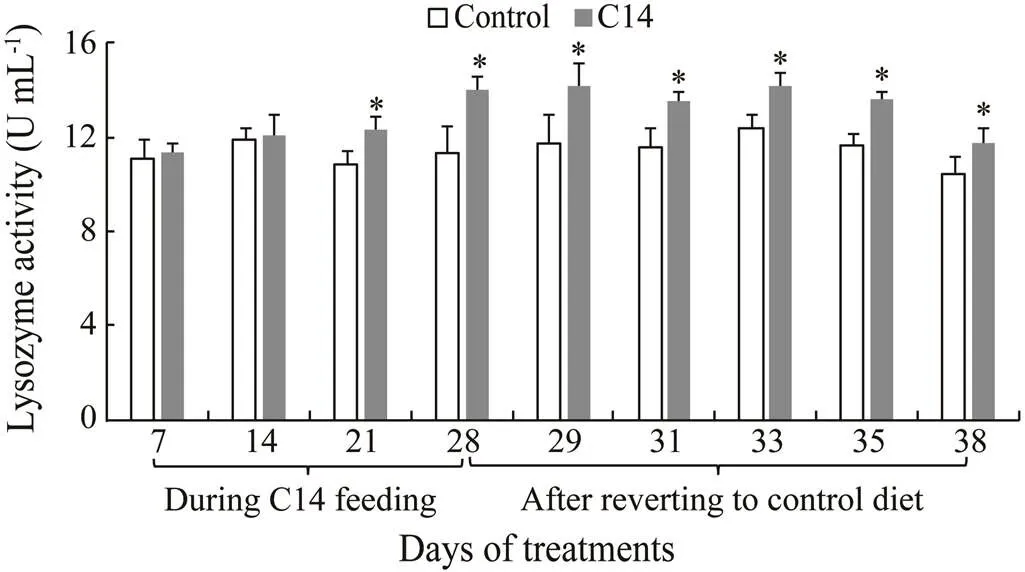
Fig.6 Lysozyme activity in coelomic fluid supernatant of sea cucumber over 38 days of feeding. Data represent means±SD (n=3). * Significantly difference (P<0.05) from the control group at the same sampling date.
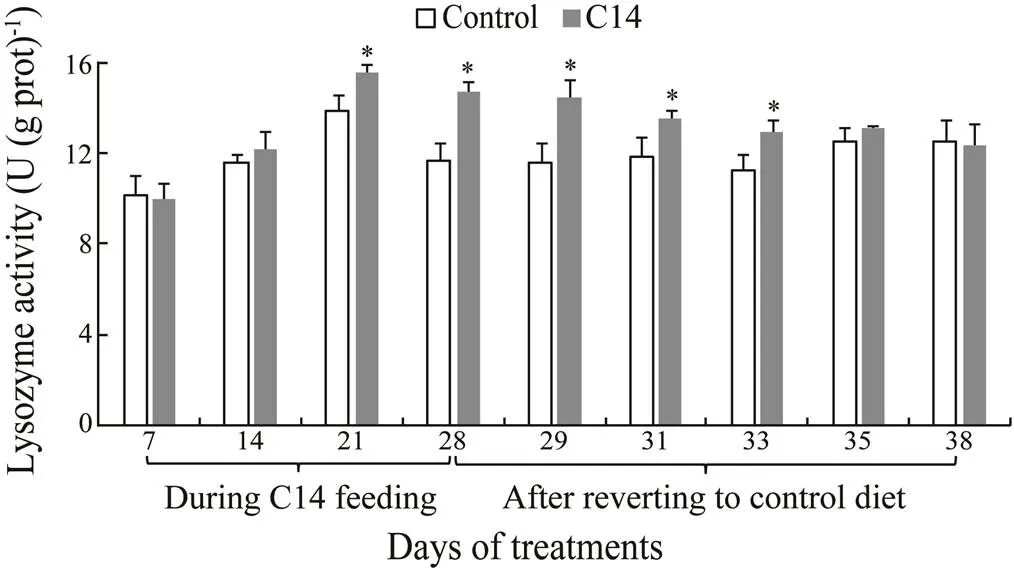
Fig.7 Lysozyme activity in coelomocyte lysate superna- tant of sea cucumber over 38 days of feeding. Data repre- sent means±SD (n=3). * Significantly difference (P<0.05) from the control group at same sampling date.
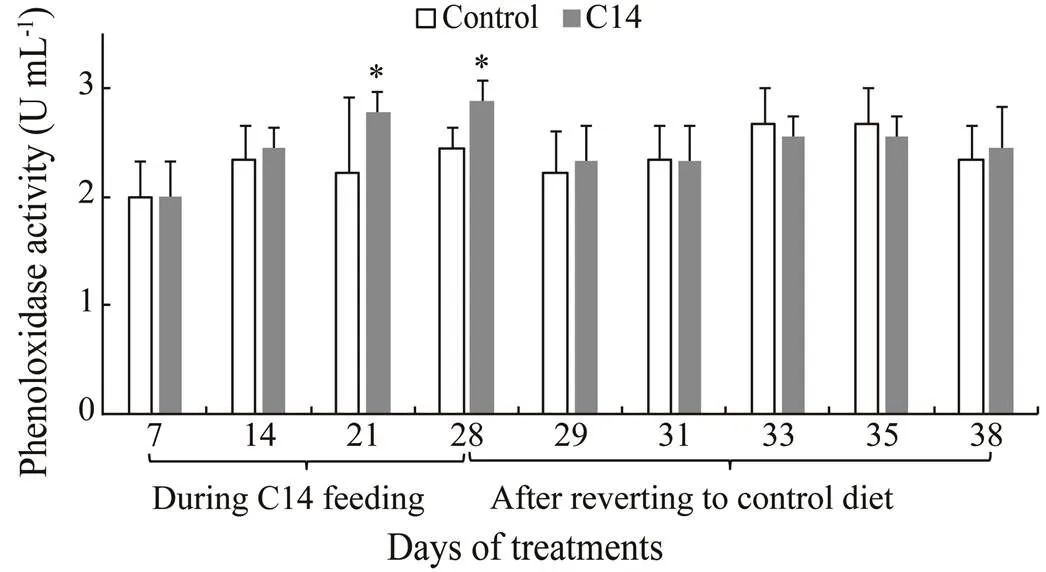
Fig.8 Phenoloxidase activity in coelomic fluid supernatant of sea cucumber over 38 days of feeding. Data represent means±SD (n=3). * Significantly difference (P<0.05) from the control group at the same sampling date.
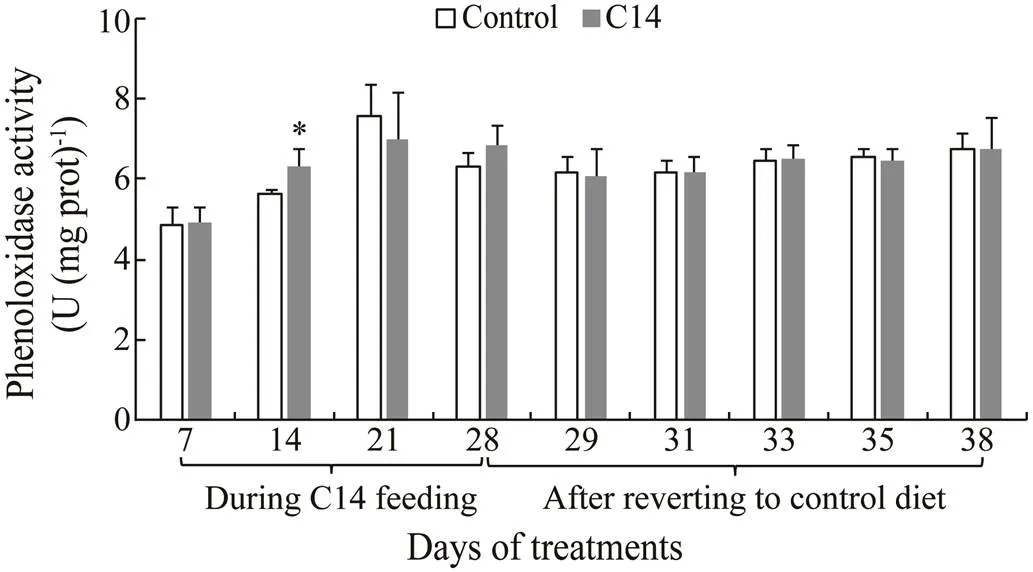
Fig.9 Phenoloxidase activity in coelomocyte lysate supernatant of sea cucumber over 38 days of feeding. Data represent means±SD (n=3). * Significantly difference (P<0.05) from the control group at the same sampling date.
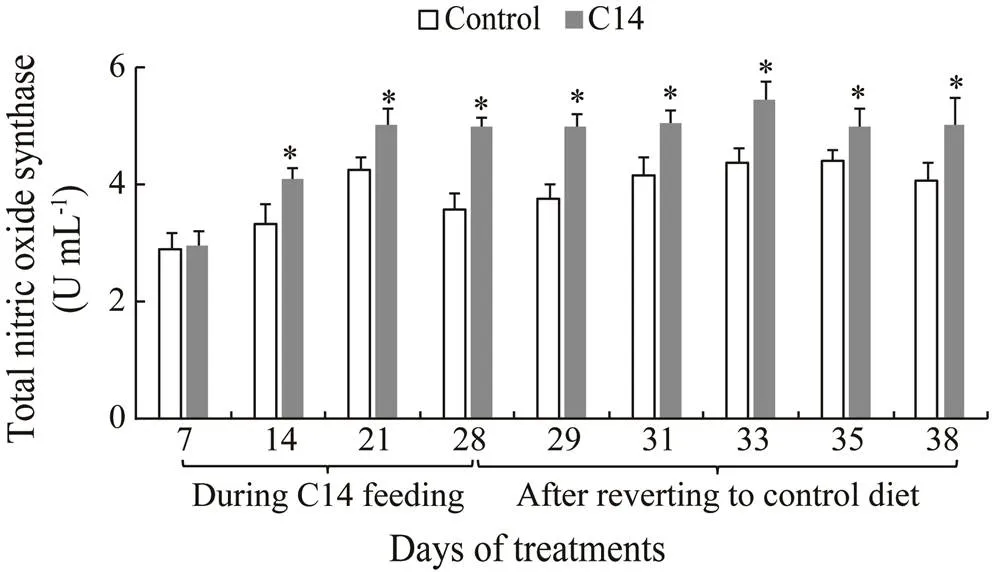
Fig.10 Total nitric oxide synthase activity in coelomic fluid supernatant of sea cucumber over 38 days of feeding. Data represent means±SD (n=3). * Significantly difference (P<0.05) from the control group at the same sampling date.
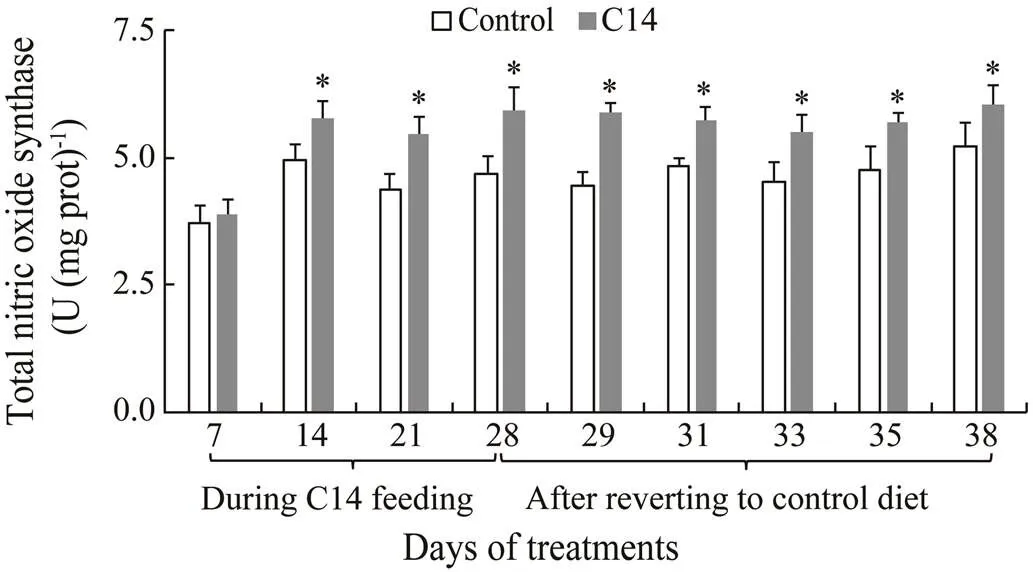
Fig.11 Total nitric oxide synthase activity in coelomocyte lysate supernatant of sea cucumber over 38 days of feeding. Data represent means±SD (n=3). * Significantly diffe- rence (P<0.05) from the control group at the same sam- pling date.
4 Discussion
In the present study, C14 was only recovered from the intestine of sea cucumber receiving the yeast diet. This suggests that C14 can survive and withstand the condition of the sea cucumber intestine. The number of C14 cells reached about 105cfug−1after sea cucumber fed with C14- supplemented diet at 105cellsg−1for 21 to 28 days. How- ever, the number of C14 cells dropped one order of magnitude 7 days after sea cucumber was shifted to the control diet. There was significant difference in trypsin activity between two groups from day 21 to day 33. We ob-served a positive correlation between the intestinal trypsin activity and the number of C14 cells while no correlation was observed between the C14 cell number and lipase/ amylase activity. Although the yeast is able to colonize the intestine of(Yang, 2014), cells must be at a concentration of approximately 105cfug−1to in- crease the trypsin activity. Similar results have also been observed in different abalone species. Feeding South Af- rican abalone with a diet containing each of the three probionts (SY9.8,sp. SS1, andAY1) at a concentration of appro- ximately 107cellsg−1for 21 days resulted in cultivatable probionts at 106to 107cfug−1gut material (Macey and Coy- ne, 2006). Once the probiotic-supplemented feeding was stopped, the number of probiont cells declined. There was a positive correlation between the number ofsp. SS1 cells and amylase activity and between the number ofSY9.8 cells and protease activity in abalone intestine while no correlation was found be- tween the number ofAY1 cells and protease and/or amylase activities (Macey and Coyne, 2006). A positive correlation was also observed between amylase or protease activity and the number ofsp. s6 cells in the gut of Japanese abalonewhereas there was no correlation between amylase or protease activity and the number ofsp. a3 cells (Iehata, 2009).
Marine yeasts have been used in sea cucumber aquaculture and were verified to have effects on non-specific immune response parameters such as phagocytic activity and LSZ, PO andT-NOS activities (Liu, 2012; Ma., 2013; Wang, 2015; Yang, 2015b; Zhang, 2017). Although different feeding durations, such as 30, 45 or 56 days, were chosen in the different reports about effects of dietary yeasts on the immune parameters of sea cucumber (Liu., 2012; Ma., 2013; Wang., 2015; Yang., 2015b; Zhang, 2017), the basis for choosing these periods was unclear. In the present study, sea cucumber fed a diet containing live cells of C14 showed significantly higher phagocytic activity/ respiratory burst of coelomocytes from day 21/14 to day 35/38, higher LSZ activity of CF/ CLS from day 21 to day 38/33, and higher T-NOS activity of CF/ CLS from day 14 to day 38 than the control. A correlation analysis suggested a positive association between colonization of C14 in the intestine andLSZ activity of CF and CLS, indicating that cultivatable C14 cells should be maintained at 105cfug−1to make a significant contribution to the cellular and humoral immune responses of sea cucumbers.
In addition, the varied enzyme activities/immune para- meters observed in the control group over the 38 days may be related to environmental factors, such as temperature, and metabolism and growth of sea cucumbers, as well as other factors.
5 Conclusions
Yeast C14 can successfully colonize sea cucumber intestine when supplemented with control diet. There is a positive correlation between the number of probiotic cells colonizing intestine and the trypsin activity/LSZ activity.
Acknowledgement
This work was supported by the Scientific Research Pro- ject from the Department of Education of Liaoning Pro- vince (No. JL201903).
Bradford, M. M., 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding., 72: 248-254.
Chi, C., Liu, J. Y., Fei, S. Z., Zhang, C., Chang, Y. Q., Liu, X. L., and Wang, G. X., 2014. Effect of intestinal autochthonous probiotics isolated from the gut of sea cucumber () on immune response and growth of., 38: 367-373.
FAO/WHO, 2001.. Food and Agriculture Organization and World Health Organization Joint report, 34pp.
Iehata, S., Inagaki, T., Okunishi, S., Nakano, M., Tanaka, R., and Maeda, H., 2009. Colonization and probiotic effects of lactic acid bacteria in the gut of the abalone., 75: 1285-1293.
Li, C., Ren, Y. C., Jiang, S. H., Zhou, S., Zhao, J. S., Wang, R. J., and Li, Y. M., 2018. Effects of dietary supplementation of four strains of lactic acid bacteria on growth, immune-related response and genes expression of the juvenile sea cucumberSelenka., 74: 69-75.
Li, J. G., Xu, Y. P., Jin, L. J., and Li, X. Y., 2015. Effects of a probiotic mixture (YB-1 andYB-2) on disease resistance and non-specific immunity of sea cucumber,(Selenka)., 46: 3008-3019.
Liu, J., Han, H., Sun, F. X., Zhang, C. Y., Cao, S. Q., Zhao, N. X., and Ma, Y. X., 2013. Effects of dietary livesp. BC26 on digestive enzyme activity, immune response and disease resistance againstinfection in juvenile sea cucumber., 28: 568-572 (in Chinese with English abstract).
Liu, N. N., Zhang, S. S., Zhang, W. W., and Li, C. H., 2017.sp. 33 a potential bacterial antagonist of, pathogenic to sea cucumber ()., 470: 68-73.
Liu, Z. M., Ma, Y. X., Yang, Z. P., Li, M., Liu, J., and Bao, P. Y., 2012. Immune responses and disease resistance of the juvenile sea cucumberinduced bysp. C14., 368-369: 10-18.
Ma, Y. X., Li, L. Y., Bao, P. Y., Li, M., Chen, W., and Chang, Y. Q., 2018. Effects of combined dietary administration ofsp. H26 andsp. BC26 on growth, immunity and intestinal microbiota in juvenile sea cucumber,., 49: 3792-3803.
Ma, Y. X., Li, L. Y., Li, M., Chen, W., Bao, P. Y., Yu, Z. C., and Chang, Y. Q., 2019. Effects of dietary probiotic yeast on growth parameters in juvenile sea cucumber,., 499: 203-211.
Ma, Y. X., Liu, Z. M., Yang, Z. P., Bao, P. Y., Zhang, C. Y., and Ding, J. F., 2014. Effects ofC21 on the growth and digestive enzyme activity of juvenile sea cucumber., 32: 743-748.
Ma, Y. X., Liu, Z. M., Yang, Z. P., Li, M., Liu, J., and Song, J., 2013. Effects of dietary live yeastC21 on the immune and disease resistance againstinfection in juvenile sea cucumber., 34: 66-73.
Macey, B. M., and Coyne, V. E., 2006. Colonization of the gastrointestinal tract of the farmed South African abaloneby the probiontsSY9,sp. SS1, andAY1., 8: 246-259.
Newaj-Fyzul, A., Al-Harbi, A. H., and Austin, B., 2014. Review: Developments in the use of probiotics for disease control in aquaculture., 431: 1-11.
Prado, S., Romalde, J. L., and Barja, J. L., 2010. Review of pro- biotics for use in bivalve hatcheries., 145: 187-197.
Sajeevan, T. P., Philip, R., and Singh, I. S. B., 2006. Immunosti- mulatory effect of a marine yeastS165 in., 257: 150-155.
Song, H. L., and Hsieh, Y. T., 1994. Immunostimulation of tiger shrimp () hemocytes for generation of microbicidal substances: Analysis of reactive oxygen species., 18: 201-209.
Verschuere, L., Rombaut, G., Sorgeloos, P., and Verstraete, W., 2000. Probiotic bacteria as biological control agents in aquaculture.,64: 655- 671.
Wang, J. H., Zhao, L. Q., Liu, F., Wang, H., and Xiao, S., 2015. Effect of potential probioticD30 on the growth performance, digestive enzyme activity and immunity in juvenile sea cucumber., 43: 330-336.
Xing, J., Leung, M. F., and Chia, F. S., 1998. Quantitative ana- lysis of phagocytosis by amebocytes of a sea cucumber,., 117: 67-74.
Yan, F. J., Tian, X. L., and Dong, S. L., 2014a. Effect ofYD13 supplemented in diets on growth performance and immune response of sea cucumber ()., 13: 805- 810.
Yan, F. J., Tian, X. L., Dong, S. L., Fang, Z. H., and Yang, G., 2014b.Growth performance, immune response, and disease resistance againstinfection in juvenile sea cucumberfed a supplementary diet of the potential probioticDB11., 420- 421: 105-111.
Yang, G., Peng, M., Tian, X. L., and Dong, S. L., 2017. Mole- cular ecological network analysis reveals the effects of probi- otics and florfenicol on intestinal microbiota homeostasis: An example of sea cucumber., 7: 4778.
Yang, G., Tian, X. L., Dong, S. L., Peng, M., and Wang, D. D., 2015. Effects of dietaryG19,BC-01, andDB11 supplementation on the growth, immune response, and expression of immune-related genes in coelomocytes and intestine of the sea cucumber (Selenka)., 45: 800-807.
Yang, Z. P., Sun, J. M., and Xu, Z., 2015a. Beneficial effects ofsp. C11 on growth and disease resistance of juvenile Japanese spiky sea cucumber., 27: 71-76.
Yang, Z. P., Sun, J. M., Xu, Z., Zhang, C. C., and Zhou, Q., 2014. Beneficial effects ofsp. C14 on growth and intestinal digestive enzymes of juvenile sea cucumber., 197: 142-147.
Yang, Z. P., Xu, Z., Zhou, Q., Zhang, C. C., and Sun, J. M., 2015b. Effects of dietary supplementation of marine yeastsp. C11 on digestive enzyme activity and immune response in juvenile sea cucumber, 36: 107-112 (in Chinese with English abstract).
Zhang, K., Liu, J. F., Dong, Q., Zhang, C. X., Xiao, S., Wang, H., and Wang, J. H., 2017. Effects of dietary addition ofon performance of, 36: 6-9 (in Chi- nese with English abstract).
Zhang, Z., Xing, R. L., Lv, Z. M., Shao, Y. N., Zhang, W. W., Zhao, X. L., and Li, C. H., 2018. Analysis of gut microbiota revealedcould be an indicative of skin ulceration syndrome in farmed sea cucumber, 80: 148-154.
Zhao, Y. C., Yuan, L., Wan, J. L., Sun, Z. X., Wang, Y. Y., and Sun, H. S., 2016. Effects of potential probioticEN25 on growth, immunity and disease resistance of juvenile sea cucumber., 49: 237-242.
Zhao, Y. C., Zhang, W. B., Xu, W., Mai, K. S., Zhang, Y. J., and Liufu, Z. G., 2012. Effects of potential probioticT13 on growth, immunity and disease resistance againstinfection in juvenile sea cucumber., 32: 750- 755.
February 14, 2019;
April 28, 2019;
November 13, 2019
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2020
. Tel: 0086-411-84763096
E-mail: mayuexin@dlou.edu.cn
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Circulation and Heat Flux along the Western Boundary of the North Pacific
- System Reliability Analysis of an Offshore Jacket Platform
- The Mineral Composition and Sources of the Fine-Grained Sediments from the 49.6˚E Hydrothermal Field at the SWIR
- Research Progress of Seafloor Pockmarks in Spatio-Temporal Distribution and Classification
- Application of the Static Headland-Bay Beach Concept to a Sandy Beach: A New Elliptical Model
- Climatology of Wind-Seas and Swells in the China Seas from Wave Hindcast
