Indoor spray and windows screens effects on dengue vector density after space spraying in a field trial
2020-02-27NapadolSudsomKuaananTechatoSuwichThammapaloNannapatPruphetkaew
Napadol Sudsom, Kuaanan Techato, Suwich Thammapalo, Nannapat Pruphetkaew,
Noodchanath Kongchouy5, Virasakdi Chongsuvivatwong4, Theerakamol Pengsakul6✉
1Nan Provincial Public Health Office, Ministry of Public Health, Thailand
2Faculty of Environmental Management, Prince of Songkla University, Thailand
3Office of Disease Prevention and Control 12 Songkhla, Thailand
4Epidemiology Unit, Faculty of Medicine, Prince of Songkla University, Thailand
5Department of Mathematics and Statistics, Faculty of Science, Prince of Songkla University, Thailand
6Faculty of Medical Technology, Prince of Songkla University, Thailand
ABSTRACT Objective: To demonstrate the effect of indoor spraying and window screens on Aedes aegypti mosquito density after space spraying.Methods: A total of 141 households (the study houses) in six communities of Songkhla City, located in Songkhla Province of southern Thailand, were randomly selected and the adult Ae. aegypti populations were assessed pre- and post-insecticide spraying from March to October, 2014. Houses close to (within a 20 m radius) the study houses were analyzed using spatial analysis tools. The Aedes aegypti density in the study houses and house density index were compared with the density in the neighbouring houses, based on three spraying conditions: (i) unsprayed (ii) only outdoor sprayed and (iii) indoor plus outdoor sprayed. Results: Only spraying houses indoors was the most effective (P<0.05). There was insufficient evidence that the source of the increase in the number of mosquitoes in unsprayed houses was due to their migration from neighbouring houses which had been sprayed. However, the study houses without screens on their windows were found to have a likely higher dengue vector population after spraying, but the difference was not significant.Conclusions: In dengue endemic areas, all houses should be fully screened and the number of houses ultra-low volume sprayed indoor plus outdoor should be increased with the cooperation of householders and communities during epidemics.
KEYWORDS: Space spraying; Dengue; Aedes aegypti; Windows screens; Indoor spray
1. Introduction
Ultra-low volume (ULV) space spraying, the aerosol application of insecticides to suppress adult female Aedes (Ae.) aegypti mosquitoes, which are the main vector of dengue virus infections, is a public health strategy adopted as an emergency measure during outbreaks of dengue[1]. However, there is insufficient evidence that ULV spraying is an effective intervention interrupting the transmission of the dengue virus[2]. Several factors, such as the time of application, vector behaviour, meteorological conditions and the skill of the spray operators, may influence the effectiveness of ULV applications[3]. Improving the effectiveness of ULV spraying can reduce adult Ae. aegypti populations and the transmission of the dengue virus[4].
Failure of space spaying interventions to control dengue outbreaks has often been associated with the non-participation of householders due to residents or building owners not facilitating access to buildings[5] and a low rate of indoor spray coverage. Based on previous studies, inadequate space-spraying in outbreak areas has been unable to stop the occurrence of secondary dengue cases[6]. Moreover, the mosquitoes’ resistance to synthetic pyrethroid insecticides and the tendency of mosquitoes to respond to pyrethroid spraying by migrating, may also have an impact on the efficacy of space spraying[7,8].
Ae. aegypti has been shown to be repelled by synthetic pyrethroids because of their property of causing irritation to the insects. These compounds produce a combination of irritation (excitation) and repellence, which results in the mosquitoes moving away from the area sprayed with the insecticide[8,9]. Study by Kongmee M et al. shows that, 78% of adult female Ae. aegypti mosquitoes rapidly exited the area within 30 min of being directly exposed to a standard field dose (0.02 g/m2) of deltamethrin[10]. In a wind tunnel, the number of ULV droplets of 1% prallethrin (range of diameter=2.5-5.0 µm) on their bodies caused an immediate increase in the duration and speed of flight of flying mosquitoes[11]. The result of an excitorepellency test system using the optimal dose of deltamethrin (0.02% concentration, LC50) produced spatial repellency of Ae. aegypti[12] with 21%-40% of the females escaping from the treated chambers. After field space spraying, recovery patterns of migration of Ae. aegypti mosquitoes from untreated to treated areas were found within a 15 m radius of the border of the sprayed areas[13]. A previous study has shown that rapid resurgence of Ae. aegypti mosquito populations were found after space spraying[14], while evidence of the escape effect from external spraying possibly increasing the vector density in nearby premises was not found. However, another study has shown that houses with window screens have the potential for integrated control of the dengue vector[15].
There have been very few ULV field studies focusing on the escape effect from external spraying. Because of the mosquitoes’ avoidance behaviour to pyrethroid insecticides, it is suspected that the mosquito density of nearby houses would increase, which might enhance the probability of dengue infection in the households thus affected. The objective of this study was to demonstrate the effect of indoor, outdoor spraying and window screens on Ae. aegypti density after space spraying. The findings from this study will be useful for dengue control measures in the future.
2. Material and methods
2.1. Study design
A field study was carried out to evaluate the effectiveness of indoor and outdoor ULV space spraying of insecticide for the prevention and control of mosquito vectors within communities where dengue infection is endemic. The study was conducted between March and October 2014 in Songkhla City, Muang District, Songkhla Province, Thailand.
2.2. Ethical approval and informed consent
Ethical approval (No. 57-068-19-9 07/03/2014) was obtained from the Human Research Ethics Committee of the Faculty of Medicine, Prince of Songkla University, Thailand. The permission, of all residents was obtained before spraying took place and they were given full details of the purpose, methods, risks and benefits of the ULV spraying intervention before they signed an informed consent form.
2.3. Study setting
Songkhla City located in southern Thailand (7° 12′ N, 100° 36′ E), is an area with endemic dengue infection[6] (Figure 1). Songkhla has a tropical monsoon climate, a dry season between February and July, and a wet season between August and January, with an average annual temperature of 28.2 ℃ (range: 19.3 ℃-36.5 ℃), an average annual relative humidity of 76% (range: 38%-97%) and an annual rainfall of 1 941 mm in 2014 with rain falling on 142 days (Southern Meteorological Center, East Coast). The municipality had a total population of 67 600 in 2014, covers an urban area of 9.27 km2with a population density of 7 300 people per km2. The residential areas consist of 37 communities with 26 600 households. In crowded communities with a mean distance between houses of less than 10 m, the occurrence of dengue fever/dengue hemorrhagic fever cases has been reported throughout the last three years, therefore, these were considered to be suitable areas for this study.
Figure 1. Study area map showing location of Songkhla City.
2.4. Field spraying operations
The field spraying operations consisted of ULV space spraying, conducted by the Office of Disease Prevention and Control 12 (Disease Prevention and Control, 12). Based on WHO recommendations[15], in six different areas, all households located in a circle of 100 m radius, geo-located using high resolution satellite images (Quick Bird, USA) and GIS software (ArcGIS 10.1, ESRI ArcGISTM, Redlands, CA, United States) were sprayed by well-trained sprayers from Disease Prevention and Control 12 with calibrated equipment and insecticides. The status of mosquito screening on all the study houses were noted. All spraying operations were carried out at the optimum time (08:00-10:00 am) and in optimum weather conditions (wind speed: <13 km/h and no rain). Based on the agreement of the house owners, all the houses targeted in the six different areas were classified into three groups of households: (1) indoor plus outdoor, (2) outdoor only and (3) unsprayed (Figure 2).
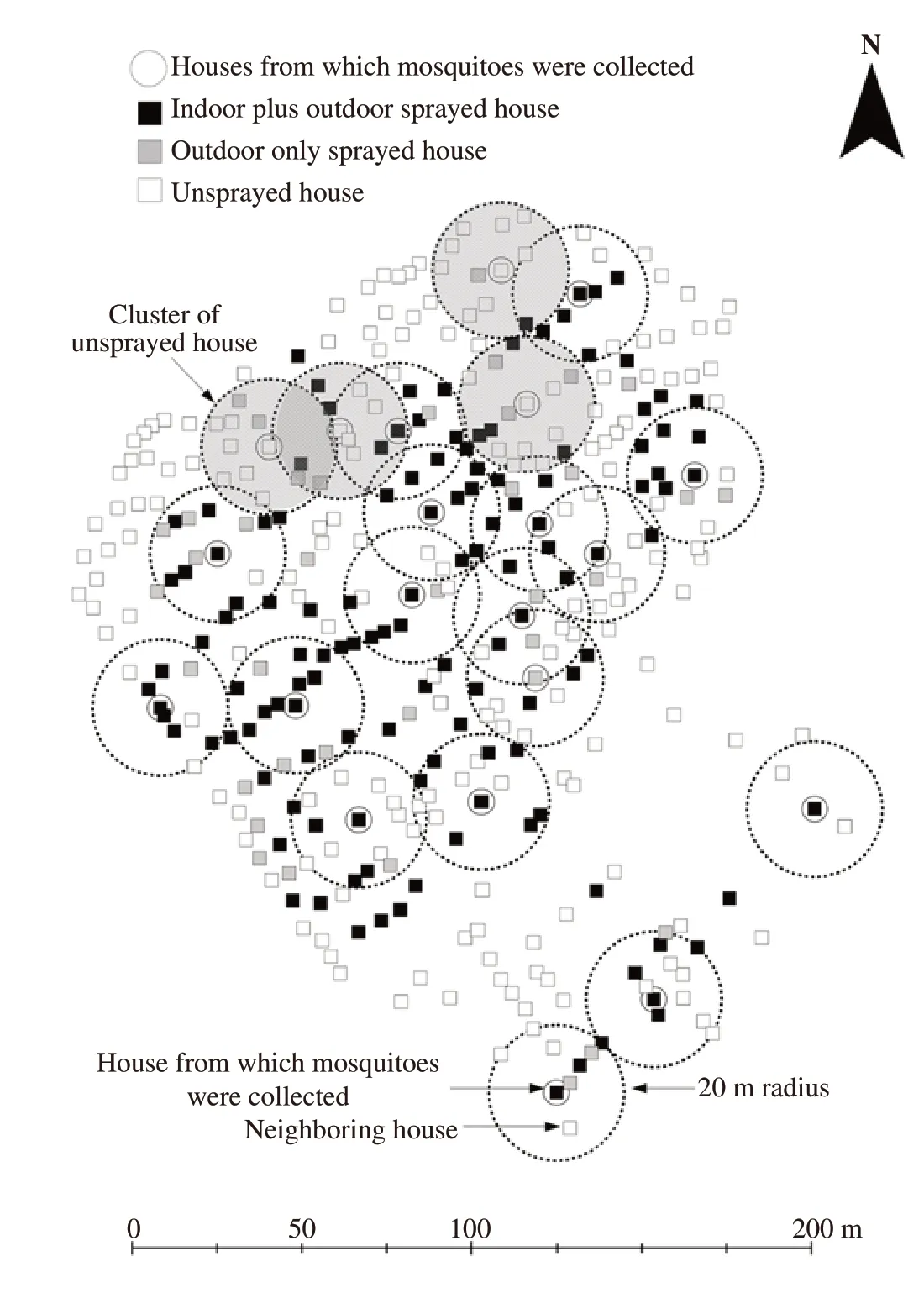
Figure 2. Distribution of houses from which Aedes aegypti were collected and clusters of nearby houses (20 m radius) at the Kaoseng study site.
2.5. ULV equipment and chemical calibration
The hand-portable ULV generators (Fontan Portastar S, Germany) and chemical (Deltamethrin 2% w/v) were calibrated at Disease Prevention and Control 12 before the field spraying operations were carried out. The four ULV generators delivered acceptable ranges of droplets with volume median diameters of 23, 25, 25 and 26 microns, and a discharge rate at 22 mL/min with a dosage of 0.5-1.0 g/ha which was in accordance with the WHO guidelines[15-17]. Further, the Ae. aegypti collected from Songkhla City were still highly susceptible to deltamethrin with 99.8% knockdown and 99.9% mortality rate based on a droplet bio-assay test conducted according to WHO guidelines[16].
2.6. Field insecticide susceptibility
Based on the WHO guidelines[16], a cage bio-assay test was applied to evaluate the field insecticide susceptibility in the ULV spraying areas. Four of the six study sites were randomly chosen and a total of 16 cages of adult Ae. aegypti mosquitoes (25-50 per cage) were placed in different locations inside four houses (four cages per house). The mortality of the mosquitoes was determined 24 h after spraying.
2.7. Adult Ae. aegypti mosquito collection
In each sprayed area, house density index was assessed in around 21-31 randomly selected houses, 2 days before (D-2), and 1, 2 and 6 days after spraying (D1, D2and D6). Adult Ae. aegypti mosquitoes were collected using hand-held nets, based on the WHO guidelines[18], in the living areas of the same houses over a period of 15 min.
2.8. Spatial analysis
All households located in a circle of 20 m radius (Figure 1) from each of the houses in which the adult mosquito population had been assayed and determined using spatial analysis tools. The number of nearby houses was determined using buffer and identity analysis with ArcGIS 10.1.
2.9. Data analysis
The numbers of Ae. aegypti pre- and post-ULV space spraying were compared between the three groups of houses (unsprayed, outdoor sprayed and indoor sprayed). The relationship between increases in Ae. aegypti density and the number of neighboring houses being sprayed was analyzed. Finally, the change of Ae. aegypti densities over time was compared between houses with and without full window screens. All analyses were performed using the R statistical program (R Development Core Team 2014).
3. Results
3.1. Mosquito collection
A total of 659 adult female Ae. aegypti mosquitoes were collected from 141 houses in the six communities assayed (number of mosquitoes, number of houses), Kaoseng (177, 21), Thasaan (40, 25), Watthasalahuayang (89, 19), Kubo (78, 20), Noksuan (87, 26) and Ruamjaiphatthana (188, 30), in pre- and post-space spraying collection operations.
3.2. Field bio-assay cage test
On the day of spraying (D0), the 24-hour mortality rate of indoor plus outdoor sprayed houses, outdoor sprayed only houses and unsprayed houses were 100%, 39% and 24%, respectively.
3.3. Clustering of sprayed houses
The study community had a relatively high house density (11 houses within a radius of 20 m). The highest mean values of neighbouring houses within 20 m of the unsprayed houses were of unsprayed and outdoor sprayed only study houses (5.3 and 4.9 houses respectively, Table 1). In contrast, for the indoor plus outdoor sprayed houses the highest mean value of neighbouring houses was of indoor plus outdoor sprayed study house (5.6 houses within a 20 m radius). Thus, the spraying had a clustered pattern.

Table 1. Analysis of relationship between the number of female Aedes aegypti collected from the three groups of study houses classified by spray status (n).
3.4. Dynamics of Ae. aegypti density
The Ae. aegypti density breakdown by spray status is summarized in Table 2. There was a significant decrease on 1, 2 and 6 days after spraying only in the indoor plus outdoor sprayed houses, while the Ae. aegypti density in the unsprayed houses increased from the baseline (D-2) but the difference was not statistically significant (P>0.05).
3.5. Changes in vector density in households not ULV sprayed
In Table 3, the 31 unsprayed houses are classified into three groups based on the direction of change in the number of mosquitoes. In each group, the mean numbers of nearby houses (within a 20 m radius of the study houses) based on those which were ULV sprayed indoors and outdoors or were only sprayed outdoors were calculated. The mean of nearby sprayed houses was consistently higher among the increasing groups but the difference was not statistically significant (P>0.05).
3.6. Relationship of vector densities between households which were not ULV sprayed and those in neighboring houses which were outdoor sprayed
The logarithm of the Ae. aegypti counts on Day 1, 2 and 6 were modeled against the number of houses sprayed within 20 m, with the offset value being the log count on D-2and household ID, based on random effects. This model was run on the glmmPQL function of the ‘MASS’ package of R. No significant effect of day and number of neighboring house sprayed was observed (detailed results omitted).
3.7. Direction of changes in Ae. aegypti populations in the fully screened houses
Table 4 shows the changes in vector density from D-2to D6broken down by whether the houses were fully screened from mosquitoes or not fully screened. There is a trend for those without screens to have higher mosquito densities but also without statistical significance based on a sign test (P>0.05).

Table 2. Comparison of number of female Aedes aegypti collected by days from three groups of study houses (n).

Table 3. Changes of vector density in the 31 unsprayed houses, neighboring houses of which were outdoor sprayed (n).
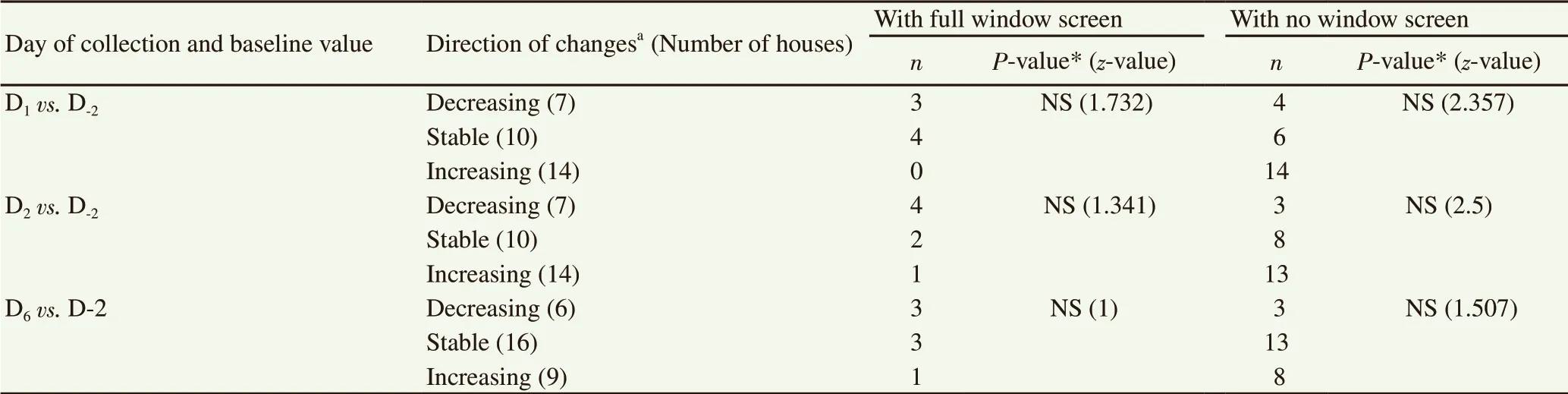
Table 4. Relationship between directions of changes in Aedes aegypti density and number of nearby outdoor sprayed houses of study houses with or with no full window screen (n).
4. Discussion
The findings indicated that the reduction of Ae. aegypti density in the houses ULV sprayed was significant only in those houses which were indoor plus outdoor sprayed. Further, although there was a trend of association between increased Ae. aegypti density in unsprayed houses with increased numbers of neighbouring houses being outdoor sprayed, the relationship did not reach statistical significance. In addition, increasing mosquito density in the study houses without windows screens was found to be likely higher than in those with screens.
In this study, we found that the acceptance of ULV spraying by householders had a clustered pattern. The clustered pattern of participation for spraying had not previously been noted probably because no spatial analysis had ever been conducted on this issue. Clustering in cooperation in health activities is, however, an expected phenomenon as neighbors may directly or indirectly influence one another to share the same attitude[19]. Incomplete coverage of spraying has been reported to be followed by secondary dengue cases[6]. Thus, risk may increase in clusters in which the majority of the houses refuse spraying as they are the destination of the mosquitoes repelled from the sprayed houses.
Our data provide supporting evidence that indoor ULV application is essential to suppress dengue vectors[3], while outdoor spraying alone was useless. This can be explained by previous findings that most Ae. aegypti rest inside houses[20]. In most dengue outbreaks, ULV spraying is believed to be the most important weapon in suppressing the number of dengue cases. However, there is no evidence of its effectiveness based on field studies. In all probability, most spraying is conducted outdoors as comprehensive indoor spraying is not completely conducted in most communities[3].
Previous studies have reported the escape behavior of the Ae.
aegypti mosquito when exposed to pyrethroids[9]. Our study failed to demonstrate a significant relationship between the density of Ae. aegypti mosquito collected from unsprayed houses whose neighboring houses being outdoor sprayed. This was probably due to the sample size being too small to test the significance of the effect. Further study of this effect is therefore needed.
Fully screened houses were found to be more likely to have a reduced mosquito density after spraying while the opposite was true in the unscreened houses. Based on the studies reviewed, full house screening has the potential to prevent incoming outdoor mosquitoes[21]. Thus it is likely that full screening was able to prevent the immigration of mosquitoes from neighboring houses which had been sprayed. Moreover, previous studies have revealed that the risk of contracting dengue viral infections is lower among people living in fully screened houses[22]. Unsprayed houses with no window-screens are an optimum habitat for Ae. aegypti mosquitoes to move to while spraying is being carried out in nearby houses. Moreover, such increases enhance the likelihood of dengue infection in the residents of such houses. Clusters of unsprayed houses within sprayed areas may become zones with a high-risk of dengue virus infection. Thus all houses should be fully screened in dengue endemic areas.
The study shows that only indoor plus outdoor spraying is effective, and outdoor spraying may cause the migration of Ae. aegypti mosquito populations. Full house screening has the potential to prevent the immigration of mosquitoes. It is recommended that in order to decrease the escape effect caused by external spraying, the number of houses ULV sprayed indoors should be increased with the cooperation of householders and communities where dengue fever is endemic.
Conflict of interest statement
The authors declare that they have no competing interests.
Acknowledgement
We would like to thank all the Disease Prevention and Control, 12 vector-borne teams, Songkhla City municipality and Muang Songkhla District Public Health Office; and also the residents in the study sites for their cooperation and support during the study. We also sincerely thank Mr. Michael Currie for improving the use of English in the manuscript.
Funding
This research was supported by Research Fund, Prince of Songkla University Contract Number MET590137S.
Authors’ contributions
N.S. and T.P. are engaged in research plan, design and conduct research, data collection, data analysis, summarize the research findings, write reports and prepare articles for publication. K.T., S.T., N.P., and N.K. are involved in conducting research and data analysis. All authors contributed to the final version of the manuscript. And V.C. supervised the project.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- The phytochemical and pharmacological properties of artocarpin from Artocarpus heterophyllus
- Prevalence and genotype distribution of hepatitis B virus among migrant workers in Lombok Island, Indonesia
- Salivary gland antigens of laboratory-bred Phlebotomus sergenti and their immunogenicity in human volunteers in laboratory condition
- Sandfly fauna and ecological analysis of Phlebotomus orientalis and Phlebotomus martini in the lowland foci of visceral leishmaniasis in Somali Regional State, southeast Ethiopia
- Orostachys japonicus ethyl acetate fraction suppresses MRSA biofilm formation
- Cryptococcal meningitis with pulmonary cryptococcoma in an immunocompetent patient: A case report
