Protective and defensive roles of non-glandular trichomes against multiple stresses: structure-function coordination
2020-01-18GeorgeKarabourniotisGeorgiosLiakopoulosDimosthenisNikolopoulosPanagiotaBresta
Geor ge Karabourniotis · Georgios Liakopoulos ·Dimosthenis Nikolopoulos · Panagiota Bresta
Abstract As superficial structures, non-glandular trichomes, protect plant organs against multiple biotic and abiotic stresses. The protective and defensive roles of these epidermal appendages are crucial to developing organs and can be attributed to the excellent combination of suitable structural traits and chemical reinforcement in the form of phenolic compounds, primarily flavonoids. Both the formation of trichomes and the accumulation of phenolics are interrelated at the molecular level. During the early stages of development, non-glandular trichomes show strong morphological similarities to glandular ones such as the balloon-like apical cells with numerous phenolics. At later developmental stages, and during secondary wall thickening, phenolics are transferred to the cell walls of the trichomes. Due to the diffuse deposition of phenolics in the cell walls, trichomes provide protection against UV-B radiation by behaving as optical filters, screening out wavelengths that could damage sensitive tissues. Protection from strong visible radiation is also afforded by increased surface light ref lectance. Moreover, the mixtures of trichome phenolics represent a superf icial chemical barrier that provides protection against biotic stress factors such as herbivores and pathogens. Although the cells of some trichomes die at maturity, they can modulate their quantitative and qualitative characteristics during development, depending on the prevailing conditions of the external biotic or abiotic environment. In fact, the structure and chemical constituents of trichomes may change due to the particular light regime, herbivore damage, wounding, water stress, salinity and the presence of heavy metals.Hence, trichomes represent dynamic protective structures that may greatly affect the outcome of many plant-environment interactions.
Keywords Non-glandular trichomes · Phenolics ·Flavonoids · Protection · Defence · Biotic stress · Abiotic stress
Introduction
Superf icial tissues (epidermis) and structures (cuticle and epidermal appendages) of plant organs play a crucial protecting role against multiple biotic and abiotic stress factors. As they comprise the outermost boundary between the plant and the environment, they mediate in a plethora of plant-environment interactions. Apart from their protective role against abiotic stress factors such as water losses,high UV and visible radiation intensities and temperature extremes, superf icial tissues represent a barrier that has to be breached before any successful pathogen or herbivore attack can be established, and hence constitute the first line of plant defence. The protective (against abiotic stresses)and defensive (against biotic stresses) roles of these tissues and structures can be attributed to an excellent combination of suitable structural traits and chemical reinforcement in the form of secondary metabolites (LoPresti 2015). Among these compounds, phenolics play pivotal roles in chemical protection and defence located in the cuticle, the epidermis and (if present), in trichomes, either glandular or non-glandular. Given that the location of a given defensive/protective compound determines its ecological function (LoPresti 2015), and the selection of the optimal superf icial protectivedefensive mechanism is probably related to the successful survival in a particular environment (Agrawal et al. 2009),differences in leaf superf icial structure and chemical composition are expected both at the inter- and intraspecif ic level,as well as along the various developmental stages. The variation in superf icial structures of young leaves of different cultivars of grapevine (Vitis viniferaL.) is a good example of this selection at the intraspecif ic level. The surfaces of young leaves of different cultivars of grapevine may be glabrous-green (‘Soultanina’, syn. ‘Thompson Seedless’), or transiently have anthocyanins (e.g., ‘Siriki’) or pubescence(e.g., ‘Athiri’). Leaves possessing anthocyanins or trichomes are better protected against photoinhibition compared to the glabrous leaves. Photoinhibition is def ined as the inhibition of photosynthesis due to damages in the photosynthetic machinery caused by the absorption of more photon energy than what can be used in the biochemical reactions and the risk of this phenomenon is higher in sunny environments(Demmig-Adams and Adams 2018). Both anthocyanins and trichome layers behave as optical filters, decreasing the intensity of the incident radiation received by the photosynthetic cells and thus decreasing the photoinhibition risk and consequently the xanthophyll cycle utilization rates (Morales et al 2002; Liakopoulos et al. 2006a; Galmés et al. 2007).In particular, the occurrence of non-glandular trichomes on leaf surfaces provides adaptive advantages under stressful environments by combining physical, mechanical and biochemical protection, especially in developing organs. To this respect, the current review focuses on the protective and defensive roles of non-glandular trichomes against multiple stresses, highlighting the coordination between trichome structural traits and phenolic deposition as a key component of the plant protective-defensive mechanism.
Non-glandular trichomes are protective epidermal appendages
Trichomes (or hairs) are def ined as “unicellular or multicellular appendages, which originate from epidermal cells only, and develop outwards on the surface of various plant organs” (Werker 2000). The term “indumentum” describes the trichome layer of an organ as a whole. Investigation either of the individual structures or of the collective properties of the trichome layers (indumenta) began early in the XVII century (Johnson 1975). The ecophysiological roles of trichomes have been reviewed by Uphof ( 1962),Johnson ( 1975), Fahn ( 1986) and recently by Bickford( 2016). The functions of trichomes and their bioinspired applications have also been recently reviewed by Liu et al.( 2017a).
Trichomes can develop on the surface of all plant organs and are characterized as "glandular" or "non-glandular".The cells comprising the glandular trichomes can synthesize and secrete large quantities of compounds of the secondary metabolism, usually mixtures of terpenoids (as the main constituents) and phenolics (Werker 2000; Wagner et al. 2004; Huchelmann et al 2017; Lange and Srividya 2019). Non-glandular trichomes (the subject of the present review), do not possess a secretory mechanism and there are no analytical studies referring to the occurrence of high concentrations of terpenoids in these structures. However, non-glandular trichomes accumulate large quantities of phenolics, mainly at the early stages of their ontogeny(see next section), without apparent secretion ability. During these stages, however, young trichomes of olive (Olea europaeaL.) and holm oak (Quercus ilexL.) leaves show high anatomical similarities to the glandular trichomes,such as the balloon-like apical cells characterised by thin cell walls (Galati 1982; Karabourniotis et al. 1998; Fig. 1).
Non-glandular trichomes are distinguished by their morphological characteristics and display a tremendous variability in their properties such as morphology, size and density often related to their functional purposes.For example, the presence of dense trichome layers is often considered a xeromorphic character (Fahn 1986;Karabourniotis et al. 1995; Körner 2003; Valkama et al.2004; Moreno et al. 2010; Mershon et al. 2015; Amada et al. 2017). According to recent unpublished data of our research group deriving from a study on a typical Mediterranean ecosystem (Acarnanic Mountains in West Greece),we recorded that 57.7% of species of dwarf shrubs and perennial herbs in the study area had leaves with trichomes,while 26.2% were species with dense trichome layers.These life forms must tolerate the stressful conditions of the Mediterranean summer, i.e., the combination of high temperatures and light intensities and drought. However,it seems that this character is not limited to species of xerothermic sites and specif ic functional groups, given that a large amount of available data (Table 1) show that a signif icant part of the tree species of tropical rain forests(7.3-30%) have also densely pubescent leaves. The occurrence of dense trichome layers even in tropical species has also been related to protection against drought and high light intensity conditions (Ichie et al. 2016). However,data from different sites, life forms and functional groups are missing, highlighting the need for further studies that could offer valuable information on the function and evolutionary history of trichomes.
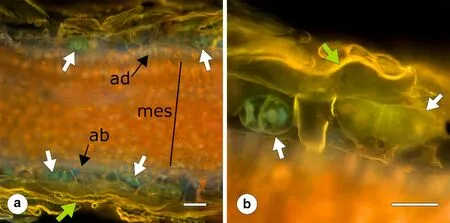
Fig. 1 a Cross section of a young expanding leaf (second node) of Olea europaea under the fluorescence microscope. Excitation light is UV (exciter filter 330-385 nm/barrier filter 420 nm) and autof luorescence emitted is red-orange (chlorophyll of mesophyll tissues) or orange-yellow and yellow-green (phenolics, mainly flavonoids, from the cell walls of developed hairs (green arrows) and from the protoplast of young undeveloped hairs (white arrows). Black arrows show adaxial epidermis (ad) or abaxial epidermis (ab). Black bar shows mesophyll area. b Detail of the trichome layers of the adaxial leaf surface showing a developed hair and two undeveloped hairs on each side of the former. The one on the left is younger than the one on the right. In both undeveloped hairs, fluorescence is emitted by the protoplasm (yellow-green) and by the nuclei (yellow or green), the latter being due to perinuclear flavonoids. Scale bars: 200 μm. Microphotographs were taken with an Olympus BX40 fluorescent microscope equipped with an Olympus DP71 digital camera (Olympus Corporation, Japan)

Table 1 Plant species with leaves bearing leaf trichomes (% of the species examined) thriving in different ecosystems of the world
Non-glandular trichomes contain phenolics associated with cell walls
The term “phenolic” is used to def ine carbon-based molecules that possess one (simple phenols) or more (polyphenols) phenolic groups (phenyl-groups), i.e. at least one hydroxyl-group bonded onto a benzyl ring (Quideau et al 2011). Phenolics are multifunctional secondary metabolites playing a wide array of defensive, protective and regulatory roles against either biotic or abiotic stress factors (Karabourniotis et al. 2014; Table 2). The phenolic content of non-glandular trichomes was analysed in a number of tree and shrub species with olive representing one of the most studied. Leaf trichomes of this species contain extractable phenolics, particularly flavonoids, non-covalently bound to the cell walls. The deposition of these compounds in the cell walls takes place during the short period of final trichome development, which corresponds to secondary wall thickening (Karabourniotis et al. 1998). Their composition differs from that of the internal pool, i.e., mesophyll and epidermal cell phenolics (Liakopoulos et al. 2006b). Although, quercetin 3-O-rutinoside and apigenin 7-O-glucoside located inleaf trichomes were also present in the lamina, quercetin,quercetin 3-O-rhamnoside and an unidentif ied flavone were located exclusively in the trichome layers (Liakopoulos et al.2006b). The phenolics of mature trichomes are usually only a minor fraction of the total leaf pool (Liakopoulos et al.2006b). Recently, molecular studies conf irmed that mature olive trichomes are transcriptionally active, coding mainly for enzymes catalysing reactions involved in the biosynthesis of phenolics playing important protective and defensive roles (Koudounas et al. 2015). Roka et al. ( 2018) identif ied 249 proteins from olive mature trichomes which were classif ied to diverse groups such as “phosphorylation”, “response to stress” and “carbohydrate metabolic process” indicating that the cells of these structures are physiologically and biochemically active.

Table 2 Summary of the roles and actions of phenolics in plants
Flavonoids and other related compounds were also detected in non-glandular trichomes of leaves and fruits of other tree species. The leaf trichome layers of holm oak contain flavonoids and the main compounds are acylated kaempferol glycosides (Skaltsa et al 1994). Also, the trichomes covering the surface of peach cv. ‛Calrico’ are filled by polysaccharide material (63%) containing hydroxycinnamic acid derivatives (p-coumaric acid and ferulic acid)and flavonoids (Fernández et al. 2011). In some cases, the leaf surfaces are covered by anthocyanic-pigmented trichomes. The trichomes of young, developing leaves of the plane tree (Platanus orientalisL.) contain peonidin (Ntef idou and Manetas 1996) and the cauline trichomes ofPlectranthus ciliatuscontain peonidin 3,5-diglucosides with aromatic acylation with p-coumaric and sometimes caffeic acids (Jordheim et al. 2016). Anthocyanic trichomes are also present on the leaf surface ofCastanopsis fissa(Zhang et al.2016). The occurrence of phenolic compounds in trichome layers has also been reported for some characteristic shrubs of the Mediterranean ecosystem. InCistus salvifoliusL.leaves, the stalk cells and channel of the arm of non-glandular trichomes contain ellagitannins (punicalagin and two galloyl derivatives of punicalagin), whereas the trichome arms contain acylated kaempferol 3-O-glycosides associated with the cell wall (Tattini et al. 2007). El-Negoumy et al. ( 1986) reported that the leaf trichomes ofPhlomisaureaandPhlomis floccosacontain 7-glucosides of naringenin, apigenin, luteolin and chrysoeriol and their acylated derivatives.
The occurrence of phenolic compounds in the trichome layers has been conf irmed not only by analytical procedures but also by microscopic observations. Flavonoids (as well as other related phenolic compounds), show a bright yellow-green fluorescence following irradiation by blue light(Goodwin 1952; Rost 1995). Thus a number of histological studies conf irmed the occurrence of these compounds in trichomes by epif luorescence microscopy (Karabourniotis and Fasseas 1996; Karabourniotis et al. 1998; Tattini et al.2007; Stavrianakou et al. 2010; Karioti et al. 2011; Fernández et al. 2014; Koudounas et al. 2015; Fig. 1), and confocal laser scanning microscopy (Hützler et al. 1998; Fernadez et al. 1999). The occurrence of phenolic compounds in the trichome layers has also been conf irmed histochemically(Tozin et al. 2016). Phenolics are not only detected in the soluble fraction of trichomes, but are also located in the cuticle (wax-bound) and in the cell wall insoluble fraction(esterif ied) of these structures (Liakopoulos et al 2006b;Fernández et al 2011; Mateu et al. 2016; Karabourniotis and Liakopoulos 2005). Moreover, flavonoids were also detected in the perinuclear region of the cells of young developing trichomes, indicating the need of special protection of this UV-sensitive organelle (Karabourniotis et al. 1998; Agati et al. 2012; Fig. 1).
The formation of trichomes and the accumulation of phenolics are interrelated at the molecular level
At the molecular level, the AtTTG1 (Arabidopsis thaliana(A. thaliana) TRANSPARENT TESTA GLABRA 1), the head of an evolutionarily conserved gene regulatory network, regulates both trichome formation and flavonoid (and anthocyanin) production throughout development (Xiao et al. 2017; Zhang and Schrader 2017). However, TTG1 was not identif ied in the trichomes of olive leaves (Koudounas et al. 2015). InBrassica rapa, the lipid transfer protein 2(BraLTP2), which is expressed in leaf epidermal cells and trichomes, may play a role in both trichome development and accumulation of secondary metabolites, especially flavonoids (Tian et al. 2018). It seems, therefore, that both the formation of trichomes and the accumulation of phenolics are interrelated at the molecular level.
Coordinated structure (trichomes)-function(phenolics deposition) relationships and protection of plants against stresses
The multifunctionality of phenolic compounds indicates that resource allocation to these compounds in superf icial tissues and especially in trichomes may render both defence against herbivores and pathogens, and protection against abiotic stresses. For example, flavonoids show a broad spectrum of functions for plants, including UV and high visible radiation protection, radical scavenging, pollination and feeding attraction, rhizosphere signalling and pathogen and herbivore defence (Iwashina 2003; Carletti et al. 2014; Mierziak et al. 2014; Agati and Tattini 2010; Siipola et al. 2015).
Protection from radiation
An important role of the trichome layer covering the surface of plant organs is the absorption of harmful UV-B radiation(Karabourniotis et al. 1992; Skaltsa et al. 1994; Ntef idou and Manetas 1996; Agati et al. 2012). The sensitivity to UV-B radiation is negatively correlated with the density of the trichomes, suggesting a UV-protective role for these structures (Liakoura et al. 1997; Yan et al. 2012). Indeed, trichomes act as shields against harmful wavelengths, offering protection to tissues against UV-B radiation (Karabourniotis et al. 1993, 1995; Grammatikopoulos et al. 1994; Skaltsa et al. 1994; Ripley et al. 1999; Manetas 2003; Tattini et al.2007). This property of the trichome is attributed to the already mentioned diffuse deposition of phenolics in the cell walls (Strack et al. 1988; Karabourniotis et al. 1992,1998; Skaltsa et al. 1994; Karabourniotis and Fasseas 1996;Ntef idou and Manetas 1996; Agati et al. 2012). Studies on olive and holm oak conf irmed the optical role of trichomes by monitoring the light microenvironment beneath leaf trichome layers with fibre-optic microprobes. The trichome layers of the leaves in these two species attenuated almost all incident UV-B (310 nm) and UV-A (360 nm) radiation and a considerable portion of blue light (430 nm) (Karabourniotis and Bornman 1999; Karabourniotis et al. 1999). These light filtering and ref lecting properties of the trichome layer may also render protection against visible radiation damage,especially in young leaves (Lang and Schindler 1994; Bisba et al. 1997; Karabourniotis and Bornman 1999; Karabourniotis et al. 1999; Zhang et al. 2016).
Plant-microorganism and plant-herbivore interactions
It is well known that trichomes represent permanent structures that offer physical protection against biotic stress factors. Concerning enemies, trichomes (including the glandular ones), inf luence insect oviposition and/or feeding in a wide range of insects and other herbivores (Levin 1973;Vermeij 2015). The occurrence of trichomes contributes to increased plant resistance against herbivores across different species of plants and affects tritrophic interactions (Riddick and Simmons 2014; Krimmel 2014). Moreover, trichomes are often composed of cellulose and other substances (such as phenolics) that are of low nutritional value for the insects(Levin 1973).
Concerning pathogens, trichomes act as a passive screen that prevent spores and other microbial structures from reaching the leaf surface or forming a water repellent surface preventing the formation of water films on which pathogens might be deposited and germinate or multiply(Johnson 1975; Allen et al. 1991; Mmbaga and Staedman 1992; Mmbaga et al. 1994; Kortekamp and Zyprian 1999;Kortekamp et al. 1999; Bradley et al. 2003; Agrios 2005;Fernández et al. 2014).
However, not only the physical but also the chemical characteristics of the superf icial tissues and structures affect the success or failure of microbial growth on, and subsequently, within the leaf (Allen et al. 1991). Microbial growth on leaf surfaces is usually favoured when rain or dew creates an aqueous film on the lamina (Romantschuk et al. 1996).The inducible plant defence mechanisms do not reach the external surface of an unwounded organ, but the presence of aqueous films on leaf surfaces may cause leaching of substances present in superf icial tissues and structures (Reigosa et al. 1999). These leachates may include substances that restrict or prevent the growth of phytopathogens such as terpenoids and phenolic compounds (Inderjit et al. 1999;Reigosa et al. 1999; Mithöfer and Maffei 2016). Flavonoids and other polyphenol constituents may be toxic to bacteria, fungi and insects and/or have allelopathic properties(Pourcel et al. 2006; Weston and Mathesius 2013). Thus the occurrence of mixtures of phenolics in trichomes may provide a preformed chemical line of defence of plant surfaces against biotic stress factors. The antimicrobial activity of these compounds may be enhanced by the synergistic action of UV-A radiation (Shirai and Yasumoto 2019). Given that these substances are non-covalently bound to the cell walls of the trichomes), they can be leached out by water(Fig. 2) and create a chemically adverse environment against the entrance and spreading of pathogens in the leaf interior.Indeed, aqueous extracts from isolated non-glandular leaf trichomes of olive and holm oak at realistic concentrations(resembling those of the leaf surface), inhibit the growth of the majority of the phytopathogenic bacteria as well as the spore germination and growth of various fungi species(Stavrianakou et al. 2010). This defensive ability seems to be age-dependent because the leached substances are not replaced (the trichomes of olive and holm oak are dead at maturity, see Fahn 1986; Karabourniotis et al. 1998). Therefore, trichomes of older leaves contain lower concentrations of polyphenol compounds per unit dry mass than younger,fully expanded leaves, probably due to leaching during the growth period (Karabourniotis et al. 1998). In a recent study,Rennberger et al. ( 2017) showed that trichome density and length, as well as polyphenol autof luorescence of epidermis and trichomes were negatively correlated with the susceptibility of members of the Cucurbitaceae family toDidymella bryoniae, the causal agent of gummy stem blight.
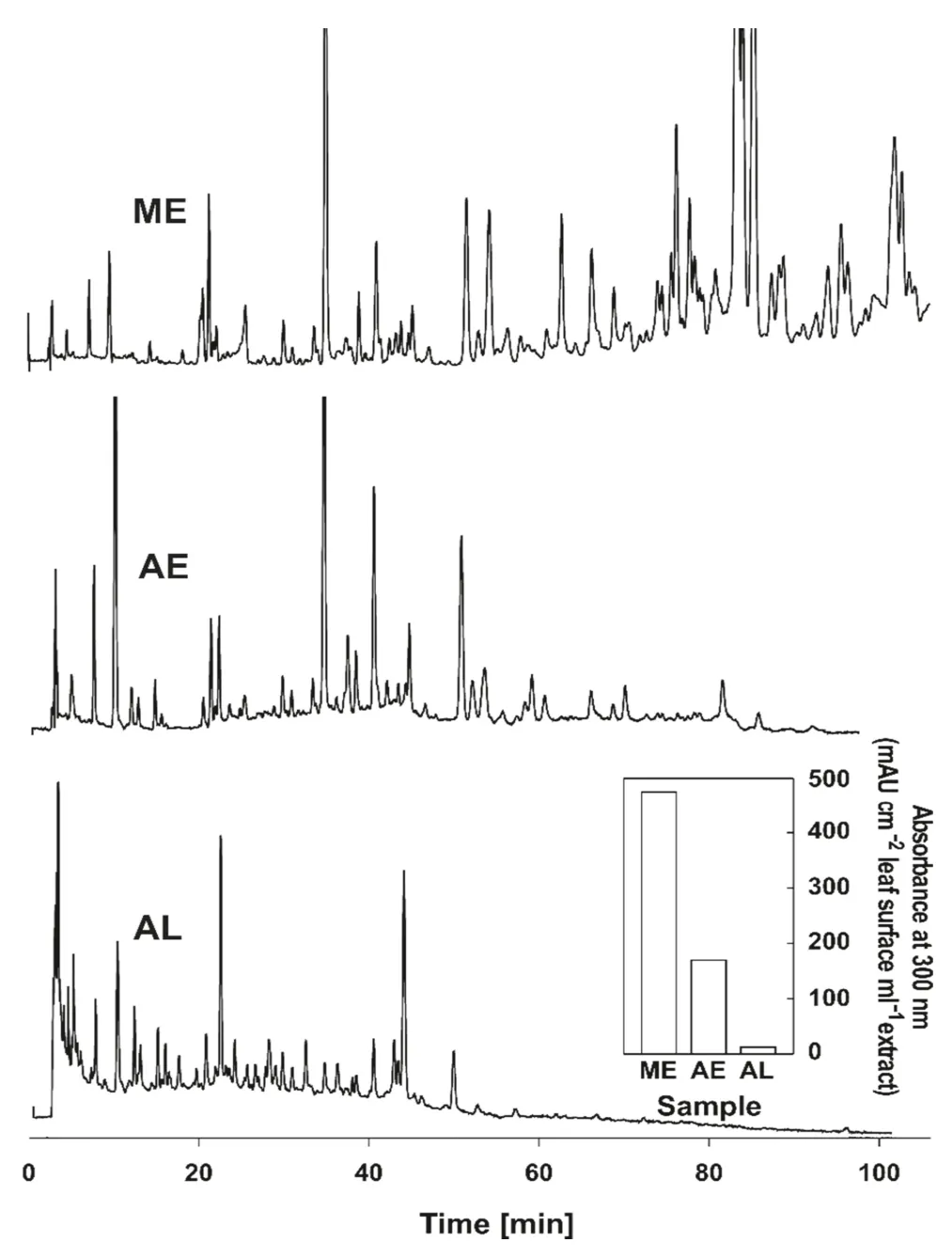
Fig. 2 HPLC prof iles of methanolic (ME) and aqueous (AE) extracts of non-glandular leaf hairs of Olea europaea, as well as of aqueous leachates (AL) of leaves after a leaching time of 4 h. Insert graph shows the relative abundance of phenolic compounds in extracts(ME, AE) and aqueous leachates (AL) by means of absorbance at 300 nm
Other functions not related to the occurrence of phenolic compounds in trichomes
A number of additional protective roles have been ascribed to trichomes, not directly related to the deposition of phenolic compounds in these structures. Dense trichome layers may prevent water losses, either directly by inf luencing the thickness of the boundary layer and hence the respective resistance to water vapour diffusion from the transpiring leaf surface, or indirectly by regulating the energy balance and thus the temperature of the lamina (Nobel 1983; Ehleringer 1984; Fahn and Cutler 1992; Schuepp 1993; Holmes and Keiller 2002; Pshenichnikova et al. 2019). Trichomes also affect the water-leaf surface interactions (leaf wettability,droplet retention or repellence), and contribute to plant water uptake and water balance (Savé et al. 2000; Fernández et al.2014; Konrad et al. 2014; Bickford 2016; Hu et al. 2019).
There is also evidence that leaf trichomes of a number of plants, includingA. thaliana, take part in the detoxif ication of heavy metals (Blamey et al. 1986; Ager et al.2003; Broadhurst et al. 2004; Domínguez-Solís et al. 2004).There are, however, reports that in certain hyperaccumulators, heavy metals are consistently concentrated at the base of leaf trichomes or epidermal cells but are excluded from trichomes (Krämer et al. 1997; Küpper et al. 2000; Psaras et al. 2000; Zhao et al. 2000). These contradictory reports could be attributed to the physiological differences between species, since some trichomes are dead at maturity (as mentioned previously), and thus unable to accumulate metals.The accumulation of toxic molecules within trichomes may decrease the nutritional value of the plant and therefore deter herbivore feeding. This function may be related to the presence of phenolics in trichome cells, as these compounds show metal chelating activity (Michalak 2006).
Leaf epidermal appendances such as glandular or nonglandular trichomes may also interact with atmospheric pollutants such as ozone. High levels of tropospheric ozone negatively affect the growth, the development as well as the productivity of plants, both in a short- and long-term basis. O3is characterized as a toxic, strong polar oxidant which is diffused to the plant interior mainly through stomata. Leaf epidermal appendances increase the active leaf area and favor the maintenance of a thick and moist boundary layer which can act as an ozone sink (Wieser 2002).Moreover, leaf surface reactions can scavenge O3,acting as an additional sink for O3before it enters the leaf. For example, semi-volatile organic compounds (such as the diterpenoidcis-abienol), secreted by the glandular trichomes ofNicotiana tabacumact as an efficient O3sink (Jud et al.2016). Thus glandular trichomes constitute a chemical barrier that reduces leaf ozone uptake and toxicity (Li et al.2018; Oksanen 2018; Prozherina et al. 2003). This function is positively correlated with the density of glandular trichomes but not with non-glandular ones (Li et al 2018).In plants growing with high levels of Ca2+, trichomes play a key role in the regulation of the apoplastic concentration of this element. Ozone has a detrimental effect on the ability of trichomes to regulate the concentration of apoplastic Ca2+,resulting in altered stomatal behavior, possibly due to the disruption of guard-cell Ca2+-mediated signal transduction(De Silva et al. 2001).
Trichomes can also act as traps, accumulating atmospheric particles and dust and thus enhance the filtering capacity of the plant species (Sæbø et al. 2012; Ram et al.2015; Muhammad et al. 2019). The indumentum represents an optimum zone of particle deposition because it increases the active leaf area and creates a rough surface (De Nicola et al. 2008). Pubescent leaves have been shown to exhibit greater entrapping ability than glabrous ones, both for inorganic and organic contaminants (Little and Wiffen 1977;Howsam et al. 2000; Hu et al. 2019). This capability gives rise to inspirations for efficient oil spill cleanup materials(Zeiger et al. 2016).
Recently, it was proposed thatA. thalianatrichomes could act as sensors responding to mechanical and acoustic stimuli. Zhou et al. ( 2016) proposed that trichomes behave as an active mechanosensor, converting physical signals such as mechanical touch from insects into chemical signals like calcium oscillation and pH shift of skirt cells to elicit various defensive reactions. The mechanic stimuli could also be combined with vibrational stimulation ofA. thalianatrichomes associated with feeding caterpillars (Liu et al.2017b).
Trichomes: preformed but not static structures
As mentione d previously, the occurrence of a dense layer of non-glandular trichomes has been considered as a preformed mechanical barrier for non-specif ic plant resistance to pathogens and herbivorous insects, or a preformed layer for protection against intense radiation and water losses.The trichome layers of mature leaves, and possibly of other organs, are considered as a fixed and static protective characteristic because usually the trichome cells are dead at maturity, hence there is no chance for further structural or biochemical alterations. However, trichome layers can change their characteristics during development (for a recent review see Hauser 2014). For example, a mature olive leaf invests up to 10% of its dry mass in the trichome layers,whereas young leaves invest more than 40% (Karabourniotis et al. 1995). Moreover, during these stages, the developing non-glandular trichomes resemble the glandular ones morphologically and possibly functionally, due to the very high concentration of phenolics contained in their cells (Karabourniotis et al. 1995; Karabourniotis and Fasseas 1996;Fig. 1). In the young leaves of olive trees, a high portion (up to 70%) of their phenolic pool is deposited in trichome layers(Karabourniotis et al. 1995). Therefore, the protective role of the trichome against biotic and abiotic factors is particularly signif icant during early stages of leaf development and may be less important at later stages, namely, when protection is taken over by the epidermis (Karabourniotis and Fasseas 1996; Valkama et al. 2004; Calixto et al. 2015). Furthermore, young leaves of many plant species are pubescent on both surfaces, but as they develop, the adaxial trichome is progressively lost and the protective role is undertaken by the epidermis (Karabourniotis et al. 1 995). The occurrence of trichome layers on the abaxial surface of mature leaves is possibly related to the protection of stomata against water losses and intense radiation (Karabourniotis et al. 1993;Grammatikopoulos et al. 1994).
Leaves can also modify trichome quantitative and qualitative characteristics according to the conditions that prevail in the external biotic or abiotic environment. The structure and chemical constituents of trichomes may change upon herbivore damage or artif icial wounding (Larkin et al. 1996;Yoshida et al. 2009). The response of some plants to herbivore damage includes the development of new leaves with an increased density and/or number of trichomes (Traw and Bergelson 2003). This inducible defence response inA. thalianais controlled by jasmonic and salicylic acid (Traw and Bergelson 2003). Insects feeding on these new acclimated leaves often consume less biomass and show limited growth compared to insects feeding on non-acclimated leaves (Baur et al. 1991; Dalin et al. 2008). In some cases, the feeding of eriophyoid mites on leaf surfaces can cause erinea formation, e.g., a hyperplasia of the leaf trichomes (Karioti et al.2011). In holm oak leaves, the hypertrophic trichomes accumulate pigments responsible for the red-brown coloration of the erineum. The cells of these trichomes have thinner walls and contain higher concentrations of proanthocyanidin B3, catechin and quercetin-3-O-glucoside, but lower concentrations of acylated flavonoid glycosides than normal ones (Karioti et al. 2011). Taking into account that the first two compounds are referred to as feeding deterrents, these changes in trichome anatomy and chemistry may restrict the damage caused by the mites (Karioti et al. 2011).
The structure and chemical constituents of trichomes may also change according to the light regime during leaf development. Exposure of developing leaves to high light intensities induces qualitative and quantitative changes in the phenolic content of trichomes, with the possible formation of new flavonoid compounds (Ntef idou and Manetas 1996;Liakoura et al. 1997). The UV-absorbing capacity and the density of the trichomes of leaves exposed to UV-B radiation were higher compared to those of shaded leaves (Liakoura et al. 1997; Václaník et al. 2017). In severalA. thalianatrichome mutants and wild-type plants, the exposure to UV-B radiation caused a signif icant increase in trichome density,suggesting that trichome formation was induced by UV-B(Yan et al. 2012). qRT-PCR analysis indicated that the control of trichome initiation by UV-B radiation is integrated through the expression of a GLABRA3 (GL3) transcription factor (Yan et al. 2012; Kulich et al. 2015). GL3 is a key transcription factor not only of UV-induced, but also of wound-induced trichome formation inA. thaliana. In the last case, GL3 acts downstream of jasmonic acid signalling (Yoshida et al. 2009; Hauser 2014). Continuous UV-B irradiation causes an increase in the number of cells and in the polyphenolic content of the trichomes (Yamasaki et al.2007; Yamasaki and Murakami 2014). Trichome formation was also increased in plants grown under water stress, such as barley (Liu and Liu 2016), aubergine (Fu et al. 2013),olive (Boughalleb and Hajlaoui 2011), eggplant (Fu et al.2013) and tomato (Galdon-Armero et al. 2018). Similar results were also observed under saline conditions (Çelik et al. 2018). In contrast, trichome density was reduced in leaves of Pb-treated plants (Koul and Bhatnagar 2017).
Conclusions
Plant trichomes constitute a superficial, protective and quite dynamic tissue that, during its development, is able to respond to an abundance of cues. During both their early life and after development, they exhibit a plethoric character,distinct optical and mechanical properties and a very rich,leachable and complex mixture of secondary metabolites.Therefore, trichomes prevail on plant surfaces exposed to the surrounding environment and determine the outcome of many abiotic and biotic interactions. Trichomes represent an important plant trait that should always be accounted in the perpetual pursuit for more stress tolerance characters in plants.
Open AccessThis article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creat iveco mmons.org/licen ses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
杂志排行
Journal of Forestry Research的其它文章
- Assessment of early survival and growth of planted Scots pine(Pinus sylvestris)seedlings under extreme continental climate conditions of northern Mongolia
- Influencing in vitro clonal propagation of Chonemorpha fragrans(moon)Alston by culture media strength,plant growth regulators,carbon source and photo periodic incubation
- Variation analysis of growth traits of four poplar clones under different water and fertilizer management
- Nodule study in Albizia chinensis in relation to nitrogen metabolism,morphology and biomass
- Comparative transcriptome analyses reveal candidate genes regulating wood quality in Japanese larch(Larix kaempferi)
- Comparative study on the mRNA expression of Pinus massoniana infected by Bursaphelenchus xylophilus
