Multicenter Randomized Double-Blind Controlled Clinical Study of Huoxue Tongluo Recipe (活血通络方) External Washing in the Treatment of Chemotherapy-Induced Peripheral Neuropathy
2020-02-20ZHANGDongHEBinSUNLingyun孙凌云YANGYufei杨宇飞
ZHANG Dong (张 栋), HE Bin (何 斌), SUN Ling-yun (孙凌云), YANG Yu-fei (杨宇飞)
1. China Academy of Chinese Medical Sciences, Beijing 100700, China
2. Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing 100091, China
ABSTRACTObjective:To objectively evaluate the efficacy of Huoxue Tongluo Recipe (活血通络方) in the treatment of chemotherapy-induced peripheral neuropathy (CIPN). Methods:A multicenter, randomized,double-blind, block, and parallel-controlled study was conducted in which 184 subjects who met the inclusion criteria were randomized into a trial group (92 cases), a control group (46 cases), a placebo group(46 cases) at a 2:1:1 ratio, respectively. Huoxue Tongluo Recipe (活血通络方) granules, Huangqi Guizhi Wuwu Decoction (黄芪桂枝五物汤) instant granules, placebo instant granules were given for hand and foot soaking, with water temperature 38 ℃-45 ℃, soaking for 20 minutes and the intervention time was 14 days. Results:(1) The total effective test drug was 41.30%, the control group was 32.61%, and the placebo group was 32.61%. There was no significant difference in the efficiency of the 3 groups by CMH chi-square test (P>0.05); (2) In the statistics of previous chemotherapy regimens including oxaliplatin or paclitaxel as stratification factors, the overall efficiency of the trial group was significantly different from the other 2 groups. Conclusion:Huoxue Tongluo Recipe (活血通络方) external washing can improve the peripheral nerve injury caused by chemotherapy to some extent, especially in patients with previous chemotherapy regimen including oxaliplatin and paclitaxel.
KEYWORDS Huoxue Tongluo Recipe (活血通络方), peripheral nerve injury, chemotherapy, clinical Study
INTRODUCTION
Chemotherapy Induced Peripheral Neuropathy(CIPN) is one of the serious toxic side effects of chemotherapy, the severity of which is doserelated. With the progress of chemotherapy, the symptoms of nerve injury will become more and more serious, seriously affecting the smooth progress of chemotherapy. The high incidence of malignant tumors and the clinical application of chemotherapy have led to this toxic side effect, and sometimes it is particularly serious in individual patients. Common chemotherapeutic drugs that can cause CIPN include oxaliplatin,paclitaxel, vincristine, thalidomide, etc. Among them, oxaliplatin and paclitaxel are the most common, and these 2 drugs are most widely used in clinical applications. The neuropathy caused by these 2 drugs is the main component of peripheral nerve injury caused by chemotherapy, and it is a problem that clinicians often face. At present, there is no clear and effective drug applied to the clinic based on the mechanism of CIPN. Based on its unique medical thinking mode, Chinese medicine practitioners are constantly exploring ways to treat the disease. According to the clinical manifestations of numbness and dysfunction of hand and foot,it can be attributed to the category of "blood bi"and "not nourished" of Chinese medicine." Plain Conversation Discussion on Bi-Syndrome said"Numbness without pain indicates chronic and deep progress of the disease, unsmooth flow of nutrient qi and defensive qi as well as occasional emptiness of the channels. That is why the patient does not feel painful. Since the skin is not nourished,it feels insensitive." According to the analysis of Chinese medicine symptoms, the chemotherapy drugs can be attributed to toxin products, which can damage essence and consume blood. Internal deficiency of yin blood cannot fill into blood vessels. The blood cannot nourish the skin, and there are symptoms such as numbness or pain in the hands and feet. Syndrome differentiation is a deficiency of qi and blood, qi stagnation and blood stasis, imbalance of nutrient qi and defensive qi,causing collaterals stasis. The treatment should be nourishing blood and promoting blood circulation,warming meridians and unblocking bi syndrome.Huoxue Tongluo Recipe (活血通络方) is mainly composed of Rhizoma Ligustici Chuanxiong (Chuan Xiong), Radix Salviae Miltiorrhizae (Dan Shen),Radix Paeoniae Rubra (Chi Shao), Flos Carthami(Hong Hua), Radix Angelicae Sinensis (Dang Gui)and other drugs. Rhizoma Ligustici Chuanxiong(Chuan Xiong) and Radix Salviae Miltiorrhizae(Dan Shen) combine to activate blood circulation and qi movement, remove stasis and stop pain.
Radix Paeoniae Rubra (Chi Shao) and Flos Carthamir (Hong Hua) activate blood circulation and unblock meridians. Radix Angelicae Sinensis(Dang Gui) activates blood and replenishes blood.All kinds of medicines play a role of activating blood and nourishing blood, unblocking meridians and relieving pain. Our department applied this prescription for the treatment of patients with CIPN symptoms, but there is still a lack of clinical studies to provide evidence-based medical evidence.Therefore, we conducted a multicenter randomized,double-blind, placebo-controlled clinical study in conjunction with 5 hospitals in Beijing to validate the efficacy of Huoxue Tongluo Recipe (活血通络方)in the treatment of CIPN.
CLINICAL INFORMATION
Research Objects
The subjects of this study were from inpatients and outpatients from March 2013 to March 2015 at Xiyuan Hospital of China Academy of Chinese Medical Sciences, Guang'anmen Hospital of China Academy of Chinese Medical Sciences, Dongzhimen Hospital of Beijing University of Chinese Medicine,China-Japan Friendship Hospital, and the People's Liberation Army No. 309 Hospital.
Diagnostic Criteria
According to CTCAE version 3.0[1], there are symptoms of numbness in the hands and feet associated with chemotherapy, and other diagnoses that cause numbness or interference in the hands and feet can be determined.
Inclusion Criteria
① Patients with malignant tumors confirmed by pathology, cytology or imaging and having grade 1 (degree) or more peripheral nerve injury after chemotherapy (Neurotoxicity NCI Rating Scale) Anti-tumor Acute and Subacute Toxicity Index of Drug Developed by WHO); ② Karnofsky score > 60 points; ③ Expected survival > 6 months; age 18 to 80 years old; ④ Voluntarily received the drug treatment and signed informed consent that agrees not to use other treatments for peripheral nerve damage caused by chemotherapy during clinical trials. ⑤ Original diabetes history,good blood sugar control, no numbness of hands and feet before chemotherapy, good blood sugar after chemotherapy, with hand and foot numbness.
Exclusion Criteria
① Does not meet the above inclusion criteria; ② Original trauma or other causes of peripheral nerve injury; ③ Diabetes complicated with neurological disease; ④ Patients with severe cardiac dysfunction, cardiac function classification sever (The New York Heart Association (NYHA) 1982); ⑤ Undergoing treatment for other non-chemotherapeutic drugs that may cause neurotoxicity; ⑥ Skin lesions in hand and foot that cannot be treated with external washing of Chinese medicine; ⑦ Poor compliance.
Sample Size Estimation
The study was designed for superiority in the placebo group, and the non-inferior design for the positive control group, and the largest sample was taken. A comprehensive report on the incidence of peripheral neurotoxicity caused by chemotherapy and the previous treatment data of our department,taking into account statistical, ethical and other issues with α = 0.05, β = 0.1 power of 90%, the cutoff value of 0.3. The quality of the test was strictly controlled during the test to ensure that the rate of loss of follow-up was within 20%. Using the SPASS system, the initial estimated sample size was 180 cases. To ensure random hiding, the sample content should be a multiple of 8, and the sample size was set to 184 cases.
RESEARCH CONTENT AND INTERVENTION METHODS
Test Drugs and Intervention Methods
According to the hospitalized or outpatient treatment plan of the enrolled patients, under the premise of not affecting the basic treatment of the patients, after the chemotherapy was stopped or after the chemotherapy was terminated, the randomized and blinded drugs were administered separately for a 14-day soaking and washing intervention. The intervention drugs were divided into 3 groups before the implementation of the blind method, namely the test group drugs, the control group drugs and the placebo group drugs.The 3 groups of drugs were all made into instant granules, all of which were caramel and dextrin as auxiliary agents. The silver pouches of the same size and shape have a scent of caramel and a yellowish brown color. The method of use is 2 bags each time, solving into 3000 mL warm water,soaking the lesion (hand or foot) once a day. The water temperature was maintained at 38-45 ℃,20 min each time, 7 days for a course of treatment,a total of 2 course observations, one follow-up at the end of each course. The test group drugs were Radix Salviae Miltiorrhizae (Dan Shen),Radix Paeoniae Rubra (Chi Shao), Flos Carthami(Hong Hua), Rhizoma Ligustici Chuanxiong (Chuan Xiong), etc., and were accompanied by ginger extract. The control group was Radix Astragali seu Hedysari (Huang Qi), Ramulus Cinnamomi (Gui Zhi), Radix Paeoniae Alba (Bai Shao), Rhizoma Zingiberis Recens (Sheng Jiang), Fructus Jujubae(Da Zao), etc. The placebo group was divided into caramel and dextrin. The test drugs used were all prepared into rapidly dissolvable granules, which exhibited the same appearance and odor in solid state and after dissolution, and unified internal and external packaging.
Randomization and Blinding Design
The random number of 256 subjects was generated by SAS 9.2 for Windows software. The 5 test centers assigned random numbers from small to large according to the order of enrollment. Each random number corresponded to the same number of drugs, and the patient was assigned a unique random number after enrollment, and used the corresponding drug. The two-stage blind method was adopted, and the two-stage blind bottoms were separately sealed, two in each.
Observation Indicators
Main observation indicators: the effectiveness of the 3 groups of drugs in the treatment of CIPN
Secondary observation indicators: symptomatic relief of peripheral nerve injury, changes in quality of life (QLQ-C30 Scale).
Effectiveness evaluation criteria
Grade 1 of the Neurotoxic NCI Rating Scale:mild sensory abnormalities, deep sacral reflexes disappeared; Grade 2: mild or moderate objective sensation disappears or moderate paresthesia;Grade 3: severe objective sensation disappears or feels abnormal, affecting functionality.
CIPN treatment effectiveness evaluation method
(1) Efficacy evaluation method: According to the classification of 4 levels, the patients were subjected to grading evaluation of neurotoxicity before treatment and after one course of treatment and after 2 courses of treatment, thereby determining the curative effect, as follows:
Cured: clinical symptoms disappear.
Significant effect: clinical symptoms were significantly relieved or neurotoxicity graded after treatment decreased by 2 levels before treatment.
Effective: Clinical symptoms improved, and the neurotoxicity graded treatment decreased by 1 or not before treatment.
Ineffective: No improvement in symptoms and signs and no decline in neurotoxicity grading.
(2) Efficient calculation method: efficiency (%) =(effective + effective + cure) / total number of cases × 100%.
Safety indicators
Possible skin allergic reactions, skin pain,itching, purpura, and other topical drug-related adverse reactions (CTCAE 4.0).
Statistical Analysis Method
Statistical analysis was performed using SAS 9.2 software. Demographics and other baseline characteristics were selected from the full analysis set, and the safety evaluation was selected as the safety set. Descriptive statistics were performed on the clinical efficacy and total effective rate of peripheral nerve injury. The frequencies and percentages were listed. The differences between groups were compared using CMH χ2considering central factors, and the 95% confidence interval for the difference in total effective rate between groups was calculated. All statistical tests were performed using a two-sided test, and P 0.05 would be considered statistically significant.
RESULTS
General Information
The study planned to enroll 184 patients,and 187 patients were enrolled in the study, and 3 subjects were enrolled repeated. The number of cases in each sub-center was 56, 32, 40, 32, and 24 (order as above). There were 184 FAS cases(including 92 test drugs, 46 positive drugs and 46 placebo drugs) included in full analysis set, which accounted for 98.40% of the enrolled cases. There were 156 cases with PPS in the protocol set (78 test drugs, 39 positive drugs, 39 placebo drugs),accounting for 83.42% of the enrolled cases; 183 cases of safety analysis SS (91 test drugs, 46 positive drugs, 46 placebo drugs), accounting for 97.86% of the cases.
Among the subjects enrolled, males accounted for 43.48%, females accounted for 56.52%; the youngest patient was 28 years old,the oldest was 80 years old, and the median age was 58 years; 35 colon cancers, 25 rectal cancers,24 gastric cancers, 1 esophageal cancer, 27 lung cancers, 23 ovarian cancers, 18 breast cancers,7 cervical cancers, and 27 others. Among them,83 patients received oxaliplatin chemotherapy,accounting for 44%, and 39 patients with paclitaxel chemotherapy, accounting for 29%. 59 patients with intermittent chemotherapy, and 125 patients finished chemotherapy.
Effectiveness Evaluation Index
FAS set results of the main observation indicators
The total effective rate was 41.30% in the test drug, 32.61% in the positive drug, and 32.61% in the placebo drug; the CMH chi-square test showed no significant difference between the 3 groups (P>0.05).See Table 1 and Table 2 for details.
Stratified Analysis
(1) The PPS set was analyzed with the previous chemotherapy regimen containing oxaliplatin as the stratification factor: the totaleffective rate was 37.14% in the test drug, 11.76%in the positive drug, and 6.25% in the placebo. After the CMH chi-square test, the difference between the 3 groups was statistically significant (P=0.02<0.05).See Table 3 and Table 4 for details.
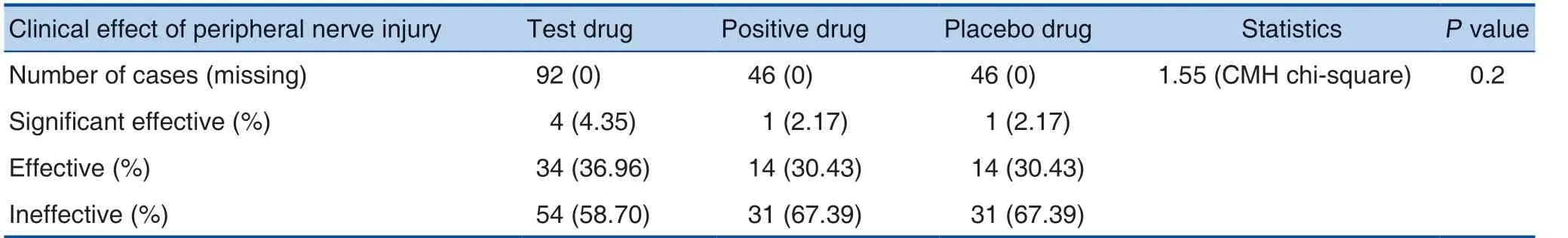
Table 1. Comparison of Therapeutic Effects between Different Groups

Table 2. Comparison of Treatment Effectiveness between Different Groups
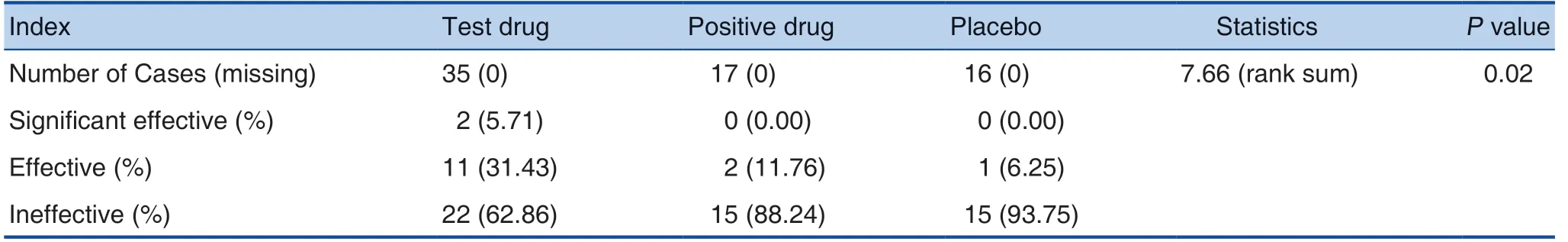
Table 3. Subgroup Analysis of Different Groups of Oxaliplatin in Previous Chemotherapy Regimens

Table 5. Subgroup Analysis of Different Groups of Paclitaxel in Previous Chemotherapy Regimens

Table 6. Effective Rate Subgroup Analysis of Different Groups of Paclitaxel in Previous Chemotherapy Regimens
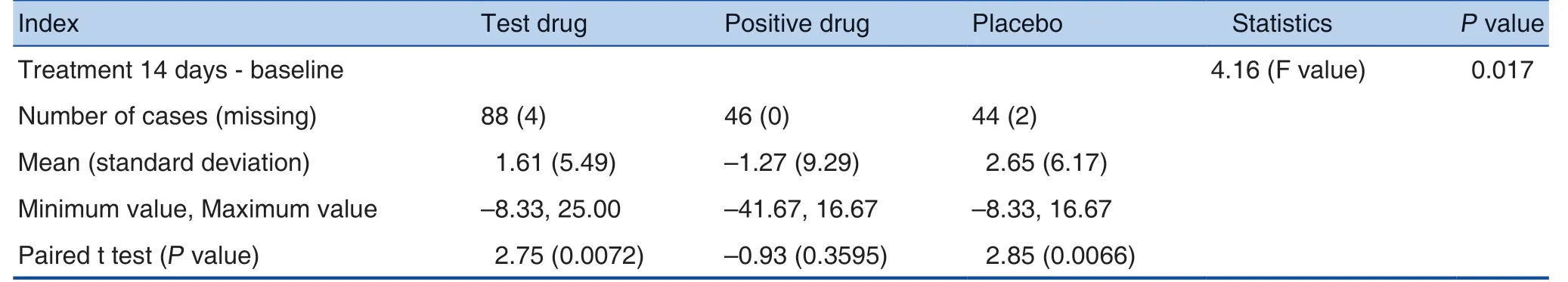
Table 7. Comparison of Emotional Changes in Different Groups at Different Times
(2) Analysis of PPS set by paclitaxel as a stratification factor in previous chemotherapy regimens: PPS set with paclitaxel as stratification factor: total effective rate was 37.14% in the test drug, 11.76% in the positive drug, and 6.25% in the placebo. After CMH chi-square test, the difference of the 3 groups was statistically significant (P<0.05).See Table 5 and Table 6 for details.
(3) Descriptive statistics on the standard scores(somatic function, emotional function, etc.) and their relative baseline changes in the QLQ-C30 scale after treatment were conducted. Paired t-test was used for intra-group comparison. The covariance analysis model was used to compare the differences between the 3 groups after treatment versus baseline. Emotional function and overall health status showed differences (FAS set). See Table 7and Table 8 for details.

Table 8. Comparison of Changes in Total Health Status of Each Group at Different Times
Adverse Events
One serious adverse event occurred during the trial, which was in the placebo group. All 8 cases of adverse events occurred 11 times in this trial, including 3 cases 4 times in the test drugs,and the incidence rate was 3.3%; 1 case 1 time in the positive drug, and the incidence rate was 2.17%; 4 cases 6 times in the placebo, and the incidence rate was 8.7%. Related adverse events:A total of 3 cases occurred 4 times, including 1 case 1 time in the test drug, and the incidence rate was 1.1%, 0 cases 0 times in the positive drug, and the incidence rate was 0%; 2 cases 3 times in the placebo, and the incidence of rate was 4.35%.
DISCUSSION
Based on the results of the FAS set population, the total effective rate of Huoxue Tongluo Recipe (活血通络方) for the treatment of CIPN was higher than that of Huangqi Guizhi Wuwu Decoction (黄芪桂枝五物汤) and placebo, but there was no statistically significant difference. Despite this, the warm water soaking method of promoting blood circulation and unblocking collaterals may still have a certain effect in improving peripheral nerve injury caused by chemotherapy. In the future, in the study design, a scale can be used to evaluate the trend of symptoms before and after treatment, and a conventional treatment control group can be established. Subgroup analysis showed that in the statistics of oxaliplatin and paclitaxel as stratification factors, the PPS set population showed that the experimental group showed significant difference compared with the other 2 groups, P<0.05, indicating that patients with CIPN caused by oxaliplatin and paclitaxel are more likely to benefit from Huoxue Tongluo Recipe (活血通络方) based on the population consistent with the program. The secondary evaluation indicators showed differences in the improvement of patients'mood and overall health status. The remaining dimensions did not show significant differences among the groups, indicating that the external washing of Huoxue Tongluo Recipe (活血通络方)in improving the patient's mood and total health(QLQ) -C30 scale) is helpful.
As a common toxic side effect of chemotherapy, CIPN only has a symptomological diagnosis. The clinical manifestations include numbness of the extremities, reduced sensation,acupuncture sensation, etc. Sever cases can affect the function, such as buckling button, writing,opening bottle cap, and other fine movements. The chemotherapy drugs that cause CIPN are mainly oxaliplatin and paclitaxel, and also included the most cases, accounting for 73%. Oxaliplatin has been used in a variety of digestive tract tumors since its introduction in Europe in 1996. About 50%of patients receiving oxaliplatin for 5-7 months have moderate-severe peripheral neuropathy[2]. Degree grading is according to the Common Terminology Criteria for Adverse Events (CTCAE)[3]. When the cumulative dose is >1000 mg/m2, 50%-75% of patients have paresthesia and dysfunction[4]. 90% of patients will be relieved after discontinuation[5], and about 40% of patients recovered completely within 6 to 8 months, leaving some sequelae[6]. The incidence of peripheral nerve injury caused by paclitaxel was 62%[7], and the incidence of severe symptoms was 6%. CIPN caused by paclitaxel also had a tendency to self-heal. A 13-year follow-up study showed that symptoms of 14% of patients disappear within a few months after the end of chemotherapy[8]. The severity of the symptoms caused by the 2 drugs is positively correlated with the dose, and will affect the smooth progress of chemotherapy in severe cases[9]. Chemotherapy with oxaliplatin can make the postoperative completion rate of rectal cancer below 50%[10-12], and a study has shown that CIPN can also seriously interfere with the quality of life of patients. Women with higher levels of neuropathy have reduced levels of psychological, emotional,cognitive and social functions, and the overall quality of life is reduced. They are more prone to fatigue,nausea/vomiting, pain, insomnia, difficulty breathing,and anxiety[13].
Many studies on its pathogenic mechanism have shown that the main targets of oxaliplatin and paclitaxel are Dorsal Root Ganglion (DRG). The central and peripheral nervous systems have bloodbrain barrier and blood-nerve barrier, respectively isolated the blood circulation system, but DRG does not. There are abundant capillary beds, which are easily damaged by chemotherapeutic drugs.The specific damage mechanism is different. The possible mechanism of oxaliplatin is the synthesis of rRNA in nucleoli, leading to protein synthesis disorders, which in turn affect function[14]. The mechanism of paclitaxel-induced peripheral nerve injury is likely to be the same as its antitumor mechanism, promoting tubulin aggregation,which can arrest cells in the G2/M phase on tumor cells, and this mechanism is no longer capable of cell division on the nerve cells. It interferes with microtubule formation and affects electrical signal transmission and energy supply disorders, causing apoptosis of DRG neurons[15].
There are currently no clear and effective drugs for clinical use in the treatment of CIPN.Based on its unique medical thinking mode,traditional Chinese medicine (TCM) is constantly exploring ways to treat and alleviate the disease.According to the clinical manifestations of numbness and dysfunction of hand and foot, it can be attributed to the category of "blood-arthralgia."
Plain Conversation Discussion on Bi-Syndrome said "Numbness without pain indicates chronic and deep progress of the disease, unsmooth flow of nutrient qi and defensive qi as well as occasional emptiness of the channels. That is why the patient does not feel painful. Since the skin is not nourished, it feels insensitive." According to the analysis of Chinese medicine symptoms,the chemotherapy drugs can be attributed to toxin products, which can damage essence and consume blood. Internal deficiency of yin blood cannot fill into blood vessels. The blood cannot nourish the skin, and there are symptoms such as numbness or pain in the hands and feet. Syndrome differentiation is a deficiency of qi and blood, qi stagnation and blood stasis, imbalance of nutrient qi and defensive qi, causing collaterals stasis.The treatment should be nourishing blood and promoting blood circulation, warming meridians and unblocking bi syndrome. The basic prescription of Huoxue Tongluo Recipe (活血通络方) is Qingxue Granules prepared by Xiyuan Hospital, which is mainly composed of Rhizoma Ligustici Chuanxiong(Chuan Xiong), Radix Salviae Miltiorrhizae (Dan Shen), Radix Paeoniae Rubra (Chi Shao), Flos Carthami (Hong Hua), Radix Angelicae Sinensis(Dang Gui) and other drugs. It has achieved many basic research and clinical results in erythrocytosis,radiation pneumonitis, radiation esophagus and oncology[16,17], won the third prize of Beijing Science Progress Prize, and is one of the "Ten Diseases and Ten Prescriptions" in Beijing scientific research project. Rhizoma Ligustici Chuanxiong (Chuan Xiong) and Radix Salviae Miltiorrhizae (Dan Shen) combine to activate blood circulation and qi movement, remove stasis and stop pain. Radix Paeoniae Rubra (Chi Shao) and Flos Carthami(Hong Hua) activate blood circulation and unblock meridians. Radix Angelicae Sinensis (Dang Gui)activates blood and replenishes blood. All kinds of medicines play a role of activating blood and nourishing blood, unblocking meridians and relieving pain.
In this study, a three-arm design with multicenter, randomized, double-blind, placebo,and positive drug controls was used to observe the efficacy of Huoxue Tongluo external washing for CIPN. The results showed no significant differences in the full data set. Patients with peripheral nerve injury caused by oxaliplatin and paclitaxel showed a certain effect. This result indicates that it is not feasible to treat all peripheral nerve injury caused by chemotherapy with Huoxue Tongluo method, but oxaliplatin and paclitaxel-induced peripheral nerve injury is worth trying, which may be related to the similarity of the 2 chemotherapy drugs to the target of nerve damage. The confounding of the enrolled patients may be the main reason for the absence of significant differences in the full data set. This also suggests that the mechanism of peripheral nerve injury caused by different chemotherapy drugs may be completely different, and the corresponding treatment should also be different.
In the study, we also noticed that the patient's numbness and pain symptoms are difficult to have more detailed assessment, especially in the early stage of neurological symptoms. The patient's selfevaluation method can more accurately reflect the change of the disease or as a supplement[18]. The way of self-evaluation, even if the score changes little, has a large hint of clinical significance[19]. The lack of a more sensitive evaluation method is also one of the places where such research is worthy of improvement. There is no Chinese version of selfevaluation. .
Due to the research and design reasons and the factors of modification of prescriptions,the traditional Chinese medicine Huangqi Guizhi Wuwu Decoction (黄芪桂枝五物汤) was only used for soaking, and showed no significant difference.The effect of oral administration still needs further study. External washing of traditional Chinese medicine has improved the quality of life and improved overall health. Due to the fact that most of the patients enrolled in the group are cancer patients, and there are many confounding factors,this results need to be further explored. The unnegligible side is that temperature stimulation will give the patient a certain comfortable experience, which can relieve the patient's emotions for a short time. This is often mentioned in the follow-up conversation with the patients.The numbness symptoms may be temporarily improved and thus recognized by most patients.This indicates that the method of external warm water soaking combined with the method of Tongluo Huoxue is worth further research in the treatment and prevention of CIPN caused by oxaliplatin and paclitaxel.
猜你喜欢
杂志排行
World Journal of Integrated Traditional and Western Medicine的其它文章
- World Federation of Chinese Medicine Societies Center for Translation
- New Year's Message
- INSTRUCTION FOR AUTHORS
- Professor YANG Yu-fei's Experience on the Integrated Chinese and Western Medicine Treatment Strategy for Advanced Colorectal Cancer
- Two Definitions of Life Will Highlight on Physiological Understanding of Six Meridians
- Effects of Shenmai Injection (参麦注射液) Combined with Meglumine Adenosine Cyclophosphate Injection on Cardiac Function and Peripheral Serum Levels of TNF-α,TGF-β1 and IFN-γ in Patients with Viral Myocarditis
