Source of mycorrhizal inoculum influences growth of Faidherbia albida seedlings
2020-01-18EmiruBirhaneMengsteabHailemariamGirmayGebresamuelTesfayArayaKirosMelesHadguLindseyNorgrove
Emiru Birhane·Mengsteab Hailemariam·Girmay Gebresamuel·Tesfay Araya·Kiros Meles Hadgu·Lindsey Norgrove
Abstract Poor land use management and practice inhibit the growth and establishment of tree seedlings in dryland areas.We assessed arbuscular mycorrhizal fungi(AM)status of Faidherbia albida(Del.)A.Chev.trees grown on different land uses.We quantified the growth and nutrient uptake of F.albida seedlings inoculated with AM from different sources.These efforts were based on soil and fine root samples from the rhizosphere soils of F.albida trees.AM root colonization was determined using the gridline intersect method.Spores were extracted by the wet sieving and decanting method and identified to genus level.The seedling experiment had a completely randomized onefactorial design with four treatments and five replications.Faidherbida albida seedlings were grown in a greenhouse.All in situ F.albida trees were colonized by AM fungi.AM root colonization of F.albida trees was significantly higher(P <0.0086)in area exclosures than on lands used for grazing or cultivation.Spore abundance was significantly higher(P <0.0014)in area exclosures followed by cultivated land and grazing land.Glomus was the dominant genus in all land-uses.AM-inoculated F.albida seedlings grew better (P <0.05) than non-inoculated controls.Seedlings inoculated with AM from area exclosure had significantly(P <0.05)higher growth and nutrient uptake than those inoculated with AM from grazing and cultivated land.This emphasizes the importance of the native soil AM potential for better establishment of seedlings to achieve optimum plant growth improvement and assist in rehabilitation of degraded arid lands.
Keywords Spore abundance·AM colonization·Inoculum types·Land-use types·Nutrient uptake·Growth parameters
Introduction
Deforestation rates in tropical drylands,such as those in northern Ethiopia,have been high due to agricultural and pastoral infringements and the removal of trees for fuelwood,charcoal production and construction material(Bishaw 2001;Jama and Zeila 2005).High temperatures combined with low rainfall also hinder tree re-establishment.Plant associations with particular soil micro-organisms can alleviate stress symptoms(Ruiz-lozano et al.2003;Smith and Read 2008;Seckbach and Grube 2010).Among these,arbuscular mycorrhizal fungi(AM)are the most common soil symbionts(Cardoso and Kuyper 2006;Smith and Read 2008; Varma and Kharkwal 2009),occurring ubiquitously due to their ability to adapt ecologically(Haselwandter and Bowen 1996;Barea et al.2011;Pellegrino et al.2015).The host plant supplies carbon for AMF growth and,in turn,the fungus enhances the uptake of relatively immobile nutrients such as phosphorus,sulfur,copper,zinc,and boron(Jayachandran and Shetty 2003;Smith and Read 2008).AM fungi can improve plant water status and growth under drought conditions.Hence,the use of AM fungi in dryland reforestation efforts can enhance long-term stability by contributing to nutrient cycling processes and improving environmental adaptability(Jasper et al.1989;Haselwandter and Bowen 1996).However,the development of AM fungi varies with host species,plant life history stage,resource availability and abiotic conditions such as soil type and depth and season(Pande and Tarafdar 2004;Gai et al.2006).The success of inoculation with AM fungi under field conditions can be determined by the level of mycorrhizal dependence of the host, the propagule density and effectiveness of any indigenous fungi,soil nutrient contents,and whether fertilizer has been applied and at what rate(Clark 1997;Camprubı´et al.2008;Seckbach and Grube 2010).
Deforestation and subsequent conversion to agriculture have degraded soil and vegetation.Drought further constrains rehabilitation efforts as it can negatively impact soil biodiversity(including beneficial root symbionts)and plant physiological processes that affect establishment and survival of seedlings(Cardoso and Kuyper 2006).Exclosures are locally adapted rehabilitation strategies that have been established by local communities or authorities with the intention to improve those communal grazing lands that have become degraded(Mekuria and Yami 2013).Such areas are protected from the interference of humans and domestic animals to facilitate natural regeneration of plants and to rehabilitate degraded land(Mekuria et al.2011;Seyoum et al.2015).Faidherbida albida(Del.)A.Chev.(Mimosoideae),a leguminous tree commonly known as apple-ring acacia or winter thorn,is used by farmers across the arid and semi-arid zones of Africa for soil fertility improvement(Nair 1993).In Ethiopia,F.albida parkland agroforestry systems have long been used for soil fertility and crop yield improvements,fodder for livestock and rehabilitation of degraded lands in the study area.It is intercropped with annual crops and used on grazing land.Inoculation of tree seedlings with AM fungi has huge potential for rehabilitation of degraded land.A site's native inoculum potential should be evaluated for the intended host plants and strategies for maintaining and enhancing native AM populations should be encouraged.The relationship between propagule abundance and plant growth response is usually not linear. Soils with the highest inoculum potential do not always have the highest plant growth response either because there are fungal speciesspecific differences in sporulation and effectiveness or because host plant ‘‘costs'' exceed ‘‘benefits'' with increasing colonization(Onguene and Kuyper 2005).To improve predictions of where inoculation is most likely to be beneficial,there is need for a better understanding of the effects of both site and management.It is important to include plant response to different inoculum densities in any assessment of land-use practices.
Studies have examined growth of trees following inoculation with AM fungi(Chev et al.2009;Stevens et al.2011;Xie et al.2014;Bati et al.2015).However,there is little information on the effects of land use on AM fungi occurrence and on the growth and nutrient uptake of F.albida seedlings.We hypothesized that:(1)field-collected inocula from the rhizosphere soil of F.albida trees grown on different land-uses could enhance growth of F.albida seedlings;and(2)growth of F.albida seedlings depends on the mycorrhizal status of soil inoculum,land-use practice and soil properties.Hence,the objectives of our study were to:(1)determine spore abundance of AM fungi from F.albida trees grown in different land-uses;(2)estimate the extent of AM fungi colonization of F.albida tree roots grown in different land-uses,(3)investigate the effect of inoculation of AM fungi on the growth and nutrient uptake of F.albida seedlings,and(4)discuss the influence of land-use practices on the occurrence of AM fungi associated with F.albida.
Materials and methods
The study site was at Kilite Awulaelo District Abraha Weatsbaha village(13°45′N-14°00′N and 39°30′E-39°45′E altitude 1980 m to 2500 m a.s.l.),in northern Ethiopia.Average daily air temperature ranges between 15 and 30°C,and mean annual rainfall is 558 mm.Based on USDA soil textural classification methods,the soils were grouped into sandy loam,clay sand and sandy clay loam and clay classes.A greenhouse experiment with F.albida seedlings was conducted at Mekelle University(13°29′N and 39°28′E,elevation 2200 m a.s.l.).The mean daily temperature of the greenhouse was 27°C during the day and 22°C during the night with a mean daily average relative humidity of 51%for the study period.
Land-use and tree selection
Faidherbida albida trees growing in different land-use types(area exclosure,grazing land and cultivated land)under the same agro-climatic conditions(mid altitude)were selected during the summer based on previous species composition studies and after doing a reconnaissance survey(Noulekoun et al.2017).Equal numbers of sample plots(5)were used in all land use types to compare their AM fungi status,assess the impact of disturbance on AM status and investigate the effect of AM fungal inocula on the growth of F.albida seedlings.Plots were 20×20 m.All individual trees were identified to species and measured for diameter at breast height(DBH),height,and crown diameter.Trees in grazing and cultivated lands were older(11-13 years)than those in area exclosure(5-6 years).The average distance between individual trees was 10 m in the land use types.
Soil and root sampling
From the rhizosphere of selected trees,samples were collected for spore analysis,assessment of AM fungal colonization, soil physical and chemical analysis and greenhouse experiment.Soil cores of 0-25 cm were taken by use of a cylindrical soil corer of 8 cm internal diameter.Soil cores included fine roots of the host plant and were collected at four directions perpendicular to each other to form a composite soil sample. Each tree species was replicated five times for sampling of soil and roots.A subsample of approximately 100 g of soil was taken for extraction of AM fungal spores.The soil samples were airdried,passed through a 2 mm sieve and stored at 4°C before analysis.
Physical and chemical analysis of soil samples
Soils were analysed for pH,electrical conductivity(EC),available P,total nitrogen(TN),organic matter(OM),exchangeable bases(Na,K,Ca and Mg),cation exchange capacity(CEC),bulk density(BD),and soil texture.OM was determined by the wet combustion procedure of Walkley-Black(Van Ranst et al.1999).TN was determined by wet-oxidation procedure of the Kjeldahl method(Bremner and Mulvaney 1982).Available P content was determined by the Olsen method (Olsen and Sommers1982).Na,K,Ca and Mg,and CEC were determined by the 1 M ammonium acetate(pH 7)method according to the percolation tube procedure(Van Reeuwijk 1995).The effective CEC was calculated as the sum of exchangeable cations extracted by the ammonium acetate buffered at pH 7 plus 1 M KCl extractable Al.BD was determined by core method (Blake and Hartge 1986). EC and pH was calculated by using a suspension of 1:2.5 soil:water ratio using EC and pH meter.Soil texture was determined using Hydrometer method(Gee and Bauder 1986).
AM fungal spore extraction
Spores were extracted from 100 g of air-dried subsamples from each land-use by the wet sieving and decanting method followed by flotation-centrifugation in 50%sucrose(Brundrett et al.1996)g=(1.118×10-5)R*S2(1)where g is the relative centrifugal force,R is the radius of the rotor in centimeters,and S is the speed of the centrifuge in revolutions per minute.The soil samples were suspended in water for 30 s and decanted over a series of sieves with 750,250,100 and 38 μm mesh sizes.Soil material was recovered from each sieve,suspended in water,and centrifuged at 2000 revolutions per minute(RPM)which deliver a centrifugal force of 538×g for 5 min(Brundrett et al.1996).After decanting the supernatant,each soil-spore mixture of the pellets was re-suspended in sucrose solution(50%)and centrifuged for 1 min at 2000 RPM.The supernatant containing spores was filtered under vacuum on filter paper and then transferred into round petri dishes that had gridlines marked at the bottom to form 1 cm squares then spores were counted using a stereoscope microscope.For observation and identification of spore characters,spores were mounted on glass slides in polyvinyl alcohol-lactoglycerol (PVLG) and PVLG+Melzer's reagent and identified to genus level using current taxonomic criteria(Brundrett et al.1996)and information by INVAM(http://www.invam.caf.wvu.edu).
Assessment of AM fungi colonization
Subsampled roots were chopped into 1 cm long segments and then treated with 10% KOH and autoclaved (for impregnation of staining chemicals)at 121°C for 15 min.Dark pigmented roots were bleached with 10%H2O2for 10 min and acidified with 3%HCl(v/v)for 30 min at room temperature.Cleared roots were transferred into a staining solution of Trypan blue (0.05% w/v) in lactoglycerol(1:1:1,lactic acid:glycerol:distilled water),and autoclaved at 121°C for 15 min(Brundrett et al.1996).Stained roots were left in a de-staining solution (50% glycerol) to remove coloration from empty root cells.Finally,six randomly selected stained roots from each replicate were prepared and examined at 100×-400× magnification under a microscope for the presence of AM fungal structures(arbuscule,vesicles and internal hyphae in the root cortex).Mycorrhizal root colonization was estimated by the gridline intersect method (Giovannetti and Mosse 1980).
AM fungal inoculum preparation and soil potting
Inocula were collected from the rhizospheres of F.albida trees from different land-uses and inoculum was subsequently produced.The inocula were propagated on Sorghum bicolor plants grown on sterilized sand and field soil autoclaved at 121°C for 2 h and filled into plastic pots of 10 kg capacity. Approximately 50 g of fungal inocula consisting of a mixture of soil,spore and root fragments produced from the rhizosphere of soil and roots of precolonized Sorghum bicolor plants was added near the roots of each experimental seedling.The controls were prepared in the same manner but without AM fungal propagules.
Seed treatment,planting and growing conditions
Seeds of F.albida trees were collected from the sampled adult trees growing on the study site.Seeds were surface sterilized and allowed to germinate on petri dishes.Four germinating seeds of uniform appearance were transplanted into each pot.Potted seedlings were maintained in a greenhouse and watered to field capacity.Twenty days after sowing,seedlings were thinned,leaving the most vigorous plant per pot.
Experimental design and treatments
The experiment had a one-factor completely randomized design(CRD)in five replicates.The factor,inoculum type,had three levels,from three land use types plus an uninoculated control(AM-).
Harvest and measurements
The plants were harvested 12 weeks after planting(WAP).For each harvested pot,the plant shoot was cut at the base,and roots were separated from the soil by washing over a 1-2 mm sieve into a container for retention. Growth parameters:plant nutrient content,plant height,shoot and root dry matter,total root length,leaf number and AM colonization percentage were determined.Dry matter was determined after drying at 65°C for 24 h.A sub-sample of 1.5 g fresh roots were removed,fixed and chopped(1 cm long),cleared and stained,and AM root colonization calculated in a similar procedure to that described before.
The nutrient content of the F.albida seedlings was determined by conducting shoot elemental analysis.Shoot samples were oven-dried at 65°C for 24 h.Samples were then wet-digested and analyzed for N,P,and K.Total N was determined using the standard Kjeldahl method,P calorimetrically by spectrophotometer,and K by flame photometry(Anderson and Ingram 1993).
Statistical analysis
Differences in the number of spores,AM fungi colonization and growth parameters were subjected to analysis of variance using SAS statistical software(SAS version 9,2002).The relationship between spore abundance and AM root colonization of field standing trees and greenhouse grown seedlings was evaluated by employing Pearson's correlation coefficient. When the analysis of variance(ANOVA)showed significant differences(at P <0.05),a mean separation was made using Duncan's multiple range test.
Results
Soil properties and vegetation characteristics
In most cases,soil samples collected from the three landuse types(area exclosure,grazing land and cultivated land)showed significant differences in total N, available P,exchangeable(exch.)Ca,exch.Mg,exch.K,OC,Na concentrations, CEC, and texture (Table 1). Soil from cultivated land had highest total N, available P, and exch.K, followed by grazing land and area exclosure.Cultivated lands had been fertilized with both chemical fertilizers and organic manure for the previous 5 years,but area exclosures and grazing lands had not been fertilized.The understory vegetation of both cultivated and grazing lands was dominated by agricultural weeds and grasses.However,the area exclosures as well as grasses,supported tree seedlings,shrubs and trees(Acacia abyssinica,A.etbaica,Becium grandiflorum,F.albida,Maytenus senegalensis,Rhus glutinosa,Rumex nervosus,and Salix subserrata).Moreover,measured F.albida trees grown on area exclosure land-use type had significantly lesser diameter at breast height (DBH), height, and crown diameter than their conspecifics grown on grazing and cultivated land-use types.Trees grown on cultivated and grazing lands were older(11-13 years)than those grown on area exclosure(5-6 years).
AM spore abundance
Spore abundance(number of spores per 100 g of dry soil)of the rhizosphere soils of F.albida trees was significantly(P <0.0014)affected by land-use type with the highest number of spores in soil from area exclosure and the lowest in soil from grazing land(Fig.1,Table 2).Spores were identified to genus level,and four genera were recorded in all land-use types.Glomus was the most abundant and most frequently recorded genus in all land-use types and wasfollowed in decreasing order of abundance by Gigaspora,Scutellospora and Acaulospora.

Table 1 Soil chemical and physical properties at 0-25 cm depth and size of Faidherbia albida trees in area exclosure,grazing land and cultivated land(Mean±SE of five replicates)
AM root colonization
The percentage of root length colonized by different AM structures varied between land-use types(Fig.1b).Significant (P=0.0086) differences in AM colonization structure(arbuscule,vesicle and hyphae)were observed among the root samples of F.albida trees grown on different land-uses(Table 2).The majority of trees showed AM structures in their roots(arbuscule,vesicle or hyphal coils).Percentage colonization was highest on roots of F.albida trees grown on area exclosure, followed in decreasing order by grazing and cultivated land.
All inoculated seedlings were colonized by AM fungal structures(arbuscule,vesicle,and hyphae).None of the plants from the control group were colonized by AM fungi.Colonization by AM fungi was highest after inoculation from area exclosure followed by grazing land and cultivated land(Table 3 and Fig.2).Growth rates of colonized plants were higher(Fig.3).Inoculated seedlings were taller,had more leaves,longer root and higher shoot and root biomass than non-inoculated seedlings (Table 4).Likewise,differences in growth parameters were observed among the seedlings inoculated with AM fungal inoculum sources with the highest growth from area exclosure followed by grazing land and cultivated land(Fig.3).The number of AM colonized roots of F.albida seedlings was not correlated(r=0.317,p=0.376)with the number of spores counted from the soil samples.
Seedling nutrient uptake
P,N,and K nutrient contents in the shoots of F.albida seedlings were significantly higher for AM inoculated seedlings from area exclosure than those from grazing land and cultivated land inoculum soils(Table 5,Fig.4).
Discussion
AM root colonization and spore abundance in native trees

Fig.1 Effects of land-use type(area exclosure,grazing land and cultivated land)on AM fungi associations.a Spore abundance,b AM root colonization structures with F.albida trees

Table 2 The Spore abundance and AM root colonization structures of F.albida trees grown on area exclosure,grazing land and cultivated land land-use types(Summary of ANOVA table result from five replications)
Under semi-arid conditions,it has already been shown that AM inoculation improves plant establishment on disturbed soils(Li and Zhao 2005;Petersen and Oberwinkler 2006;Smith and Read 2008;Martinez and Johnson 2010;Barea et al.2011;Carvalho et al.2015).The role of AM fungi in agroforestry systems and rehabilitation of degraded areas is well documented(Haselwandter and Bowen 1996;Pande and Tarafdar 2004;Hailemariam et al.2013).In the current work,the first step was to study the existing vegetation dominating the area and determine the mycorrhizal infectivity for the plant roots.We observed that AM colonization of F.albida trees greatly varied among land-use types.Trees in area exclosure exhibited the highest level of AM colonization followed by grazing land and cultivated land.This could be due to the low level of disturbance(Cardosoand Kuyper 2006;Gillespie and Allen 2006;Ndoye et al.2012),high under-story vegetation(Birhane et al.2010),lack of nutrient addition(Hamel and Moutoglis 2005;Smith and Read 2008;Taffouo and Ngwene 2014),and younger age of the trees(Diop et al.1994).Use of agricultural inputs reduced the infectivity of indigenous mycorrhizal populations(Eason et al.1999;Friberg 2001;Martinez and Johnson 2010;Onguene et al.2011;Gosling et al.2014;Ortas 2015).Thinner lateral roots on young F.albida trees in the upper soil profiles of area exclosure could explain the more frequent occurrence of indigenous AM fungi in these roots compared to those of older trees.Diop et al.(1994)reported that young F.albida trees had 16%more mycorrhizal roots than older trees.However,observed differences between land-uses could also be due to factors such as soil nutrient content,soil moisture,changes in microhabitat,and acclimatization of a particular AM genus/species to a particular location(Ndoye et al.2012).For example,Kumar et al.(2008)showed that AM colonization of the roots of the subshrub Sida cordifolia varied significantly between sites. In our study, trees growing on cultivated land had low AM colonization,which suggests that indigenous fungi have a low colonization potential and a slow rate of development,possibly due to high fertilization and soil disturbance.

Table 3 The effect of AM inoculation(AM+and AM-)and inoculum sources from area exclosure,grazing land and cultivated land land-uses on the AM root colonization with different fungal structures of F.albida seedlings(summary of ANOVA table result from five replications)

Fig.2 Effect of AM inoculation(AM+,AM-)and inoculum sources(area exclosure,grazing land and cultivated land)on AM root colonization with different fungal structures of F.albida seedlings under greenhouse conditions.The control is not depicted in the figure,as there was no colonization

Fig.3 Effect of AM inoculation(AM +,AM-)and inoculum sources(area exclosure,grazing land and cultivated land)on plant size and growth of F.albida seedlings under greenhouse conditions.Different letters indicate significant differences(P <0.05)among AM inoculation and inoculum sources

Table 4 The effect of AM inoculation(AM+and AM-)and inoculum sources from area exclosure,grazing land and cultivated land land-uses on the size and growth of F.albida seedlings(summary of ANOVA table result from five replications)
Our study also showed that AM fungi are not evenly distributed in soils but rather depend on current land use.Spore density can vary depending on edaphic-climatic conditions(Chalk et al.2006),soil texture(Pande and Tarafdar 2004),and management practices(Gosling et al.2014).The greater number of spores found in area exclosure soil could be due to lower levels of soil disturbance,higher vegetation cover and thus more active biological conditions(Birhane et al.2010)compared with cultivated lands. Management practices such as tillage disruptmycelia,reducing AM fungi colonization(Miransari et al.2009;Brito et al.2012;Brito and Goss 2012).Fertilizer inputs reduce spore density(Smith and Read 2008;Wright et al.2009).Diop et al.(1994)demonstrated that high levels of available nutrients,especially P,have a negative effect on the propagation of AM fungi.
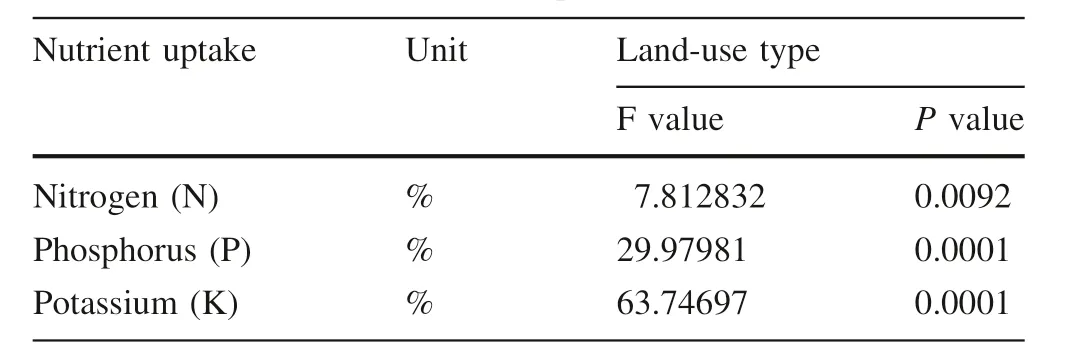
Table 5 The effect of AM inoculation(AM+and AM-)and inoculum sources from area exclosure,grazing land and cultivated land land-uses on the nutrient uptake of F.albida seedlings(summary of ANOVA table result from five replications)
AM spore density and root colonization were strongly influenced by land-use type.Vegetation cover increases soil microbial biomass and activity by facilitating a vast network of AM hyphae and spores that interconnect plant roots(Graves et al.1997).Higher herbaceous cover in exclosures indicates the site potential to support relatively higher biological activity for the recovery of mycorrhizal inoculum potential(Birhane et al.2010).Under low disturbance and continuous root growth,mycelia are the most dominant infective propagules.Hence,the lower number of spores and high root colonization of trees grown on grazinglands compared to cultivated lands indicated that there were high percentages of infective vegetative propagules(Jefwa et al. 2009). This may explain the low spore abundance and high colonization in soils under natural forest (Graves et al. 1997). To ensure efficient plant growth,especially under semiarid regions often characterized by low availability of P in soil,the enhancement of native soil mycorrhiza through the culture of highly mycotrophic plants or through controlled mycorrhization is a strategy to increase productivity(Ndoye et al.2012).

Fig.4 Effect of AM inoculation(AM+, AM-)and inoculum sources(area exclosure,grazing land and cultivated land)on nutrient uptake of F.albida seedlings under greenhouse conditions.Different letters indicate significant differences(P <0.05)among AM inoculation and inoculum sources
Growth
We documented a positive effect of AM fungi on the growth and biomass increment of F.albida seedlings.Increased growth and development in AM plants compared to nonmycorrhizal ones,has been reported for other plant species(Onguene and Kuyper 2005;Onguene et al.2011;Birhane et al.2012;Doley and Jite 2012;Hailemariam et al.2013).The higher biomass increment could be a result of increased root surface area leading to enhanced nutrient uptake that enables increased growth rates(Jayachandran and Shetty 2003;Onguene and Kuyper 2005;Perner et al.2007;Birhane et al.2012).Inflows of phosphorus to mycorrhizal roots can exceed inflows to comparable non-mycorrhiza roots up to 2-5 fold(Sanders and Sheirh 1983).However,AM colonization of F.albida seedlings was not significantly correlated with the number of spores counted.Although spore numbers from grazing land were fewer,AM colonization and plant biomass were greater than from cultivated land soil samples which had more spores.This implies that plants grown in soils with the highest inoculum potential do not always have the most rapid growth(Onguene and Kuyper 2005).This could be due to fungal species-specific differences in sporulation and effectiveness or to plant costs rising more sharply than plant benefits with increasing colonization(Onguene and Kuyper 2005).In contrast,positive correlations between sporulation or colonization percentage and growth(Hetrick and Bloom 1986;Giovannetti et al.1988;Hailemariam et al.2013)or dry matter production(Clark and Zeto 1996)of mycorrhizal plants have been reported.On our study site,seedlings with inocula from area exclosures had higher growth increases than those with inocula from cultivated land.These differences could be attributed to differences in the number of infective AM fungal spores and hyphae in the colonized roots of the trees grown on different land-use types.These results support the contention that sporulation is positively correlated with the growth of mycorrhizal plants(Hetrick and Bloom 1986;Giovannetti et al.1988).Generally,native mycorrhizal potentials of rhizosphere soils of F.albida trees grown on area exclosures and grazing lands would have a tremendous impact on plant growth improvements and environmental protection.
Nutrient uptake
Our results confirmed that AM fungi contributed significantly to the acquisition of nutrients(N,P and K)by seedlings and therefore their increased biomass compared with non-inoculated seedlings. This confirms previous research on acquisition of P and other nutrients after inoculation of plants with AM(Jayachandran and Shetty 2003;Mardukhi et al.2011;Castagno et al.2014;Heidari 2014).P,an essential macronutrient,is absorbed by plants in the form of an inorganic phosphate anion(Pi)and organically bound phosphate is hydrolyzed into Pi by entering the plant root(Xie et al.2014).Enhanced acquisition of P by the plant occurs due to the AM fungal hyphae secreting extracellular phosphate enzymes(Javot et al.2007).Koide and Kabir(2000)confirmed that AM fungi could produce extracellular as well as intracellular phosphatase,and extracellular phosphatase could improve the ability of the host plant to obtain P directly from organic P sources. This study confirmed that AM inoculation increased P uptake and higher accumulation occurred when plants were inoculated with AM from area exclosures.The non-inoculated seedlings had the lowest levels of nutrient acquisition.This might have been because native AM in area exclosures had more infective potential than the other inoculum sources and increase the stomatal conductance of plants,improving nutrient uptake.Increases in stomatal conductance due to inoculation has previously been correlated with increases in nutrient uptake in native plant species(Birhane et al.2012).
Conclusions
Disturbance reduced colonization even when spore density was high.Higher spore abundance resulted in a higher AM colonization of trees in area exclosure.Soil samples collected from cultivated land had higher numbers of spores yet lower AM colonization than those from grazing lands,implying a lower level of infective AM populations in cultivated lands.Faidherbida albida seedlings had significantly increased growth and nutrient acquisition when inoculated with AM compared to non-inoculated controls.Faidherbida albida seedlings inoculated with AM fungal inoculum collected from area exclosures had the highest growth and nutrient uptake followed by grazing land and cultivated land inocula soils.This emphasizes the importance of the source for mycorrhizal inoculum and the need to screen for better combinations of F.albida seedlings with AM fungal species to achieve optimum plant growth improvement and environmental protection. Exclosure areas are good sources of AM fungi inoculum when growing seedlings for reforestation efforts.
AcknowledgementsThe write-up of the paper is supported by the Steps towards sustainable forest management with the local communities in Tigray,northern Ethiopia at Mekelle University funded by NORAD/NORHED(ETH 13/0018)program.We are grateful to the anonymous referees for constructive comments on an earlier version of this manuscript.
杂志排行
Journal of Forestry Research的其它文章
- Protective and defensive roles of non-glandular trichomes against multiple stresses: structure-function coordination
- Assessment of early survival and growth of planted Scots pine(Pinus sylvestris)seedlings under extreme continental climate conditions of northern Mongolia
- Influencing in vitro clonal propagation of Chonemorpha fragrans(moon)Alston by culture media strength,plant growth regulators,carbon source and photo periodic incubation
- Variation analysis of growth traits of four poplar clones under different water and fertilizer management
- Nodule study in Albizia chinensis in relation to nitrogen metabolism,morphology and biomass
- Comparative transcriptome analyses reveal candidate genes regulating wood quality in Japanese larch(Larix kaempferi)
