Quality Assessment of Frozen Solenocera crassicornis Treated with Sodium Metabisulphite by Soaking or Spraying
2020-03-09ZHUJingpingCHENYuJINLeiandZHUJunxiang
ZHU Jingping, CHEN Yu, JIN Lei, 4), , and ZHU Junxiang
Quality Assessment of FrozenTreated with Sodium Metabisulphite by Soaking or Spraying
ZHU Jingping1), 2), 3), CHEN Yu1), JIN Lei1), 2), 3), 4),*, and ZHU Junxiang1)
1),316021,2),316021,3),316021,4),316021,
The present work was carried out to evaluate the safety of shrimp () treated with different concentrations of sodium metabisulfite (SMB) by soaking or spraying during frozen storage. Shrimps soaked in higher concentrations of SMB showed higher sensory scores, lower total color differences, and better anti-melanosis effects than shrimps in the control and other treatment groups throughout frozen storage (−18℃). Lower total volatile basic nitrogen and thiobarbituric acid reactive substances and higher salt soluble protein contents were detected in shrimp soaked with high doses of SMB compared with other samples. In addition, lower counts of total aerobic plates and psychrotrophic bacteria were observed in shrimp treated by soaking with higher doses of SMB than those in control shrimp and shrimp treated with other methods during frozen storage (−18℃). However, the SO2content of 5% SMB-soaked samples exceeded the maximum allowable limit of 100mgkg−1. Overall, the use of 1.5% SMB soaking to treat shrimp results in good antioxidant and antimicrobial effects and, thus, may be suggested to preserveunder frozen conditions. The results of this study present important guidance on the use of SMB to maintain the quality of marine-trawling shrimp from manufacturing to consumption.
; frozen storage; SMB; soaking; spraying; quality changes
1 Introduction
The marine-trawling red shrimp () isan important commercial species mainly distri- buted in China, India, Malaysia, Indonesia, the Arafura Sea, and Japan. China is one of the world’s topexporters, and frozen peeled prawns, as the main processed products of this species, are exported to over 10 countries.is a rich source of astaxan- thin, and possesses a series of biological properties, in- cluding immunostimulation, anti-tumor, anti-oxidation, anti- hypertension, and anti-cardiovascular disease activities. Furthermore, as a delicious and nourishing aquatic prod- uct,contains large amounts of protein, fatty acids, and mineral elements (., calcium, iron, phos- phorus, iodine) (Wang, 2014; Liu, 2016). Thus,is an ideal food resource with high nutritional value.
Shrimp has a limited shelf-life and is highly perishable due to microbial spoilage and melanosis (Gokoglu and Yerlikaya, 2008; Farajzadeh., 2016). Melanosis is a biochemical reaction triggered by polyphenoloxidase (PPO). PPO oxidizes phenols into quinones, which subsequentlyundergo further oxidation or polymerization to form high- molecular weight black pigments (Benjakul.,2006; Sae-Leaw., 2017). Melanosis significantly reduces the market value of shrimp and results in considerable eco- nomic loss (Martínez-Álvarez., 2005). Freezing is usually employed as an effective method to extend the shelf-life of shrimp (Tsironi., 2009; Wachirasiri.,2016). However, even with freezing, melanosis may still occur in shrimp, albeit slowly, because PPO remains slightly active under freezing conditions (Nirmal and Benjakul,2010). Thus, traditional on-board freezing techniques com- bined with value-added on-board processing techniques using small amounts of sulfites and its derivatives isin- dispensable for offshore fisheries to overcome or alleviate melanosis (Bono., 2012, 2016).
Sulfites and their derivatives are widely used to inhibit melanosis in seafood (Montero., 2001; Martínez- Álvarez., 2008). These chemical substances prevent browning by combining irreversibly with quinones and interfering with their polymerization to melanins; they have also been demonstrated to possess antimicrobial activity and retard microbial spoilage (Nirmal and Benjakul, 2009). Sodium metabisulfite (SMB, Na2S2O5), a known sulfite, is applied to harvest marine shrimp by soaking with ice. However, SMB reacts with the oxygen dissolved in water to release SO2, which produces acidic substances and so- dium acid sulfate, while these compounds are toxic che- mical agents for human consumption (Rencüzoǧullari., 2001).
At present, direct dusting of SMB, due to its convenient operation, is widely used by many fishermen to preserve marine-trawling shrimp. However, SMB is often used in excess owing to the difficulty of spreading evenly, which frequently results in SO2residues exceeding estab- lished limits in shrimp. If the shrimp is reasonably treated by soaking or spraying with SMB solution, the problem of uneven spreading and excessive use can be avoided. Therefore, the aim of the present work is to study the quali- ty changes ofsoaked with 1.5%, 3.0%, or 5.0% SMB respectively, or sprayed with 5.0% SMB, and evaluate the safety of SMB applied at different concentra- tions and by using different treatment methods during frozen storage (−18℃). The results of this study will pro- vide a theoretical basis for the specific use of SMB as a preservative for.
2 Materials and Methods
2.1 Shrimp Collection and Preparation
Fresh red shrimp () treated without che- micals was provided by Dongqing Marine Fisheries Co- operative. The shrimps were immersed in ice-cold water after harvest and transported to the laboratory within 12h. In the laboratory, the shrimps were soaked in 1.5%, 3.0%, or 5.0% SMB solution at 4℃ for 5min. The shrimps were then taken out and drained. A batch ofwas sprayed evenly with 5.0% SMB solution and allowed to sit for 5min as another treatment group.without any chemical treatment was considered the con- trol. All procedures were performed at 4℃. Finally, all shrimp samples were frozen and stored at −18℃. The samples were frozen for 6 months. During this period, the qualities of shrimp in different groups were analyzed with an interval of 30 days. Sensory quality, color, melanosis, total volatile basic nitrogen (TVB-N), salt soluble protein (SSP), thiobarbituric acid (TBA), and microbial counts were selected as quality indices. At the beginning of the analysis, shrimps were manually deheaded and de-shelled. Then they were thawed at 4℃ and analyzed immediately when the core temperature was 0℃. A schematic of the experiment is shown in the supplementary material.
2.2 Determination of SO2
Usage amount of SMB was calculated in terms of SO2residue. SO2was determined according to GB 5009.34- 2016 (Chinese National Standard). A 5g sample was placed in a distillation flask with 250mL of distilled water and then installed in a condensing device. One end of the condenser was immersed in 25mL of lead acetate ab- sorbing solution in an iodometric bottle, capped immedi- ately, and then heated to distill. The end of the condenser was taken out the liquid level when the distillate was about 200mL, and distilled again for 1min. The device in the lead acetate solution was rinsed with a small amount of distilled water. A blank test was also conducted. Exactly 10mL of HCl and 1mL of starch indicator solution were added to the iodine bottle. After shaking, the mixtures were titrated with iodine standard solution until the solution could maintain a blue coloration for 30s. The volume of iodine standard solution used to achieve this endpoint was recorded, and SO2content was calculated as follows:
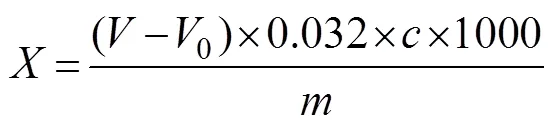
whererepresents the total SO2content (gkg−1);and0represent the titration volumes of the iodine standard solution in the test sample (mL) and blank (mL), respectively;represents the concentration of the iodine standard solution (molL−1); andrepresents the weight of the shrimp sample (g).
2.3 Sensory Analysis
Sensory evaluation was performed as described by Fa- rajzadeh. (2016) and Wu (2014). A panel of 10 trained panelists with basic sensory knowledge and test skills was used in this study to evaluate the sensory quality of the shrimp samples. Appearance, flavor, odor, texture, and over- all acceptability in appropriate forms with descriptive terms were used to describe the organoleptic evolution of quality deterioration. Appearance, texture, and odor were evaluated in raw shrimp, whereas flavor was analyzed in cooked shrimp which was boiled for 5min. Panelists were asked to score the samples independentlya 9-point descriptive hedonic scale (9=highest score, 1=lowest score) for each descriptor. The scores were calculated as mean values, and overall acceptability was defined as the average of these sensorial attribute values as weighted.
2.4 Instrumental Color Analysis
The color of shrimp was measured with a Hunter Lab colorimeter (SC-80C, Kangguang Optical Instrument Co., Ltd., Beijing, China) according to the method of Jin. (2018). The middle section of the shrimp meat was used for color analysis, and CIE Lab values were measured for color quantization. In the CIE Lab system, ‘L’ represents lightness from black and white, ‘a’ represents redness or greenness, and ‘b’ represents yellowness or blueness. Three different single shrimp specimens were used for each measurement, and average values were recorded. Color change was also evaluated by using the total color difference (TCD) during frozen storage (Dai, 2016; Yuan, 2016). TCD values (Δ) were calculated as follows:
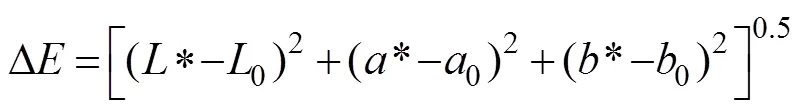
where0,0, and0respectively represent the values of*,*, and* color parameters at a storage time of zero.
2.5 Total Volatile Basic Nitrogen (TVB-N) Analysis
TVB-N was determined according to GB 5009.228-2016 (Chinese National Standard). A 20g sample was blended evenly with 100mL of distilled water, stirred thoroughly, and sonicated for 1min. After impregnated for 30min, the homogenate was filtered through filter paper and added with boric acid (10mL, 20gL−1) and mixture indicators (5 drops). The filtrate was slowly transferred into the re- action chamber, the beaker was washed, and the eluentswere collected. After adding 5mL of magnesia (10gL−1, MgO) to the reaction system, steam distillation was carried out for 5min using a Kjeldahl distillation unit. The distillate was titrated with 0.01molL−1HCl. Measurements were con- ducted in triplicate, and a blank assay was performed. TVB- N content was calculated as follows:
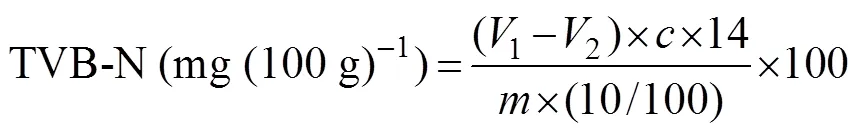
where1and2represent the titration volumes (mL)of the tested sample and blank, respectively;represents the weight of the shrimp sample (g); andrepresents the actual concentration of HCl (molL−1).
2.6 Salt Soluble Protein (SSP) Contents
SSP content was determined using the method of Al- Bulushi. (2013). Approximately 6.67g of sample was blended with 100mL of chilled NaCl (5%, w/v), adjusted to pH 7−7.5 using 0.02molL−1NaHCO3(pH 7.2), and then homogenized for 2min. The homogenate was centrifuged at 4000rmin−1for 30min at 4℃, and the supernatant was collected. Finally, 10mL of chilled trich- loroacetic acid (15%, TCA) was added to the supernatant, and non-protein nitrogen compounds were removed by centrifugation as described above. The biuret method (Gor- nall.,1949) with bovine serum albumin as the standard was used to determine SSP contents. All experiments were performed at less than 10℃.
2.7 Lipid Oxidation
TBA reactive substances (TBARS) were determined for lipid oxidation analysis of shrimp using the method of Dong. (2018) with slight modifications. Shrimp sam- ples (5g) were homogenized with 15mL of distilled water for 2min. Then, 2.5mL of TBA solution containing 0.25molL−1HCl, 15% TCA, and 0.375% TBA was added to 1mL of the homogenates. The reaction was conducted in a boiling water bath for 35min. The mixture was cooled to room temperature and then centrifuged at 4000rmin−1for 20min. The absorbance of the solution at 532nm was de- termined with a digital spectrophotometer (Cary 50, Varian Australia Pty Ltd., Australia). Malondialdehyde (MDA) levels based on 1,1,3,3 tetraethoxypropane at gradient con-centrations of 0−10μgmL−1were used for standard curve pre- paration. TBARS values were calculated and expressed as mg MDA per kg of sample.
2.8 Microbiological Analysis
Approximately 25g of shrimp samples were homoge- nized with 225mL of sterile phosphate-buffered saline (PBS) at 10Hz for 2min. Decimal dilutions were carried out using sterile PBS. Three suitable gradients of the di- luted homogenates were spread on the surface of sterile Petri dishes, pour-plated with 15−20mL of plate count agar,and then incubated at 30℃ for 3d to determine total aero- bic plate counts (TPCs) and at 7℃for 10d to determine psychrotrophic bacterial counts (PBC) (FDA, 2001). Two replicates of three appropriate dilutions were performed. Microbial counts were calculated as follows:
,
whererepresents the total microbial count in all enumerated plates;represents the dilution factor of the first dilution gradient;1(30≤≤300) represents the numbers of the flat plate in the first dilution gradient; and2(30≤≤300) represents the numbers of the flat plate in the second dilution gradient.
2.9 Statistical Analysis
In this work, an apparent first-order equation was used to model TVB-N and SSP values and clarify protein changes, which could be considered failure/formation phenomena (Tsironi., 2009). The relevant equation may be writ- ten as follows:
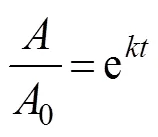
whererepresents the kinetic rate;represents the frozen storage time; and0andrepresent the TVB-N values at frozen storage times of zero and, respectively.
Analysis of variance was performed, and Duncan’s mul- tiple range test was used to determine mean comparisons. Statistical analysis was conducted using SPSS 19.0 for Win- dows (SPSS Inc., Chicago, IL, USA). A probability value of<0.05 was considered significant. All experiments were carried out thrice, and the results are reported as mean±standard deviation.
3 Results and Discussion
3.1 SO2 Contents Analysis
SO2contents in shrimps subjected to different treatments at −18℃ for 180d of storage are presented in Fig.1. Ini- tially, the 1.5% SMB-soaked shrimps had lower SO2con- tents than the 3.0% and 5.0% SMB-soaked shrimps (<0.05). However, the SO2levels of 5.0% SMB-sprayed shrimp were between those of the 1.5% and 3.0% SMB-soaked shrimp (<0.05). Thus, soaking allows better penetration of SMB into the shrimp than spraying. In general, decreased SO2contents were found in all samples as the storage time increased (<0.05). On day 180, the SO2contents of the 1.5%, 3.0%, and 5.0% SMB-soaked and SMB-sprayed samples were 29.4, 44.9, 103.4 and 32.5mgkg−1, respec- tively. Among the samples, the 1.5% SMB-soaked sample possessed the lowest SO2at all storage times (<0.05).
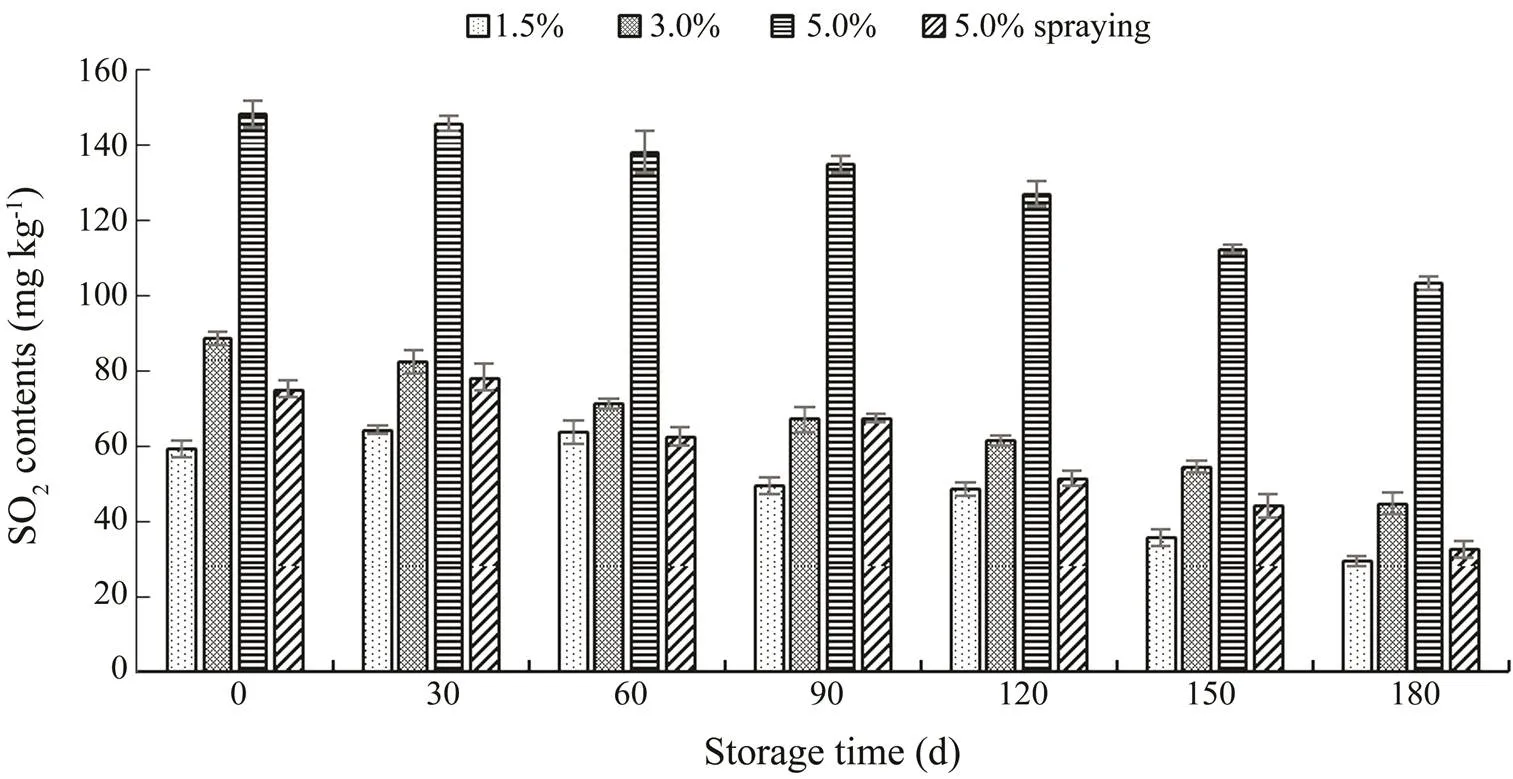
Fig.1 SO2 content of S. crassicornis treated with SMB at different concentrations using different methods over 180d of frozen storage. Bars represent the standard deviation (n=3) with three parallel samples. Soaking method: 1.5%, 3.0%, and 5.0% SMB; spraying method: 5.0% SMB.
SO2is widely used as an indicator of food safety, especially in seafoods. Food and Agriculture Organization of the United Nations (FAO) (1999), U.S. Food and Drug Administration (FDA) (Taylor, 1986), and China (GB 2760−2014) have stipulated a threshold value of 100mgkg−1SO2in fresh shrimp meat. SO2contents in the 1.5% and 3.0% SMB-soaked and 5.0% SMB-sprayed samples were consistently lower than 100mgkg−1over 180d of fro- zen storage (<0.05), but the SO2contents of the 5% SMB- soaked sample were in the range of 103.4−148.3mgkg−1, which exceeds maximum limits (<0.05). Thus, from a food safety perspective, soaking with 5.0% SMB is unsuitable for short-term freezing preservation of.
3.2 Sensory Evaluation
Fig.2 shows the changes in the sensory quality of. All indices declined with increasing storage time. In general, SMB treatment improved the gloss of shrimp, and, therefore, the appearance scores of shrimp in the treatment groups were higher than those in the control group (<0.05). Moreover, the observed effects became more significant as the storage time increased (<0.05). The appearance scores of 5.0% SMB-soaked shrimp were higher (<0.05) than those of 5.0% SMB-sprayed shrimp and samples soaked with SMB at lower concentrations. Thus, treatment of shrimp by soaking with SMB, especially at higher doses, could effectively improve appear- ance scores during frozen storage. These results are con- sistent with previous studies (Gonçalves and Ribeiro, 2008;Wachirasiri., 2016), showing that sulfites can improveappearance properties and increase consumers’ preference for red shrimp.
No difference in odor was noted in all samples (>0.05). Therefore, the use of SMB does not potentially affect the odor of shrimp. At the beginning of frozen storage, slightly lower texture scores were noted in all treated samples compared with the control (<0.05), and no difference in texture was observed among the SMB-treated samples (>0.05). Similarly, no difference in texture was noted among all samples as the storage time increased (>0.05). SMB treatment may increase the initial moisture content of the shrimps and affect their texture. However, loss of moisture occurs during frozen storage, and, thus, the influence of SMB is weakened. Flavor was analyzed using cooked shrimp after tasting. Some panelists noted a slight sourness after tasting the treated samples, which also directly reflects the lower flavor scores of treated samples compared with the control at all storage times (<0.05). Indeed, the 5.0% SMB-soaked shrimp received lower flavor scores than those soaked with SMB at lower concentrations or sprayed with 5.0% SMB at all storage times (<0.05). This result is in agreement with the high SO2content of 5.0% SMB-soaked shrimp.
Overall acceptability was calculated by taking the average of all four parameters. The overall acceptability of the samples changed from the initial values of 8.62, 8.56, 8.65, 8.65, and 8.88 to 6.61, 6.57, 6.59, 6.54, and 6.45 in the 1.5%, 3.0%, and 5.0% SMB-soaked, 5.0% SMB-sprayed, and control samples, respectively, after 180d of frozen storage. At the beginning without freezing, slightly higher scores of overall acceptability were noted in the control sample compared with those of all treated samples (<0.05). The overall acceptability of all samples decreased as the storage time increased (<0.05). Nonetheless, the rate of decrease varied depending on the SMB concentrations and treatment methods. Compared with other samples, the 1.5% SMB-soaked shrimp possessed slightly higher overall acceptability at the end of storage (>0.05). Thus, the results suggest that treatment by soaking with 1.5% SMB could ideally improve the sensory quality of red shrimp during frozen storage.
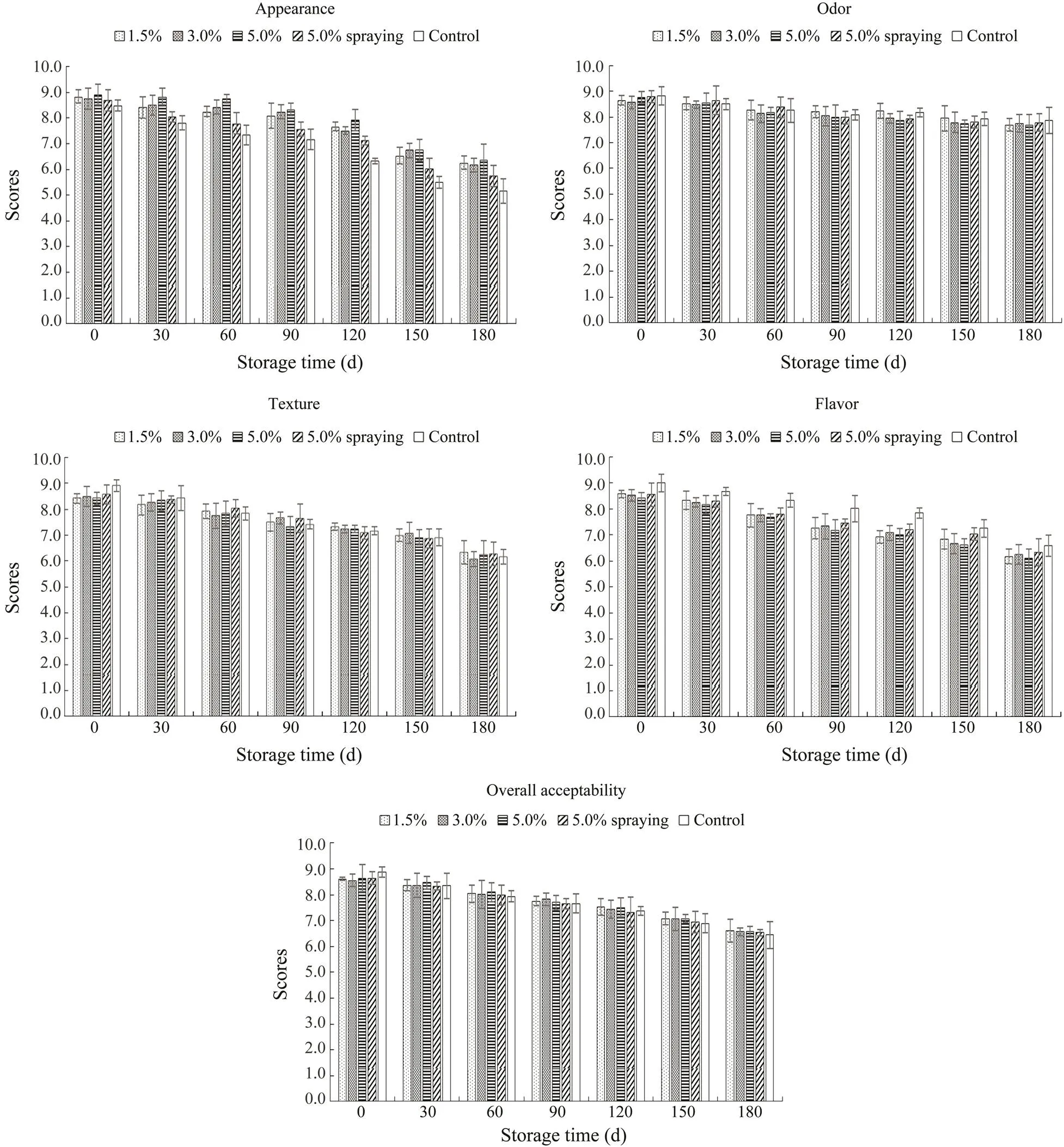
Fig.2 Appearance, odor, texture, flavor, and overall acceptability of S. crassicornis treated with SMB at different concentrations using different methods over 180d of frozen storage. Bars represent the standard deviation (n=3). Soaking method: 1.5%, 3.0%, and 5.0% SMB; spraying method: 5.0% SMB; control: treated without chemicals.
3.3 Total Color Difference (TCD) Changes (ΔE) Evaluation
Color fading is an important quality change in shrimp during frozen storage (Solval.,2014). TCD changes (Δ) in the control and treated shrimp during storage are shown in Fig.3. On day 30, no difference in Δwas noted in all samples (>0.05), and the corresponding values were determined as 3.38, 3.17, 3.40, 3.25 and 3.27 in the 1.5%, 3.0%, and 5.0% SMB-soaked, 5.0% SMB-sprayed, and con- trol samples, respectively. The TCD values of all samples increased during storage. Interestingly, a faster increase in TCD was observed in the control (<0.05) compared with the treated samples. However, shrimp treated with 1.5%, 3.0%, and 5.0% SMB soaking and 5.0% SMB spray- ing showed no difference in TCD until after 90d of frozen storage (>0.05). Shrimp treated with 5.0% SMB soaking had lower TCD values than shrimp treated with other means after 90 days (<0.05), and no difference was found among other treatment groups (>0.05). At the end of storage, the 5.0% SMB-soaked shrimp showed the lowest TCD (5.15), followed by those treated with 3.0% SMB soaking (5.40), 5.0% SMB spraying (5.59), and 1.5% SMB soaking (5.78). By comparison, the control showed the highest TCD (6.75) on day 180. The low TCD of SMB- treated shrimp is likely related to the SO2content of shrimp meat (Fig.1). In the present study, treatment ofwith SMB, especially by soaking at higher doses, could delay color changes effectively during frozen storage, which is in agreement with the work reported by Chantarasuwan. (2011).
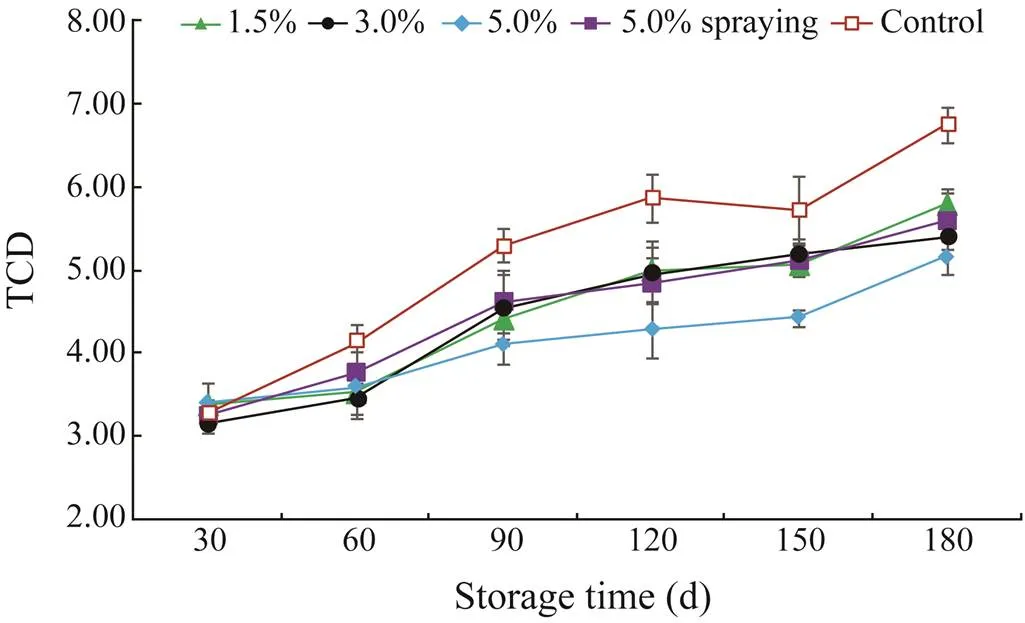
Fig.3 Total color difference (TCD) values (ΔE) changes in S. crassicornis treated with SMB at different concentrations using different methods over 180d of frozen storage. Bars represent the standard deviation (n=3). Soaking me- thod: 1.5%, 3.0%, and 5.0% SMB; spraying method: 5.0% SMB; control: treated without chemicals.
Evaluation of melanosis was performed by visual inspection of postharvest images captured by a digital camera (Canon, Japan) at the end of frozen storage (Fig.4). Black spots on the heads of shrimp treated with 1.5% SMBsoaking and 5.0% SMB spraying were visualized, than othertreated shrimp (3.0% and 5.0% SMB soaking) that showed only slight melanin deposition visually. However, among the samples, the control shrimps showed the largest black centers, and the tails of these shrimps darkened slightly. The mechanism of melanosis development is related to the enzymatic action of PPO. Application of SMB at high doses by soaking may allow penetration of the sulfite into PPO active sites underneath the shrimp shell more effectively than through other means (Shiekh.,2019). Thus, SMB is capable of preventing melanosis inkept under frozen conditions. The effect of soaking, especially at high concentrations, is especially significant.
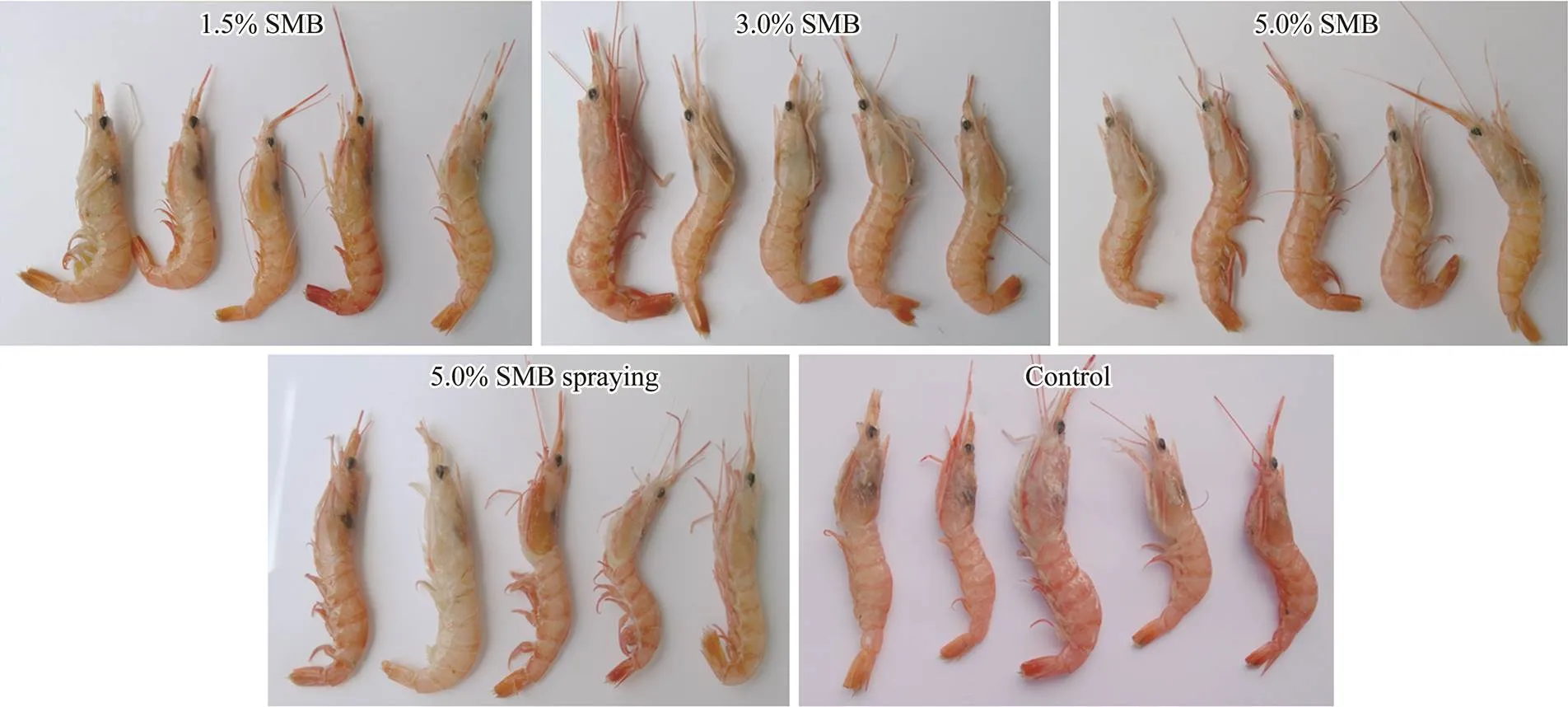
Fig.4 Photographs of S. crassicornis treated with SMB at different concentrations using different methods on day 180. Soaking method: 1.5%, 3.0%, and 5.0% SMB; spraying method: 5.0% SMB; control: treated without chemicals.
3.4 Changes in Chemical Parameters
The TVB-N contents of all samples over 180d of storage are depicted in Fig.5. Initially, no significant difference in TVB contents was observed in all samples (>0.05), and TVB-N contents ranged from 11.7 to 12.2mg(100g shrimp)−1meat. During frozen storage, the TVB-N contents of all samples showed a gradual increase (<0.05). However, TVB-N contents in the control (=0.0027) increased more significantly throughout the storage (<0.05) than those in the SMB treatments. Shrimp soaked with SMB at higher concentrations (5.0%) showed lower increase rate (=0.0016) of TVB-N contents than those soaked with SMB at lower concentrations (1.5%, 3.0%) or sprayed with 5.0% SMB (<0.05). No difference in TVB-N contents was noted among shrimp soaked with 1.5%, 3.0%, and 5.0% SMB (>0.05; final values, 16.9, 16.4, and 15.6mg(100g)−1, respectively) after 180d of frozen storage. By comparison, higher TVB contents were found in the control and sprayed shrimp (<0.05; final values, 19.2 and 17.7mg(100g)−1, respectively) on day 180. Hence, soaking shrimp with SMB could retard the formation of TVB-N more effectively than spraying during frozen storage, and the degree of in- hibition depends on the treatment dose. These results may be ascribed to high SMB contents decreasing the capacity of bacteria to induce oxidative deamination of non-pro- tein nitrogen compounds. TVB-N, as a product of protein breakdown, is an important indicator of chemical spoilage, and increases in TVB-N value are related to bacterial spoilage and the activity of endogenous enzymes (Soncin., 2009;Arancibia., 2015). In general, a TVB-N value of 30mg TVB-N per 100g in fresh shrimp is considered to reflect spoilage (GB 2733-2015). In the present study, none of the samples exceeded the spoilage limit of shrimp after 180d of frozen storage. Nevertheless, among the samples, the 5.0% SMB-soaked shrimp revealed the lowest TVB content on day 180 (<0.05). This result agrees with the high SO2content of 5.0% SMB-soaked shrimp.
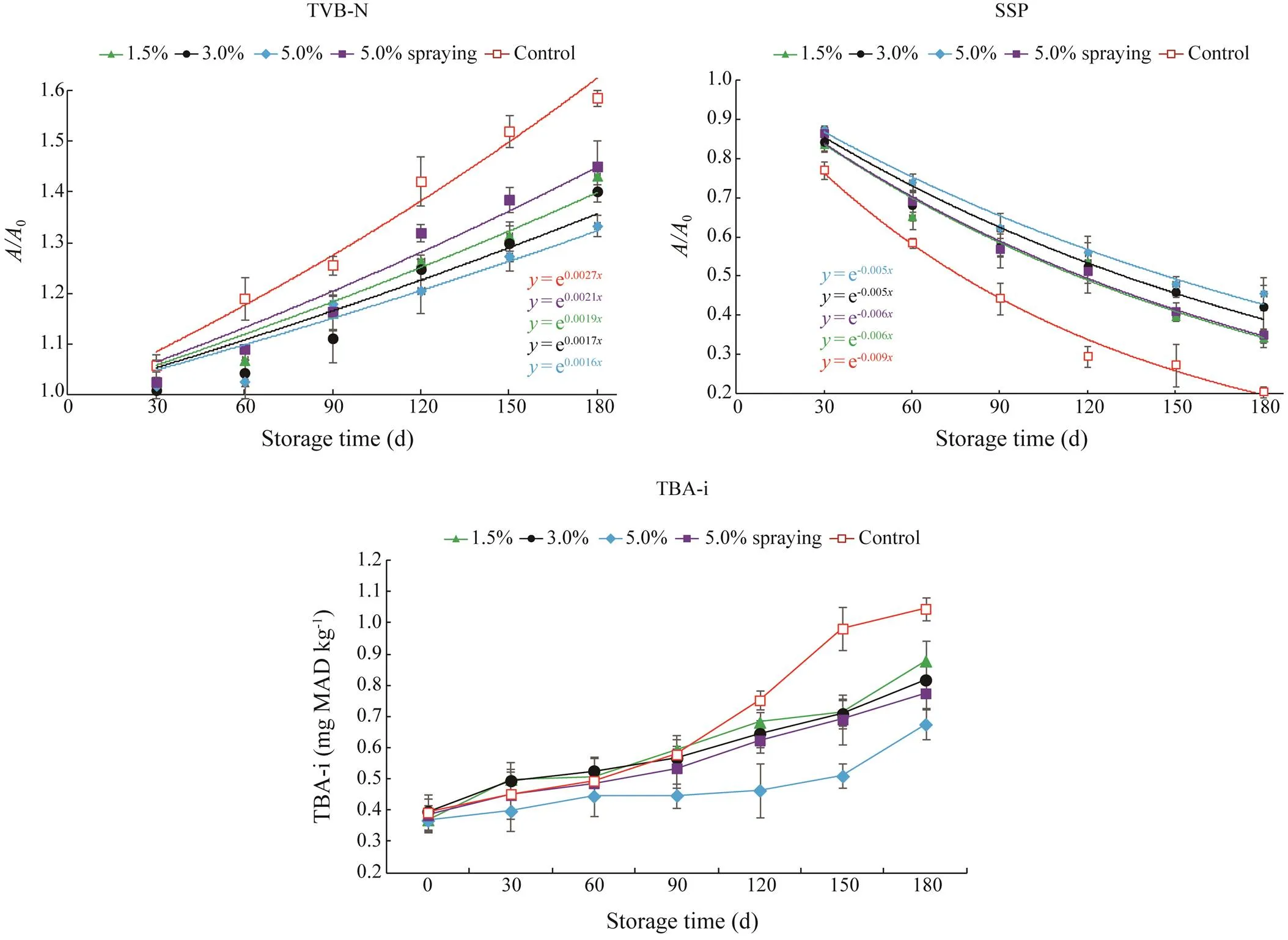
Fig.5 Total volatile basic nitrogen (TVB-N), salt soluble protein (SSP) and thiobarbituric acid reactive substances (TBARS) changes in S. crassicornis treated with SMB at different concentrations using different methods over 180d of frozen storage. Bars represent the standard deviation (n=3). Soaking method: 1.5%, 3.0%, and 5.0% SMB; spraying method: 5.0% SMB; control: treated without chemicals.
SSP refers to the myofibrillar fraction of shrimp protein and influences the water holding capacity and texture of shrimp greatly during frozen storage. Loss of protein solu- bility can indicate myofibrillar protein denaturation (Remya., 2015). On day 0, no significant difference in SSP was found among all samples (>0.05), which revealed initial SSP contents of 29.16, 30.02, 29.70, 29.97, and 30.60μgkg−1in the 1.5%, 3.0%, and 5.0% SMB- soaked, 5.0% SMB-sprayed, and control samples, respectively. Extractible SSP decreased with increasing storage time. However, SSP contents in the control showed a faster decrease (=−0.009) than those in the treatment groups.Among the SMB treatments, the decrease rates of SSP (=−0.005) in the 3.0% and 5.0% SMB-soaked shrimp weresignificantly lower (<0.05) than those in the 1.5% SMB- soaked and 5.0% SMB-sprayed shrimp (=−0.006). At the end of the storage, shrimp soaked at higher SMB doses revealed higher SSP contents (<0.05). However, no sig- nificant difference was observed between 3.0% and 5.0% SMB-soaked shrimp (>0.05; final SSP contents, 12.71 and 13.52μgkg−1, respectively). Furthermore, 1.5% SMB-soaked and 5.0% SMB-sprayed shrimp revealed SSP con- tents of 9.96 and 10.47μgkg−1on day 180, and no differ- ence was noted between these two samples (>0.05). The lowest SSP content (final value, 6.27μgkg−1) was observed in the control group. The results suggest that SMB treat- ment exerts beneficial effects during shrimp storage by re- taining a high percentage of SSP content. Moreover, soak- ing with high SMB concentrations suppresses SSP dena- turation more effectively than other methods. It is well known that decreases in myofibrillar content can affect the quality of shrimp. As previously reported by Remya. (2015) and Yoon and Lee (1990), decreases in myo- fibrillar protein content could be attributed to the denaturation and aggregation of proteins due to the formation of disulfide bonds followed by rearrangement into hydrophobic and hydrogen-bonded regions on an intra- and inter- molecular basis (Buttkus, 1974). Considering the findings in the present study, high levels of SMB may be speculated to reduce the exposure of hydrophopic residues to the protein surface and maintain the structural stability of myofibrillar proteins, ultimately slowing down the kinetics of protein aggregation (Herrera and Mackie, 2004; Al- Bulushi., 2013).
The TBARS content reflects the concentration of secondary lipid oxidation products in a sample. Changes in TBARS content in the samples are presented in Fig.5. Initially, no difference in TBARS contents was observed among all samples (>0.05). Thereafter, TBARS contents increased up to day 180 (<0.05). The lowest increase in TBARS content was observed in the 5.0% SMB-soaked shrimp (<0.05), reaching 0.676mgMDAkg−1after 180d of frozen storage. However, shrimp treated with SMB (soaking with 1.5% and 3.0% SMB and spraying with 5.0% SMB) and the control showed no difference in TBARS contents until day 120 of the storage (>0.05). On day 120, a rapid increase in TBARS content was found in the control; indeed, the TBARS content of the control reached a maximum value of 1.046mgMDAkg−1at the end of the storage (<0.05). Soaking with 5% SMB prevented lipid oxidation, as shown by the lower TBARS content observed in this sample on day 180 (<0.05) compared with those of the 1.5% SMB-soaked, 3.0% SMB- soaked, and 5.0% SMB-sprayed shrimp (0.878, 0.818, and 0.775mgMDAkg−1, respectively). Since shrimp treated with SMB had lower TBARS contents than those without treatment, SMB may be speculated to function as an antioxidant that effectively retards lipid oxidation in shrimp during frozen storage. Higher antioxidant activity was found in shrimp soaked with higher levels of SMB. Previous studies (Cacciuttolo., 1993; Solval., 2014) reported that several reactive oxygen species, especially hydroxyl radicals (·OH), which have strong chemical acti-vity, can easily react with biomolecules, such as DNA, pro- teins, amino acids, and fatty acids. SMB may react with ·OH to reduce concentrations of free ·OH and inhibit lipid oxi- dation.
3.5 Microbiological Analysis
Fig.6 shows the evolution of microbial counts in all in- vestigated samples plotted as a function of storage time, and all values are reported as log(CFUg−1) of fresh weight. The TPC values of all samples were in the range of 4.11− 4.16 log(CFUg−1) on day 0, and no significant difference in TPC was found (>0.05). TPC showed an increasing trend during frozen storage, and the highest TPC was obtained in the control samples (<0.05). TPC increased rapidly in the control shrimp over the first 30d of frozen storage, decreased slightly, and then increased once more up to the end of storage. The TPC of 5.0% SMB-soaked shrimp waslower (<0.05) than those of SMB-sprayed and low-con- centration SMB-soaked shrimp (<0.05). On day 180, the TPCs of the control, 1.5%, 3.0%, and 5.0% SMB-soaked,and 5.0% SMB-sprayed shrimp were 5.01, 4.64, 4.61, 4.53, and 4.68 log(CFUg−1), respectively. Overall, the ability of SMB to inhibit increases in TPC was dependent on the treatment method and dose, which correlates well with the TVB-N levels of the corresponding samples.
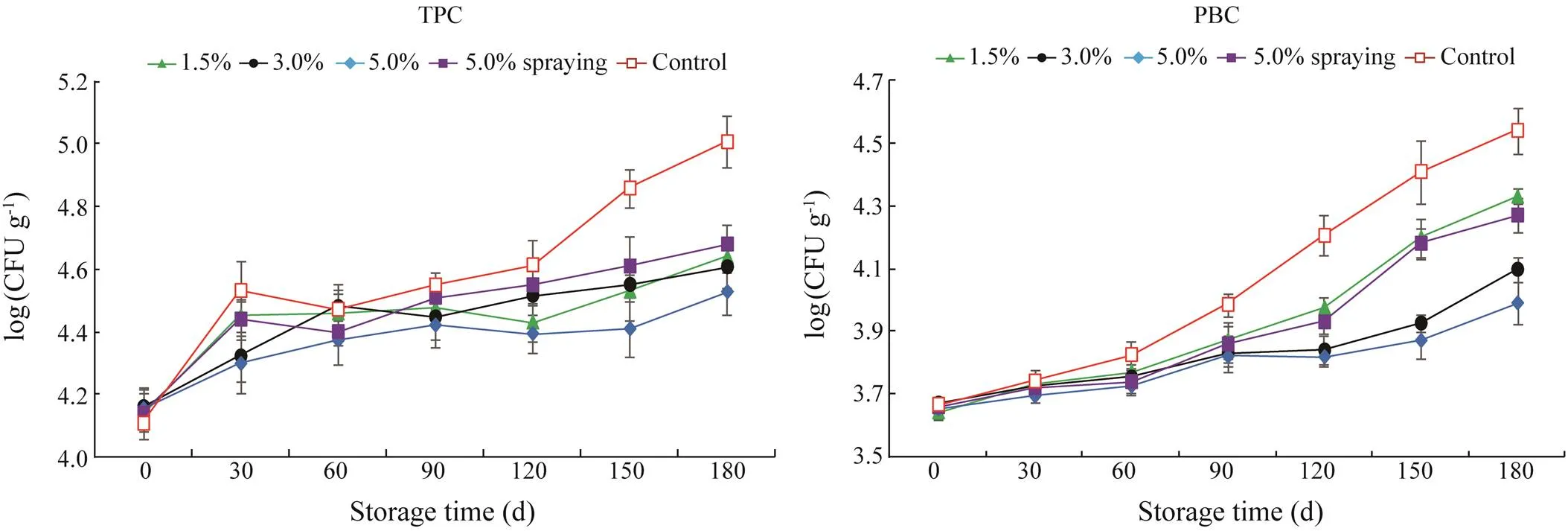
Fig.6 Total aerobic plate count (TPC) and psychrotrophic bacteria count (PBC) of S. crassicornis treated with SMB at different concentrations using different methods over 180d of frozen storage. Bars represent the standard deviation (n=3). Soaking method: 1.5%, 3.0%, and 5.0% SMB; spraying method: 5.0% SMB; control: treated without chemicals.
PBC, as a major cause of spoilage in raw shrimp during frozen storage, remains active even in a frozen environ- ment. Increased PBC was found in all samples as the stor- age time increased. On days 0 and 30, no obvious differ- ence in PBC was found among all samples (>0.05). Thereafter, a rapid increase in PBC was observed in the control shrimp (<0.05), which revealed a higher PBC after 60−180d of storage compared with treated shrimp (<0.05). No significant difference in PBC was observed among SMB treatments over 0−90d of storage (>0.05). During 120−180 days, however, differences in PBC were noted, and significantly lower PBC values were found in 3.0% and 5.0% SMB-soaked shrimp (<0.05). At the end of the storage, the control, 1.5%, 3.0%, and 5.0% SMB- soaked, and 5.0% SMB-sprayed shrimp revealed PBCs of 4.54, 4.33, 4.10, 3.99, and 4.27 log(CFUg−1), respectively. Thus, treatment of shrimp with SMB could retard the growth of PB during frozen storage, and soaking with higher con- centrations is a more effective treatment than spraying or soaking with lower SMB concentrations.
Interestingly, the TPC and PBC of all samples did not exceed the maximum permission limit of 7.0 log(CFUg−1) recommended by the ICMSF (1986) over the entire observation period. Therefore, the use of 1.5% SMB soaking treatment demonstrates good antimicrobial effects in preservingunder frozen conditions.
4 Conclusions
During frozen storage, soaking shrimp with high dose of SMB can improve sensory scores, delay color changes, and prevent melanosis more effectively than soaking shrimp with lower doses of SMB or by spraying SMB. Moreover, shrimp soaked with SMB at higher concentrations revealed lower increases in TVB, TBARS content, and microbial growth and lower decreases in SSP over 180d of frozen storage. However, the SO2contents of 5% SMB- soaked samples exceeded the limit of 100mgkg−1through- out the storage. In conclusion, soaking with 1.5% SMB revealed good antioxidant and antimicrobial effects and may be recommended in efforts to preserveunder frozen conditions.
Acknowledgements
This work was supported by the Science and Technology Plan Projects of Zhejiang Province, China (Nos. 2017 C37009, 2017F50018).
Al-Bulushi, I. M., Kasapis, S., Dykes, G. A., Al-Waili, H., Guizani, N., and Al-Oufi, H., 2013. Effect of frozen storage on the characteristics of a developed and commercial fish sausages., 50 (6): 1158- 1164.
Arancibia, M. Y., Lopez-Caballero, M. E., Gómez-Guillén, M. C., and Montero, P., 2015. Chitosan coatings enriched with active shrimp waste for shrimp preservation., 54: 259- 266.
Benjakul, S., Visessanguan, W., and Tanaka, M., 2006. Inhibitory effect of cysteine and glutathione on phenoloxidase from ku- ruma prawn ()., 98 (1): 158- 163.
Bono, G., Badalucco, C. V., Cusumano, S., and Palmegiano, G. B., 2012. Toward shrimp without chemical additives: A combined freezing-MAP approach.–, 46 (1): 274-279.
Bono, G., Okpala, C. O. R., Alberio, G. R., Messina, C. M., San- tulli, A., Giacalone, G., and Spagna, G., 2016. Toward shrimp consumption without chemicals: Combined effects of freezing and modified atmosphere packaging (MAP) on some quality characteristics of giant red shrimp () during storage., 197: 581-588.
Buttkus, H., 1974. On the nature of the chemical and physical bonds which contribute to some structural properties of protein foods: A hypothesis., 39 (3): 484- 489.
Cacciuttolo, M. A., Trinh, L., Lumpkin, J. A., and Rao, G., 1993. Hyperoxia induces DNA damage in mammalian cells., 14 (3): 267-276.
Chantarasuwan, C., Benjakul, S., and Visessanguan, W., 2011. Effects of sodium carbonate and sodium bicarbonate on yield and characteristics of Pacific white shrimp ()., 17 (4): 403- 414.
Chinese National Standard (GB 2760-2014).. Chinese National Hygiene Ministry, Beijing.
Chinese National Standard (GB 2733-2015).. Chinese National Hygiene Ministry, Beijing.
Chinese National Standard (GB 5009.228-2016).. Chinese National Hygiene Ministry, Beijing.
Chinese National Standard (GB 5009.34-2016).r Dioxide (SO) in Food.Chinese National Hygiene Ministry, Beijing.
Dai, X. Y., Zhang, M. X., Wei, X. Y., Hider, R. C., and Zhou, T., 2016. Novel multifunctional hydroxypyridinone derivatives as potential shrimp preservatives., 9 (7): 1079-1088.
Dong, Z., Xu, F., Ahmed, I., Li, Z., and Lin, H., 2018. Characterization and preservation performance of active polyethylene films containing rosemary and cinnamon essential oils for Pacific white shrimp packaging., 92: 37-46.
Farajzadeh, F., Motamedzadegan, A., Shahidi, S. A., and Hamzeh, S., 2016. The effect of chitosan-gelatin coating on the quality of shrimp () under refrigerated condition., 67: 163-170.
FDA (U. S. Food and Drug Administration), 2001.. Silver Spring, MD, https://www.fda.gov/food/ laboratory-methods-food/bam-aerobic-plate-count.
Gokoglu, N., and Yerlikaya, P., 2008. Inhibition effects of grape seed extracts on melanosis formation in shrimp ()., 43 (6): 1004-1008.
Goncalves, A. A., and Ribeiro, J. L. D., 2008. Do phosphates im- prove the seafood quality? Reality and legislation., 3 (3): 237-247.
Gornall, A. G., Bardawill, C. J., and David, M. M., 1949. Deter- mination of serum proteins by means of the biuret reaction., 177 (2): 751-766.
Herrera, J. R., and Mackie, I. M., 2004. Cryoprotection of frozen- stored actomyosin of farmed rainbow trout () by some sugars and polyols., 84 (1): 91-97.
International Commission on Microbiological Specifications for Foods (ICMSF), 1986. Sampling plans for fish and shellfish, In:2nd edition. University of Toronto Press, Toronto, 181-196.
Jin, L., Ding, G., Li, P., Gu, J., and Zhang, X., 2018. Changes in quality attributes of marine-trawling shrimp () during storage under different deep-frozen temperatures., 55 (8): 2890- 2898.
Liu, D., 2016. Study on different drying process of. Master thesis. Zhejiang Gongshang University.
Martínez-Álvarez, Ó., Gómez-Guillén, M. D. C., and Montero, P., 2008. Chemical and microbial quality indexes of Norwegian lobsters () dusted with sulphites., 43 (6): 1099-1110.
Martínez-Alvarez, O., Montero, P., and del Carmen Gómez- Guillén, M., 2005. Controlled atmosphere as coadjuvant to chilled storage for prevention of melanosis in shrimps ()., 220 (2): 125-130.
Montero, P., Lopez-Caballero, M. E., and Perez-Mateos, M., 2001. The effect of inhibitors and high pressure treatment to prevent melanosis and microbial growth on chilled prawns ()., 66 (8): 1201-1206.
Nirmal, N. P., and Benjakul, S., 2009. Effect of ferulic acid on inhibition of polyphenoloxidase and quality changes of Pacific white shrimp () during iced storage., 116 (1): 323-331.
Nirmal, N. P., and Benjakul, S., 2010. Effect of catechin and ferulic acid on melanosis and quality of Pacific white shrimp subjected to prior freeze-thawing during refrigerated storage., 21 (9): 1263-1271.
Remya, S., Basu, S., Venkateshwarlu, G., and Mohan, C. O., 2015. Quality of shrimp analogue product as affected by addition of modified potato starch., 52 (7): 4432-4440.
Rencüzoǧullari, E., İla, H. B., Kayraldiz, A., and Topaktaş, M., 2001. Chromosome aberrations and sister chromatid exchanges in cultured human lymphocytes treated with sodium metabi- sulfite, a food preservative., 490 (2): 107-112.
Sae-Leaw, T., Benjakul, S., and Simpson, B. K., 2017. Effect of catechin and its derivatives on inhibition of polyphenoloxidase and melanosis of Pacific white shrimp., 54 (5): 1098-1107.
Shiekh, K. A., Benjakul, S., and Sae-leaw, T., 2019. Effect of Cha- muang (Roxb.) leaf extract on inhibition of melanosis and quality changes of Pacific white shrimp during refrigerated storage., 270: 554-561.
Solval, K. M., Rodezno, L. A. E., Moncada, M., Bankston, J. D., and Sathivel, S., 2014. Evaluation of chitosan nanoparticles as a glazing material for cryogenically frozen shrimp.–, 57 (1): 172-180.
Soncin, S., Chiesa, L. M., Panseri, S., Biondi, P., and Cantoni, C., 2009. Determination of volatile compounds of precooked prawn () and cultured gilthead sea bream () stored in ice as possible spoilage markers using solid phase microextraction and gas chromatography/ mass spectrometry., 89 (3): 436-442.
Taylor, S. L., Higley, N. A., and Bush, R. K., 1986. Sulfites in foods: Uses, analytical methods, residues, fate, exposure assessment, metabolism, toxicity, and hypersensitivity.In:. Vol. 30. Academic Press, New York, 1-76.
Tsironi, T., Dermesonlouoglou, E., Giannakourou, M., and Taou- kis, P., 2009. Shelf life modelling of frozen shrimp at variable temperature conditions.–, 42 (2): 664-671.
Wachirasiri, K., Wanlapa, S., Uttapap, D., and Rungsardthong, V., 2016. Use of amino acids as a phosphate alternative and their effects on quality of frozen white shrimps ().–, 69: 303-311.
Wang, X., 2014. Study on the preservation effect of acidic elec- trolyzedwater for. Master thesis. Zhe- jiang Gongshang University.
World Health Organization, 1999. Sulfur Dioxide and Sulfites Fifty First Report of the Joint FAO/WHO. Expert Committee on Food Additives, WHO Technical Report Series No. 891.
Wu, S. J., 2014. Effect of chitosan-based edible coating on pre- servation of white shrimp during partially frozen storage., 65: 325- 328.
Yoon, K. S., and Lee, C. M., 1990. Cryoprotectant effects in surimi and surimi/mince-based extruded products., 55 (5): 1210-1216.
Yuan, G., Lv, H., Tang, W., Zhang, X., and Sun, H., 2016. Effect of chitosan coating combined with pomegranate peel extract on the quality of Pacific white shrimp during iced storage., 59: 818-823.
April 28, 2019;
July 2, 2019;
November 29, 2019
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2020
. Tel: 0086-580-2299871
E-mail: jinlei2388@126.com
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Circulation and Heat Flux along the Western Boundary of the North Pacific
- System Reliability Analysis of an Offshore Jacket Platform
- The Mineral Composition and Sources of the Fine-Grained Sediments from the 49.6˚E Hydrothermal Field at the SWIR
- Research Progress of Seafloor Pockmarks in Spatio-Temporal Distribution and Classification
- Application of the Static Headland-Bay Beach Concept to a Sandy Beach: A New Elliptical Model
- Climatology of Wind-Seas and Swells in the China Seas from Wave Hindcast
