拟穴青蟹致病性副溶血弧菌分离鉴定及药敏试验
2020-02-22陈小龙程长洪邓益琴马红玲苏友禄冯娟郭志勋
陈小龙 程长洪 邓益琴 马红玲 苏友禄 冯娟 郭志勋
摘要:【目的】確定引起拟穴青蟹发病死亡的病原菌,并了解其致病性及药敏特性,为该病的临床诊断及科学防控提供理论依据,同时为保障拟穴青蟹养殖业的健康发展打下基础。【方法】采用常规细菌分离纯化方法从患病拟穴青蟹的病料组织中分离优势菌株,通过形态特征观察、生理生化特性鉴定及16S rDNA序列分析进行分类学鉴定,并进行人工感染试验、组织病理学观察及药敏特性分析。【结果】从患病拟穴青蟹肝胰腺中分离得到1株优势菌株(编号NS1SP18),菌株NS1SP18感染拟穴青蟹48 h的半数致死剂量(LD50)为3.18×104 CFU/g,感染发病拟穴青蟹呈现出多体液、偶有黑鳃及肝胰腺暗黄等症状,与自然发病拟穴青蟹的症状相似。综合菌株NS1SP18的形态特征、生理生化特性及16S rDNA序列分析结果,可鉴定为副溶血弧菌(Vibrio parahemolyticus)。患病拟穴青蟹的肝胰腺细胞发生变性;心肌纤维肿大,细胞坏死,有血淋巴浸润;网状鳃腔结构消失,脱落细胞阻塞鳃腔;肌纤维形变、断裂,局部坏死。菌株NS1SP18对氟苯尼考、恩诺沙星、四环素、多四环素、诺氟沙星、复方新诺明和庆大霉素等7种抗生素敏感,对麦迪霉素、万古霉素和阿莫西林已产生耐药性;对诃子、乌梅、苏木和八角茴香等4味中药极敏,对地榆、女贞子、山楂、五倍子、黄连、半枝莲、赤芍和救必应等8味中药高敏,对应的最小抑菌浓度(MIC)为7.81~31.25 mg/mL,最小杀菌浓度(MBC)为15.62~125.00 mg/mL。【结论】从患病拟穴青蟹分离获得的副溶血弧菌具有较强致病性,能造成严重的组织损伤而导致拟穴青蟹发病死亡。在拟穴青蟹养殖生产中,可适度选用氟苯尼考和恩诺沙星等抗生素抑制副溶血弧菌病暴发流行,或以诃子、乌梅、苏木和八角茴香等中药进行副溶血弧菌病防治。
关键词: 拟穴青蟹;副溶血弧菌;分离鉴定;致病性;组织病理变化;药敏试验
中图分类号: S945.6 文献标志码: A 文章编号:2095-1191(2020)11-2846-10
Isolation,identification and drug sensitivity of pathogenic Vibrio parahemolyticus of mud crab(Scylla paramamosain)
CHEN Xiao-long1,2, CHENG Chang-hong2, DENG Yi-qin2, MA Hong-ling2,
SU You-lu3, FENG Juan2, GUO Zhi-xun2*
(1Shanghai Ocean University/Key Laboratory of Exploration and Utilization of Aquatic Genetic Resources, Ministry of Education/National Demonstration Center for Experimental Fisheries Science Education/National Pathogen Collection Center for Aquatic Animals, Shanghai 201306, China; 2Tropical Fisheries Research and Development Center, South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences/Key Laboratory of South China Sea Fishery Resources Development and Utilization, Ministry of Agriculture and Rural Affairs, Guangzhou 510300, China; 3Guangdong Water Environment and Aquatic Product Safety Engineering Technology Research Center/College of Animal Science and Technology, Zhongkai University of Agriculture and Engineering, Guangzhou 510225, China)
Abstract: 【Objective】Identified a pathogenic strain that caused the death of Scylla paramamosain and clarified its pathogenicity and drug sensitivity in order to provide a theoretical basis for the clinical diagnosis and scientific prevention and control of Scylla paramamosain disease, and lay a foundation for the healthy development of the S. paramamosain breeding industry. 【Method】The dominant strain was isolated from naturally infected S. paramamosain by conventional bacterial isolation and purification methods. Then, it was identified by the morphological observation, biochemical characteristics and 16S rDNA sequence analysis. In addition, artificial infection test, histopathological observation and drug sensitivity analysis were performed. 【Result】A dominant strain(numbered as NS1SP18) was isolated from the hepatopancreas of di-seased S. paramamosain. The median lethal dose(LD50) of strain NS1SP18 infected with S. paramamosain for 48 h was 3.18×104 CFU/g. The artificial infected S. paramamosain showed symptoms of multi-body fluid, occasional black gills, dark yellow hepatopancreas, which were similar to the naturally infected S. paramamosain. According to the morphological characteristics, physiological and biochemical characteristics and 16S rDNA sequence analysis, it was identified as Vibrio parahaemolyticus. The diseased crabs showed degenerated hepatocytes, swelled myocardial muscle fibers and necrotic cells, and infiltrated hemolymph. Additionally, the structure of the reticular gill cavity were disappeared, and the exfoliated cells blocked the gill cavity; muscle fiber filaments were deformed, broken, and localized necrosis. The NS1SP18 was sensitive to florfenicol, enrofloxacin, tetracycline, doxycycline, norfloxacin, cotrimoxazole, and gentamicin, and resistant to midecamycin, vancomycin, and amoxicillin. Further, the NS1SP18 was extremely sensitive to four kinds of Chinese herbal medicines, including Terminalia chebula,Fructus mume,Caesalpinia sappan linn,and Illi-cium verum, and highly sensitive to eight kinds of Chinese herbal medicines, including Sanguisorba officinalis, Fructus Ligustri Lucidi, Crataegus pinnatifida, Rhus chinensis, Coptis chinensis, Scutellaria barbat, Radix paeoniae rubra, Ilicis rotundae cortex. The minimum inhibitory concentration(MIC) was 7.81-31.25 mg/mL, and the(minimum bactericidal concentration) MBC was 15.62-125.00 mg/mL. 【Conclusion】The V. parahaemolyticus strain NS1SP18 isolated from diseased crabs is highly pathogenic which causes serious tissue damage and leads to the deathof S. paramamosain. In the breeding of S. paramamosain,antibiotics such as florfenicol and enrofloxacin can be used to suppress the outbreak of V. parahaemolyticus, or Chinese herbal medicines such as T. chebula,F. Ligustri Lucidi,C. sappan linn,and I. verum can be used for V. parahaemolyticus disease prevention.
Key words: Scylla paramamosain; Vibrio parahemolyticu; isolation and identification; pathogenicity; histopathological changes; drug sensitivity test
Foundation item: Special Project of National Shrimp and Crab Industry Technology System Construction(CARS-48); Guangdong Marine Fishery Science and Technology Promotion Special Project(A201701B01); General Project of Guangdong Natural Science Foundation(2019A1515011548)
0 引言
【研究意义】拟穴青蟹(Scylla paramamosain)俗称青蟹,隶属于梭子蟹科(Portunidae)青蟹属(Scy-lla),是我国东南沿海地区主要的养殖品种之一(Ye et al.,2011)。据统计,2018年我国青蟹产量达15.77万t,其产值超过200亿元(农业农村部渔业渔政管理局等,2019)。随着养殖规模的不断扩大,拟穴青蟹养殖环境持续恶化,各种疾病频繁发生(毛芝娟和卓华龙,2001;丁朋晓,2007;彭银辉等,2012;张迪等,2013;刘顺等,2014),其中弧菌(Vibrio)是引起海水蟹细菌性疾病的主要病原菌之一(陈华,2007;Weng et al.,2010;彭慧婧等,2012;周俊芳等,2014)。因此,加强拟穴青蟹弧菌病原研究,对确保其产业持续健康发展具有重要意义。【前人研究进展】弧菌是海水养殖环境中的常见优势细菌类群,其中副溶血弧菌(V. parahemolyticus)是水产养殖过程中最常见的条件性致病菌(李毅财等,2012;毕水莲等,2017)。副溶血弧菌隶属于弧菌科(Vibrionaceae)弧菌属(Vibrio),为革兰氏阴性短杆菌(Mohamad et al.,2019),于1950年在调查食物中毒事件中首次发现,1963年正式命名为副溶血弧菌(Shinoda,2011)。Letchumanan等(2015)研究发现副溶血弧菌可引发人体流行性肠胃炎。此后,副溶血弧菌在水产养殖业中暴发流行。Dong等(2016)对从湖北某养殖区患病克氏原螯虾(Procambarus clarkii)体内分离获得副溶血弧菌,并进行多位点序列分型和毒力基因筛选;郝景伟等(2019)利用分子生物学检测方法对山东某养殖场患病的三疣梭子蟹(Portunus trituberculatus)进行病原检测,结果显示样品扩增序列与致病副溶血弧菌质粒pirAvp毒力基因片段具有99%的相似性;刘杰等(2019)从广西凡纳滨对虾(Litopenaeus vannamei)体内分离出67株副溶血弧菌,与标准副溶血弧菌的相似度均在98.8%以上。此外,有研究证实副溶血弧菌对水生生物具有较强的致病性(阎斌伦等,2010;Soto-Rodriguez et al.,2015)。阎斌伦等(2010)研究发现,以3×107 CFU/mL副溶血弧菌JGX080708-1菌株浸泡或注射三疣梭子蟹,其死亡率均达100%;Soto-Rodrigue等(2015)研究证实,以8.10×106 CFU/mL副溶血弧菌M09-04菌株感染凡纳滨对虾21 h可全部致死;贾丹(2017)研究表明,副溶血弧菌对凡纳滨对虾具有较强的致病性,浸泡感染的半数致死剂量(LD50)为7.96×103 CFU/mL;黄倞等(2019)研究证实,副溶血弧菌感染凡纳滨对虾48 h的LD50为1.73×103~1.32×105 CFU/mL;李妍等(2019)研究发现,副溶血弧菌人工感染斑马鱼的LD50约为5.00×105 CFU/尾,使用剂量达5.00×107 CFU/尾时其死亡率达100%。【本研究切入点】副溶血弧菌作为水生生物致病菌已有较多研究报道(Abdel-Aziz et al.,2013;Liu et al.,2017;郝景伟等,2019),但在广东地区鲜见副溶血弧菌感染拟穴青蟹发病的相关报道。2018年广东省广州市南沙某养殖区的拟穴青蟹突然发病,其临床症状主要表现为反应迟钝、活动乏力、步足关节处肌肉呈乳白色,剖检可见多体液、肝胰腺暗黄及偶有黑鳃等病理变化。因此,急需明确其发病原因并采取有效的防治措施,以确保广东省拟穴青蟹养殖业健康发展。【拟解决的关键问题】对患病拟穴青蟹进行病原菌分离鉴定,通过人工感染、组织病理学分析和药敏试验确定其致病性及药敏特性,为该病的临床诊断及科学防控提供理论依据,同时为保障拟穴青蟹养殖业的健康发展打下基础。
1 材料与方法
1. 1 试验材料
患病拟穴青蟹于2018年8月采自广东省广州市南沙养殖区;健康拟穴青蟹购自广东江门五邑平家海鲜批发市场,平均体重约50.0 g/只,经实验室暂养7 d后选择健康、无损伤、活力强的个体用于人工感染试验。采集患病和健康拟穴青蟹的肝胰腺、心脏、鳃组织及肌肉,以4%多聚甲醛固定液固定24 h后更换为70%乙醇长期保存。MH琼脂培养基、硫代硫酸盐柠檬酸盐胆盐蔗糖琼脂(TCBS)培养基、营养肉汤(NB)培养基、革兰氏染色试剂盒及弧菌科细菌生化鉴定试剂盒购自广东环凯生物科技有限公司;细菌基因组DNA提取试剂盒、DL2000 Plue DNA Marker和2×Taq Master Mix購自TaKaRa公司;西药药敏纸片购自杭州天和微生物试剂有限公司;中药购自珠海斗门信康大药房。
1. 2 病原菌分离与纯化
对患病拟穴青蟹进行寄生虫镜检及青蟹呼肠孤病毒(MCRV)和青蟹双顺反子病毒(MCDV)检测均呈阴性。在超净台上以无菌接种环蘸取患病拟穴青蟹肝胰腺样品,涂布于MH琼脂培养基和TCBS培养基上,28 ℃恒温培养24 h。观察计数后,挑取优势单菌落进行纯化培养;对纯化菌株进行革兰氏染色,并以甘油保种,置于-80 ℃冰箱保存备用。
1. 3 人工感染试验
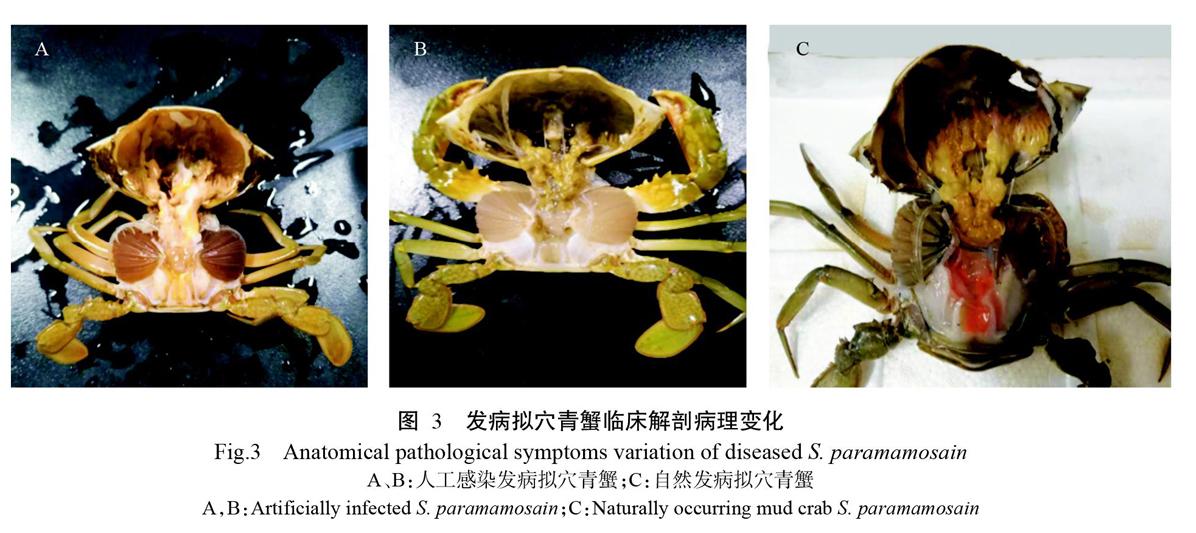
将健康拟穴青蟹随机分成7组(6个感染组和1个对照组),每组3个平行,每个平行10只拟穴青蟹。以肌肉注射方式进行人工感染,菌悬液浓度分别为2×104、2×105、2×106、2×107、2×108和2×109 CFU/mL,即感染浓度分别为4×102、4×103、4×104、4×105、4×106和4×107 CFU/g,试验组分别注射100 μL不同浓度的菌悬液,对照组注射等量的无菌生理盐水。每天记录各组拟穴青蟹的发病及死亡情况,并对濒死拟穴青蟹进行剖检和病原菌再次分离鉴定;使用SPSS 19.0计算病原菌对拟穴青蟹的LD50。
1. 4 病原菌鉴定
将纯化培养的菌落接种至MH琼脂培养基上,28 ℃恒温培养24 h后,按照弧菌科细菌生化鉴定试剂盒说明进行鉴定。将纯化菌株接种到NB液体培养基中,28 ℃下200 r/min恒温摇床上培养24 h,然后根据细菌基因组DNA提取试剂盒说明提取DNA。参照王云新和彭华林(2014)的方法,以细菌通用引物8F(5'-AGAGTTTGATCCTGGCTCAG-3')和1942R(5'-GGTTACCTTGTTACGACTT-3')扩增纯化菌株的16S rDNA序列。PCR反应体系25.0 μL:2×Taq PCR Master Mix 12.5 μL,10 mmol/L正、反向引物各1.0 μL,DNA模板2.0 μL,无菌ddH2O 8.5 μL。扩增程序:94 ℃预变性3 min;94 ℃ 15 s,55 ℃ 15 s,72 ℃ 1 min,进行30个循环;72 ℃延伸5 min。PCR扩增产物经1.0%瓊脂糖凝胶电泳检测后,送至广州天一辉远基因科技有限公司进行测序。将测序获得的目的序列与NCBI数据库中的已知核酸序列进行BLAST比对分析,并采用MEGA 6.0中的邻接法(Neighbor-joining,NJ)构建系统发育进化树。
1. 5 组织病理学观察
取出保存好的拟穴青蟹组织样品,根据组织学分析方法制作病理切片(Zhang et al.,2014)。利用TP1020自动脱水机进行脱水、透明及浸蜡;然后以ES-500组织包埋机进行石蜡包埋,Leica轮式切片机进行切片,Autostainer XL自动染色机进行苏木精—伊红(HE)染色;经中性树胶封片后在Olympus BX51显微镜下进行观察。
1. 6 药敏试验
1. 6. 1 菌悬液制备 取出甘油冻存的分离菌株,接种至MH琼脂培养基上,28 ℃恒温培养箱培养24 h后挑取单菌落接种至3 mL已灭菌的NB培养基中,28 ℃下200 r/min恒温培养12 h。采用平板菌落计数法确定菌悬液浓度,并以无菌生理盐水将其稀释至2×107 CFU/mL,备用。
1. 6. 2 抗生素药敏试验 根据美国临床实验室标准委员会(CLSI)推荐的抗菌药物敏感性试验执行标准,采用K-B纸片扩散法进行药敏试验,并根据杭州天和微生物试剂有限公司《纸片药敏试验抑菌圈直径判断标准》判定分离菌株对氟苯尼考等12种抗生素的敏感性。
1. 6. 3 中药药敏试验 将诃子等25味中药超微粉碎后过80目筛,称取100 g药粉,用纱布包裹,浸泡过夜,置于全自动煎药壶中煎煮3次,合并药液并定容至100.0 mL,浓度为1 g/mL。高压蒸汽灭菌15 min,4 ℃保存。中药药敏试验参照马绪荣和苏德模(2000)的方法稍作改动:取0.1 mL菌悬液均匀涂布在MH琼脂培养基上,用直径6 mm的无菌打孔器在培养基上垂直打孔,每孔加入150.0 μL药液,每味药设3个平行,28 ℃培养24 h,采用十字交叉法测定抑菌圈。根据抑菌圈直径判断分离菌株对中药的敏感性,判断标准(曹红峰等,2007):平均抑菌圈直径≥20 mm为极敏;15 mm≤平均抑菌圈直径<20 mm为高敏;10 mm≤平均抑菌圈直径<15 mm为中敏;平均抑菌圈直径<10 mm为低敏。采用微量二倍稀释法测定最小抑菌浓度(MIC)和最小杀菌浓度(MBC)(马绪荣和苏德模,2000)。在黑色背景光源下观察孔内细菌的生长情况,弥漫性浑浊则有菌生长,澄清透明则细菌生长受抑制;无细菌生长的各孔中,浓度最低者即为MIC。从无细菌生长的各孔中移出20.0 μL液体,均匀涂布在NB培养基上,28 ℃培养24 h,观察有无菌落生长;无菌落生长的最低药物浓度即为MBC。
2 结果与分析
2. 1 病原菌分离情况
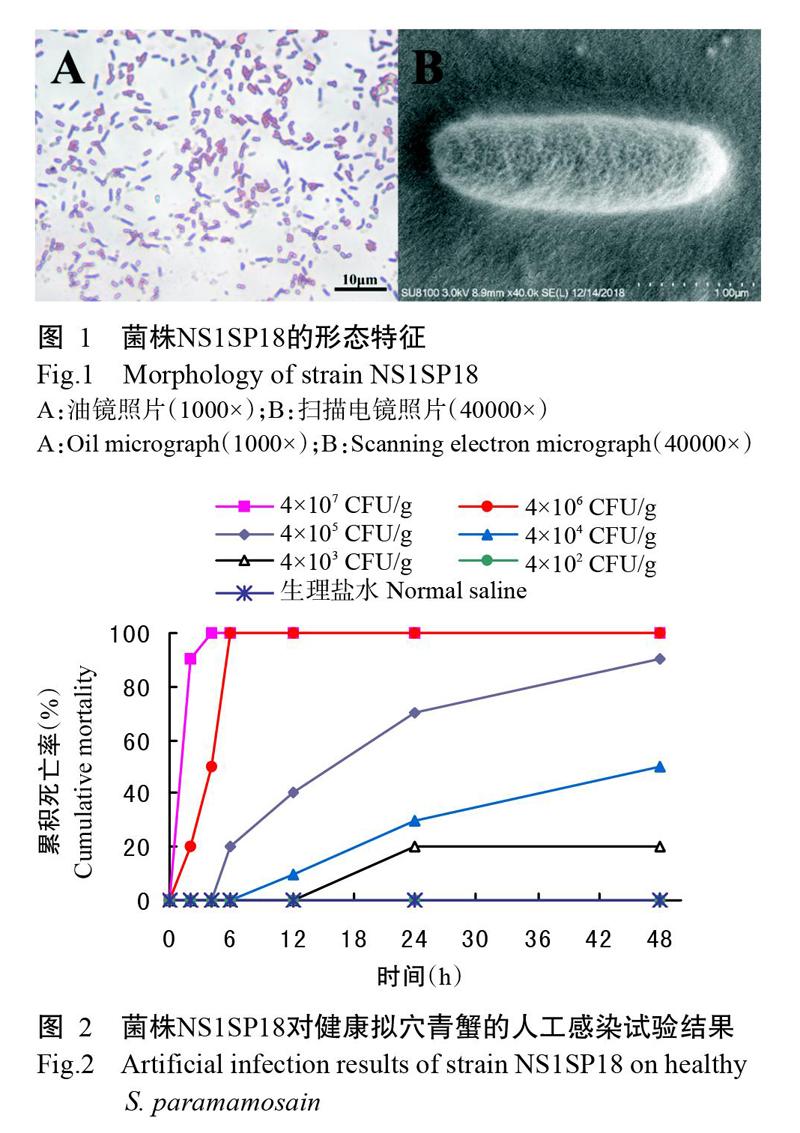
从患病拟穴青蟹肝胰腺中分离得到1株优势菌株,编号NS1SP18。菌株NS1SP18在TCBS培养基上的菌落呈圆形、绿色、半透明、表面光滑、边缘整齐,菌落直径在3 mm左右;菌株NS1SP18在MH琼脂培养基上的菌落偏黄、较大。光学显微镜下观察为革兰氏阴性菌(图1-A);扫描电镜下观察,菌体呈杆状结构,大小约0.87 μm×2.69 μm(图1-B)。
2. 2 人工感染试验结果
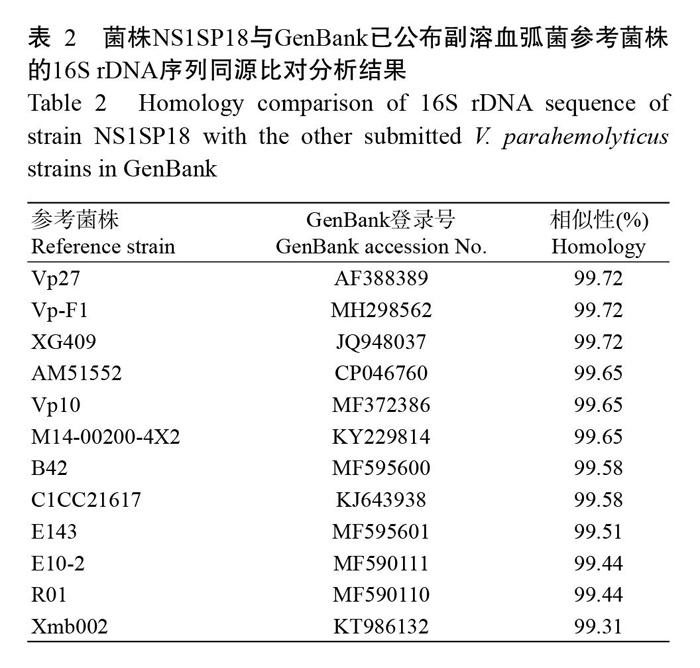
人工感染试验结果如图2所示。当菌悬液浓度为4×106 CFU/g时,感染拟穴青蟹48 h的死亡率为100%;菌悬液浓度为4×102 CFU/g时,其死亡率为0。试验期间,对照组拟穴青蟹无发病死亡现象。根据SPSS 19.0计算,菌株NS1SP18感染拟穴青蟹48 h的LD50为3.18×104 CFU/g。临床剖检发现,感染组发病拟穴青蟹呈现出多体液、偶有黑鳃(图3-A)、肝胰腺暗黄(图3-B)等症状,与自然发病拟穴青蟹的症状相似(图3-C)。从感染组发病拟穴青蟹肝胰腺中再次分离到与菌株NS1SP18生理生化特性一致的菌株。
2. 3 病原菌鉴定结果
由表1可知,菌株NS1SP18的生理生化特性与副溶血弧菌的基本一致,参照《常见细菌系统鉴定手册》《伯杰细菌鉴定手册》及弧菌科细菌生化鉴定试剂盒说明,初步鉴定为副溶血弧菌。PCR扩增获得菌株NS1SP18的16S rDNA序列片段为1446 bp,目的序列在NCBI数据库中进行BLAST比对分析,结果发现菌株NS1SP18与GenBank已公布副溶血弧菌参考菌株的16S rDNA序列相似性均在99.00%以上(表2)。选取16S rDNA序列相似性较高的弧菌构建系统发育进化树,结果显示菌株NS1SP18与副溶血弧菌聚为一支(图4)。
参考文献:
毕水莲,罗永文,关小莺. 2017. PCR衍生技术快速检测副溶血弧菌的研究进展[J]. 江西农业学报,29(8):105-109. [Bi S L,Luo Y W,Guan X Y. 2017. Research advances in rapid detection of Vibrio parahaemolyticus by derivative techniques of PCR[J]. Acta Agriculturae Jiangxi,29(8):105-109.]
曹红峰,宋靖芳,李國庆,黄文芳. 2007. 中草药对嗜水气单胞菌ST-3-3抑菌作用的研究[J]. 中医药导报,13(5):86-88. [Cao H F,Song J F,Li G Q,Huang W F. 2007. Study on bacteriostatic action of Chinese herbal medicine on Aeromonas hydrophilia ST-3-3[J]. Guiding Journal of Traditional Chinese Medicine and Pharmacy,13(5):86-88.]
柴贝贝,姜维佳,王莉贞,胡梅,赵雨川,吴永继,司红彬. 2018. 原儿茶酸或绿原酸与抗菌药联用对鱼源链球菌的抑菌效果[J]. 南方农业学报,49(3):580-585. [Chai B B,Jiang W J,Wang L Z,Hu M,Zhao Y C,Wu Y J,Si H B. 2018. Antibacterial effects of protocatechuic acid and chlorogenic acid combined with antibiotics against fish-source Streptococcus[J]. Journal of Southern Agriculture,49(3):580-585.]
陈华. 2007. 锯缘青蟹副溶血性孤菌分离鉴定及抗TLH单克隆抗体的制备[D]. 福州:福建农林大学. [Chen H. 2007. Isolation and identification of Vibrio parahamolyticus of Scylla serrata and preparation of monoclonal antibody against thermolabile hemomysin[D]. Fuzhou:Fujian Agriculture and Forestry University.]
丁朋晓. 2007. 养殖锯缘青蟹暴发性流行病的初步研究[D]. 贵阳:贵州大学. [Ding P X. 2007. Research on fulminant epidemic disease of cultured mud crab Scylla serrate[D]. Guiyang:Guizhou University.]
郝景伟,高保全,王崇,孟宪亮,万晓媛,李小平,刘萍,张庆利. 2019. 致急性肝胰腺坏死病副溶血弧菌(VpAHPND)自然感染三疣梭子蟹[J]. 水产学报,43(7):1647-1660. [Hao J W,Gao B Q,Wang C,Meng X L,Wan X Y,Li X P,Liu P,Zhang Q L. 2019. Natural infection of Portunus trituberculatus with acute hepatopancreas necrosis disease causing by Vibrio parahaemolyticus(VpAHPND)[J]. Journal of Fisheries of China,43(7):1647-1660.]
黄倞,王元,马清扬,英娜,赵姝,吴越,房文红. 2019. 2株副溶血弧菌不同盐度下致病性和毒力基因差异分析[J]. 海洋渔业,41(6):704-711. [Huang L,Wang Y,Ma Q Y,Ying N,Zhao S,Wu Y,Fang W H. 2019. Expression of pathogenic and virulent genes on two Vibrio parahaemolyticus strains[J]. Marine Fisheries,41(6):704-711.]
贾丹. 2017. 2013—2016年沿海地区虾源副溶血弧菌的特性分析及致病性研究[D]. 上海:上海海洋大学. [Jia D. 2017. Characterization and pathogenicity analysis of Vibrio parahaemolyticus isolated from penaeid shrimps in China (2013-2016)[D]. Shanghai:Shanghai Ocean University.]
李妍,李培海,刘长水,马庆军. 2019. 一株副溶血弧菌D3112致病性和抗药性的表征[J]. 海洋科学,43(6):25-33. [Li Y,Li P H,Liu C S,Ma Q J. 2019. Characterization of pathogenicity and drug resistance in Vibrio parahaemolyticus D3112[J]. Marine Sciences,43(6):25-33.]
李毅财,赵峰,朱兰兰,赵晓君,周德庆. 2012. 黄渤海区域贝类中副溶血弧菌污染调查及血清学分型[J]. 南方农业学报,43(2):241-244. [Li Y C,Zhao F,Zhu L L,Zhao X J,Zhou D Q. 2012. Investigations on contamination and serotype of Vibrio parahaemolyticus in shellfish obtained from Yellow Sea and Bohai Sea areas[J]. Journal of Southern Agriculture,43(2):241-244.]
刘杰,曾令泽,贺晓晨,许瓢尹,盛雪晴,梁静真,黄钧. 2019. 广西凡纳滨对虾源副溶血弧菌的分离鉴定及其耐药性[J]. 广西畜牧兽医,35(5):197-202. [Liu J,Zeng L Z,He X C,Xu P Y,Sheng X Q,Liang J Z,Huang J. 2019. Isolation,identification and drug resistance of Vibrio parahaemolyticus from Penaeus vannamei in Guangxi[J]. Guangxi Journal of Animal Husbandry and Veterinary Medicine,35(5):197-202.]
劉顺,戴瑜来,周素明,郑晓叶,王国良. 2014. 拟穴青蟹(Scy-lla paramamosain)血卵涡鞭虫病的流行病学分析[J]. 海洋与湖沼,45(3):595-601. [Liu S,Dai Y L,Zhou S M,Zheng X Y,Wang G L. 2014. Epidemiology analysis of Hematodinium sp. disease in Scylla paramamosain[J]. Oceanologia et Limnologia Sinica,45(3):595-601.]
马驰. 2011. 不同中药对3种细菌耐药质粒的消除作用研究[D]. 雅安:四川农业大学. [Ma C. 2011. Study on R-plasmid curing effect of different traditional Chinese medicine to three kinds of bacteria[D]. Yaan:Sichuan Agriculture University.]
马绪荣,苏德模. 2000. 药品微生物学检验手册[M]. 北京:科学出版社:230-233. [Ma X R,Su D M. 2000. Pharmaceutical microbiology inspection manual[M]. Beijing:Science Press:230-233.]
毛芝娟,卓华龙. 2001. 锯缘青蟹细菌性传染病的病原菌研究[J]. 水产科学,20(1):8-11. [Mao Z J,Zhuo H L. 2001. Studies on the pathogen of the bacteria epidemic against mud crab Scylla serrata[J]. Fisheries Science,20(1):8-11.]
南京中医药大学. 2006. 中药大辞典[M]. 上海:上海科学技术出版社:150-253. [Nanjing University of Chinese Medi-cine. 2006. Dictionary of traditional Chinese medicine[M]. Shanghai:Shanghai Scientific and Technical Publishers:150-253.]
农业农村部渔业渔政管理局,全国水产技术推广总站,中国水产学会. 2019. 2019中国渔业统计年鉴[M]. 北京:中国农业出版社. [Fishery Administration Bureau of Ministry of Agriculture and Rural Affairs,National Aquatic Technology Extension Station,China Society of Fisheries. 2019. 2019 China fishery statistical yearbook[M]. Beijing:China Agriculture Press.]
彭慧婧,邹杰,蔡德建,刘海娟,曾梦清. 2012. 两种弧菌对拟穴青蟹溞状幼体致病性研究[J]. 水产科学,31(8):473-476. [Peng H Q,Zou J,Cai D J,Liu H J,Zeng M Q. 2012. Pathogenicity of Vibrio parahaemolyticus and V. alginolyticus to zoeal mud crab Scylla paramamosain[J]. Fisheries Science,31(8):473-476.]
彭银辉,郭昱嵩,裴琨,蔡小辉,檀宁,陆志款,宋忠魁,刘旭佳,董兰芳,张琴. 2012. 广西养殖拟穴青蟹携带病原体状况的初步调查[J]. 广东海洋大学学报,32(4):78-83. [Peng Y H,Guo Y S,Pei K,Cai X H,Tan N,Lu Z K,Song Z K,Liu X J,Dong L F,Zhang Q. 2012. Preliminary investigation on status of culturing Scylla paramamosain with pathogen in Guangxi[J]. Journal of Guangdong Ocean University,32(4):78-83.]
王云新,彭华林. 2014. 水产苗种产地检疫实验操作图解[M]. 广州:广东科技出版社:33-36. [Wang Y X,Peng H L. 2014. Diagram of quarantine experiment in aquatic seed origin[M]. Guangzhou:Guangdong Science and Technology Press:33-36.]
閻斌伦,秦国民,暴增海,张晓君,毕可然,秦蕾. 2010. 三疣梭子蟹病原副溶血弧菌的分离与鉴定[J]. 海洋通报,29(5):560-566. [Yan B L,Qin G M,Bao Z H,Zhang X J,Bi K R,Qin L. 2010. Isolation and identification of Vibrio parahaemolyticus from diseased Portunus trituberculatus L.[J]. Marine Science Bulletin,29(5):560-566.]
张迪,杨铿,苏友禄,冯娟,郭志勋. 2013. 青蟹呼肠孤病毒和青蟹双顺反子病毒-1双重巢式PCR检测方法的建立[J]. 中国水产科学,20(4):808-815. [Zhang D,Yang K,Su Y L,Feng J,Guo Z X. 2013. A duplex nested-PCR assay for detection of mud crab reovirus and mud crab dicistrovirus-1[J]. Journal of Fishery Sciences of China,20(4):808-815.]
赵峰,罗家刚,张泽俊,杨永胜. 2014. 25种中草药的药理作用研究[J]. 安徽农业科学,42(14):4234-4235. [Zhao F,Luo J G, Zhang Z J,Yang Y S. 2014. Pharmacological ef-fects of 25 kinds of Chinese herbal medicine[J]. Journal of Anhui Agricultural Sciences,42(14):4234-4235.]
周俊芳,李新苍,王江勇,王元,万夕和,房文红. 2014. 中国海水蟹主要流行性疾病及其病原分析[J]. 海洋科学,38(6):102-106. [Zhou J F,Li X C,Wang J Y,Wang Y,Wan X H,Fang W H. 2014. Analysis of major infectious diseases and their causative agents of Chinese sea crabs[J]. Marine Sciences,38(6):102-106.]
Abdel-Aziz M,Eissa A E,Hanna M,Okada M A. 2013. Identifying some pathogenic Vibrio/Photobacterium species during mass mortalities of cultured Gilthead seabream (Sparus aurata) and European seabass(Dicentrarchus labrax) from some Egyptian coastal Provinces[J]. International Journal of Veterinary Science and Medicine,1(2):87-95.
Choudhury M D,Talukdar A D,Chetia P,Bhattacharjee A,Choudhury D. 2016. Screening of natural products and derivatives for the identification of RND efflux pump inhibitors[J]. Combinatorial Chemistry and High Throughput Screening,19(9):705-713.
Dong X X,Li Z,Wang X H,Zhou M,Li L,Zhou Y,Li J Q. 2016. Characteristics of Vibrio parahaemolyticus isolates obtained from crayfish(Procambarus clarkii ) in freshwater[J]. International Journal of Food Microbiology,238:132-138.
Elmahdi S,Dasilva L V,Parveen S. 2016. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries:A review[J]. Food Microbiology,57:128-134.
Hai V N. 2015. The use of medicinal plants as immunostimulants in aquaculture:A review[J]. Aquaculture,446:88-96.
Letchumanan V,Yin W F,Lee L H,Chan K G. 2015. Prevalence and antimicrobial susceptibility of Vibrio parahaemolyticus isolated from retail shrimps in Malaysia[J]. Frontiers in Microbiology,6:33. doi:10.3389/fmicb. 2015. 00033.
Li N,Zhou X J,Song Q F,Zhou M,Shi X M. 2019. Antimicrobial resistance,virulence,and molecular characterization of aquatic,clinical,and environmental Vibrio parahaemolyticus isolated from Ningbo,China[J]. Journal of Food Safety,39(4):e12650.
Liu S W,Xu X Y,Xu J,Yuan J Y,Wu W K,Zhang N,Chen Z L. 2017. Multi-drug resistant uropathogenic Escherichia coli and its treatment by Chinese medicine[J]. Chinese Journal of Integrative Medicine,23(10):763-769.
Marudhupandi T,Ajith K T T,Prakash S,Balamurugan J,Dhayanithi N B. 2017. Vibrio parahaemolyticus a causa-tive bacterium for tail rot disease in ornamental fish,Amphiprion sebae[J]. Aquaculture Reports,8:39-44.
Mohamad N,Amal M N A,Yasin I S M,Saad M Z,Nasru-ddin N S,Al-Saari N,Mino S,Sawabe T. 2019. Vibriosis in cultured marine fishes:A review[J]. Aquaculture,512:734289. doi:10.1016/j.aquaculture.2019.734289.
Ottaviani D,Leoni F,Talevi G,Masini L,Santarelli S,Rocchegiani E,Susini F,Montagna C,Monno R,D'Annibale L,Manso E,Oliva M,Pazzani C. 2013. Extensive investigation of antimicrobial resistance in Vibrio parahaemolyticus from shellfish and clinical sources,Italy[J]. International Journal of Antimicrobial Agents,42(2):191-193.
Pu H Y,Li X Y,Du Q B,Cui H,Xu Y P. 2017. Research progress in the application of Chinese herbal medicines in aquaculture:A review[J]. Engineering,3(5):731-737.
Shinoda S. 2011. Sixty years from the discovery of Vibrio parahaemolyticus and some recollections[J]. Biocontrol Science,16(4):129-137.
Silva I P,Carneiro C D S,Saraiva M A F,de Oliveira T A S D,de Sousa O V D,Evangelista-Barreto N S. 2018. Antimicrobial resistance and potential virulence of Vibrio parahaemolyticus isolated from water and bivalve mo-llusks from Bahia,Brazil[J]. Marine Pollution Bulletin,131(A):757-762.
Soto-Rodriguez S A,Gomez-Gil B,Lozano-Olvera R,Betancourt-Lozano M,Morales-Covarrubias M S. 2015. Field and experimental evidence of Vibrio parahaemolyticus as the causative agent of acute hepatopancreatic necrosis di-sease of cultured shrimp (Litopenaeus vannamei) in northwestern Mexico[J]. Applied and Environmental Microbio-logy,81(5):1689-1699.
Weng S P,Guo Z X,Sun J J,Chan M S,He J G. 2010. A reovirus disease in cultured mud crab,Scylla serrata,in southern China[J]. Journal of Fish Diseases,30(3):133-139.
Ye H H,Tao Y,Wang G Z,Lin Q W,Chen X L,Li S J. 2011. Experimental nursery culture of the mud crab Scylla paramamosain (Estampador) in China[J]. Aquaculture International,19(2):313-321.
Zhai Q Q,Li J,Feng Y Y,Ge Q Q. 2019. Evaluation of combination effects of Astragalus polysaccharides and florfeni-col against acute hepatopancreatic necrosis disease-cau-sing strain of Vibrio parahaemolyticus in Litopenaeus vannamei[J]. Fish and Shellfish Immunology,86:374-383.
Zhai Q Q,Li J. 2019. Effectiveness of traditional Chinese herbal medicine,San-Huang-San,in combination with enrofloxacin to treat AHPND-causing strain of Vibrio parahaemolyticus infection in Litopenaeus vannamei[J]. Fish and Shellfish Immunology,87:360-370.
Zhang Q L,Liu Q,Liu S,Yang H L,Liu S,Zhu L L,Yang B,Jin J T,Ding L X,Wang X H,Liang Y,Wang Q T,Huang J. 2014. A new nodavirus is associated with covert mortality disease of shrimp[J]. The Journal of Gene-ral Virology,95(12):2700-2709.
Zhao S,Ma L C,Wang Y,Fu G H,Zhou J F,Li X C,Fang W H. 2018. Antimicrobial resistance and pulsed-field gel electrophoresis typing of Vibrio parahaemolyticus isola-ted from shrimp mariculture environment along the east coast of China[J]. Marine Pollution Bulletin,136:164-170.
(責任编辑 兰宗宝)
收稿日期:2020-01-16
基金项目:国家虾蟹产业技术体系建设专项(CARS-48);广东省海洋渔业科技推广专项(A201701B01);广东省自然科学基金面上项目(2019A1515011548)
作者简介:*为通讯作者,郭志勋(1970-),博士,研究员,主要从事水产病害防治研究工作,E-mail:guozhixun1@163.com。陈小龙(1995-),研究方向为青蟹病害防治,E-mail:chenxiaolong361@163.com
