Population structures and spatial patterns of two unpalatable Arisaema species(Araceae)with and without clonal reproduction in a riparian forest intensively grazed by Sika deer
2020-01-18TetsuyaMatsumotoMunetoHirobeYasuakiAkajiYukoMiyazaki
Tetsuya K.Matsumoto·Muneto Hirobe·Yasuaki Akaji,2·Yuko Miyazaki
Abstract General decline of understory cover can result from increased abundance of and foraging pressure by deer.But population size and degree of aggregation can increase for unpalatable understory plants that escape foraging pressure.Clonal reproduction can enable unpalatable plant species to increase their population sizes while trending toward spatially aggregated distributions.However,the details of the relationship between clonal reproduction in unpalatable plants and their dynamics under intensive deer herbivory are not clear.We compared the population structures and spatial patterns of two coexisting unpalatable plant species,Arisaema ovale(with clonal reproduction)and A.peninsulae(without clonal reproduction)in a riparian forest intensively grazed by Sika deer,and examined the null hypothesis that the extent of spatial aggregation and local population size would not differ between the clonal and non-clonal Arisaema species.In a 0.36-ha plot,A.ovale had a larger population size(1087 individuals)with a higher abundance ratio of small plants (p <0.01) than A. peninsulae (84 individuals).Analyses of spatial point processes showed that both populations were spatially aggregated (p <0.05). The spatial aggregation of A.peninsulae,however,became weaker than that of A.ovale,when we excluded one dense patch originating from irregular seed dispersion.These results,excluding the aggregated distribution observed in A.peninsulae,suggested a substantial contribution of clonal reproduction to the expansion of the local A.ovale population following intensive grazing by Sika deer.
Keywords Arisaema ovale·Arisaema peninsulae·Deer herbivory·Spatial pattern·Unpalatable plant
Introduction
Ungulate herbivory can alter the structure and composition of forest communities. High grazing pressure reduces vegetation cover,and leads to indirect changes that may be reflected in plant-plant interactions,trophic cascades,and habitats(Augustine and McNaughton 1998;Rooney and Waller 2003;Heckel et al.2010;Hirobe et al.2013).For instance,selective browsing by ungulates decreases the populations of palatable plants,resulting in the population expansion of unpalatable plants and/or browse-tolerant plants,because they are released from competition with the consumed plants(Augustin and McNaughton 1998;Rooney and Waller 2003).In a northern mesic forest in midwest USA,the average population density of white-tailed deer(Odocoileus virginianus Zimmermann)was 10 individuals km-2(Rooney et al.2002),and the abundant deer population reduced the diversity of understory herbs,resulting in an increased proportion of unpalatable plant species(Rooney and Waller 2003).
The populations of unpalatable plant species can increase through seed propagation and/or clonal reproduction.Generally,clonal reproduction is regarded as a more efficient system than sexual reproduction for the following reasons. First, clonal reproduction does not require pollination processes that depend on other organisms (Abrahamson 1980). Second, clonally produced daughter ramets have lower mortality than seedlings because ramets are larger than seedlings (Kleijn and Steinger 2002;Ohara et al.2006).Finally,clonal plant species can maintain their populations even when sexual reproduction is inhibited by the shortage of resources for flowering under severe environmental conditions(Takasu 1987;Kudoh et al.1999),lack of pollinators(Bierzychudek 1987),and self-incompatibility(Vandepitte et al.2010).Thus,clonally reproducing unpalatable plant species could show more rapid population growth than nonclonal reproducers after the suppression or elimination of palatable plants by herbivores.
Shiyomi et al.(2000)and Yasuda et al.(2003)reported that the spatial distribution of clonal plants has a more aggregated pattern than that of non-clonal plants in grazed grassland communities.Specifically,clonal plants without highly dispersible spacers(e.g.,long stolons,runners,and rhizomes)tend to exhibit spatial aggregation,because they produce daughter ramets at short distances from their parents(Lovett-Doust 1981;Benot et al.2013).Additionally,seed dispersal pattern is a major factor influencing spatial distribution.Spatial aggregation is generally weaker in species with long-distance seed dispersal(e.g.,animal dispersal)than in those with limited seed dispersal(e.g.,gravity dispersal)(Nanami et al.1999;Seidler and Plotkin 2006).Therefore,spatial distribution patterns might differ between unpalatable plants with and without clonal reproduction if they have the same mode of seed dispersal.
One of the areas in Japan affected by intensive herbivory by Sika deer(Cervus nippon Temminck)is the Ashiu Forest Research Station of Kyoto University(Fujiki et al.2014).High grazing pressure has eliminated virtually all understory plant species except unpalatable species(Kato and Okuyama 2004;Fujii 2010;Sakaguchi et al.2012).In this forest,two unpalatable herbaceous species,Arisaema ovale Nakai and A.peninsulae Nakai,were distributed within the same plant community(Sakaguchi et al.2008;Fujii 2010;Sakaguchi et al.2012).These two species were minor members of the understory plant community prior to intensive herbivory(Yoshimura 1965;Miyawaki 1984).Seed dispersal by both species appears to be mediated by frugivorous birds(Suzuki and Maeda 2014a,b;Kobayashi 2016)and/or dropping of infructescence(Bierzychudek 1982;Takasu 1987).A.ovale can propagate by producing cormlets around corms (Murata 1996), while clonal reproduction does not appear to occur in A.peninsulae,according to a study on the allied species,A.serratum(Thunb.)Schott(Kinoshita 1987).
In this study,we investigated the relationship between clonal reproduction and population expansion of unpalatable plant species under high grazing pressure based on the following two predictions: relative to A. peninsulae(without clonal reproduction), A. ovale (with clonal reproduction)has(1)a large population size with a high abundance ratio of small individuals and(2)a highly spatially aggregated distribution pattern,owing to rapid population growth through the active production of young ramets near parent individuals under high grazing pressure.
Materials and methods
Study site
The study site was located in a cool-temperate coniferhardwood mixed forest in the Ashiu Forest Research Station of Kyoto University(35°21′N,135°45′E;659 m a.s.l.).Mean annual precipitation and temperature are approximately 2800 mm and 9.0°C,respectively,and the maximum depth of snow cover is greater than 2 m(Ando et al.1989).The dominant tree species are Cryptomeria japonica(Thunb. ex L.f.) D. Don and Fagus crenata Blume(Yoshimura 1965).In this forest,feeding damage caused by the Sika deer has increased sharply since the late 1990s(Fukuda and Takayanagi 2008),and virtually all plants in the understory have disappeared except the unpalatable species(Kato and Okuyama 2004;Fujii 2010;Sakaguchi et al.2012).The population density of Sika deer was estimated to be 2-10 deer km-2in this forest(Fukuda and Takayanagi 2008).
Plant materials
We focused on the populations of two Arisaema species:A.ovale and A.peninsulae(Fig.1a,b).Both species belong to Arisaema sect.Pistillata(Engl.)Nakai of the family Araceae(Murata 2011).Arisaema spp.are perennial herbs(Murata 2011),and are unpalatable,owing to their high content of calcium oxalate crystals(Bierzychudek 1982;Knight and Walter 2001). Individual Arisaema plants reversibly change from vegetative to flowering states and from male to female with increasing plant biomass(Atkinson 1898;Schaffner 1922;Maekawa 1924).Arisaema seeds are mainly dispersed by frugivorous birds(Suzuki and Maeda 2014a,b;Kobayashi 2016)and/or dropping of infructescence(Bierzychudek 1982;Takasu 1987).A.ovale reproduces clonally by producing cormlets around corms(Murata 1996),while A.peninsulae probably does not reproduce clonally,according to a study of theallied species A.serratum(Kinoshita 1987).As the generated Arisaema cormlets separate from the mother plant before the subsequent growing season(Bierzychudek 1982;Takasu 1987;Murata 1996),there is no physiological integration between A.ovale ramets.

Fig.1 Photographs of studied Arisaema species:Arisaema ovale(a),Arisaema peninsulae(b),corm of A.ovale(c),and that of A.peninsulae(d).Arrowheads indicate the generated cormlets.Scale bar=1 cm
Arisaema ovale is endemic to the Sea of Japan coast,and A.peninsulae is distributed in Japan,Korea,China,and Russia(Murata 2011).Specifically,A.ovale is rare in Kyoto Prefecture(including the study site),and designated as a vulnerable species in Red Data Book of Kyoto Prefecture 2002 and 2015(Tsugaru 2015).Although specimens of the two Arisaema species were occasionally collected from this area(Yasuda and Nagamasu 1995),both species were absent or relatively rare in nine plots(0.1 ha)established in Aesculus turbinata Blume stands(Yoshimura 1965),and three plots(0.4-0.5 ha)established in C.japonica and A.turbinata stands(Miyawaki 1984).Therefore,the two Arisaema species appeared to have been minor in this area prior to the onset of intensive Sika deer herbivory.
Study plot
We established a 0.36-ha plot(30×120 m)in a riparian forest on a riverbank.The riparian forest was dominated by A.turbinata and C.japonica,which was the major vegetation type in this area(Yoshimura 1965).The western side of the plot was adjacent to a small mountain stream.The topography was approximately flat;however,the northern side of the plot sloped gently downward from the base of a mountain ridge.The understory vegetation of the plot was extremely sparse,and dominated by four unpalatable plant species:Pterostyrax hispidus Siebold et Zucc.,Hypolepis punctata(Thunb.)Mett.,Scopolia japonica Maxim.,and Aconitum sanyoense Nakai.
Evaluation of clonal reproduction outside the 0.36-ha plot
In June 2016,we collected 50 A.ovale plants and 15 A.peninsulae plants(fewer A.peninsulae plants due to its low density)from outside the 0.36-ha plot,and counted the number of cormlets. Additionally, sex expression and pseudostem diameter were recorded for each individual.Since females were absent on our 0.36-ha study plot,we explored a wide area(~few kilometers)outside the plot in search of female samples.
Census of Arisaema in the 0.36-ha plot
In May and August 2015,we evaluated the species,position(x-and y-coordinates at the centimeter scale),sex expression(non-flowering:asexual,flowering:male and female),and pseudostem diameter of the plants of the two Arisaema species.The sex expression of flowering individuals(male or female)was based on spadix morphology.We measured pseudostem diameter as an index of plant size, since it shows high correlation with whole-plant biomass(Kinoshita 1986;Heckel et al.2010).In May 2015 and June 2016,we searched for new seedlings of A.ovale.These were identifiable from the older seedlings because they differed in leaf morphology(Kobayashi 2016).
Statistical analyses
We examined the relationship between flowering/nonflowering states and pseudostem diameter using a generalized linear model(GLM)with binomial error and logit link function. We considered flowering (non-flowering=0,flowering=1)as a response variable and pseudostem diameter as an explanatory variable.We compared Akaike's Information Criterion(AIC)with the null model,and considered the pseudostem diameter as an important predictor when the AIC of the full model was higher than that of the null model.
We analyzed the difference in the ratio of the number of asexual to flowering individuals between the two species using a Fisher's exact test.Significance was determined at p ≤0.05.
We characterized the univariate(distribution)spatial patterns of each species using an L-function modified from Ripley's K-function (Ripley 1977; Besag 1977). We defined the function K(r)as the expected number of plants within distance r of an arbitrary plant divided by plant density.K(r)was estimated as follows:
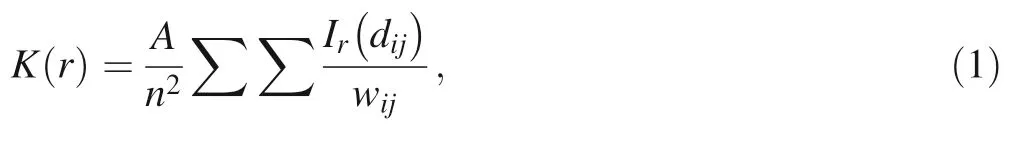
where A is the plot area,n is the number of plants in a plot,dijis the distance between the ith and jth individuals,Ir(dij)is equal to 1,if d ≤r,and 0 otherwise,wijis a correlation factor for edge effects(Goreaud and Pe´lissier 1999),and summation was performed for all possible pairs of plants in the plot.We estimated the L-function as follows:

Spatial distribution was designated as an aggregated,random,or regular distribution at distance r when the estimated L(r)was greater,equal to,or less than zero,respectively.The statistical significance of the deviation from zero was determined using Monte Carlo simulations(Besag 1977;Besag and Diggle 1977).The null hypothesis was complete spatial randomness.The 95%confidence interval of the null hypothesis was calculated using frequency distributions of the L(r) values obtained from 10,000 generations of random point distribution.All analyses were performed with the free software R(version 3.1.2.; R Development Core Team 2014), and spatial analyses were performed with the ads package(Pe´lissier and Goreaud 2015).
Results
Clonal reproduction
For A.ovale,36 of 50 plants had cormlets around the corms(Fig.1c),and 14 plants without cormlets were extremely small(pseudostem diameter <1.8 mm).The numbers of cormlets(mean±SE)were 2.55±1.97,12.14±2.63,and 15.00±2.65 in asexual,male,and female plants,respectively(Table 1).No A.peninsulae plant bore cormlets,irrespective of its sex expression or size(Fig.1d,Table 1).
Sex expression and size structure
Within our study plot we recorded no female plant of either of the two Arisaema species(Table 2).Of the A.ovale population(total:1087 plants),male plants constituted 3.8%,and asexual plants constituted 96.2%,while of the A.peninsulae population(total:84 plants),male plants constituted 10.7%,and asexual plants were 89.3%.The ratio of asexual to flowering(i.e.,male)individuals was significantly higher in A.ovale than in A.peninsulae(p <0.01,Fisher's exact test).In the A.ovale population,no new seedling was observed for 2 years.
In the A.ovale population,the pseudostem diameters of asexual and male plants(mean±SE)were 1.48±0.66 and 4.74±1.25 mm,respectively(Table 2).Similarly,in the A.peninsulae population,the mean pseudostem diameters of asexual and male plants (mean±SE) were 1.58±0.98 and 5.31±1.17 mm,respectively(Table 2).The GLM analysis suggested that flowering ability increased with increase in the pseudostem diameter of the two Arisaema species(Table 3).
Spatial distribution
Arisaema ovale showed aggregated distributions(p <0.05)at the examined spatial scale(~15 m)(Figs.2a,3a),while A. peninsulae showed strongly aggregated distributions(p <0.05) at the examined spatial scale (~15 m)(Figs.2b,3b).In the A.peninsulae population,we recordedone dense patch of more than 50 small individuals(pseudostem diameter ≤1.56 mm)(Fig.2b).When we excluded this patch from the spatial analysis,A.peninsulae showed weakly aggregated distribution(p <0.05)at a short distance(<7 m)and random distribution(p >0.05)at a long distance(≥7 m)(Fig.3c).
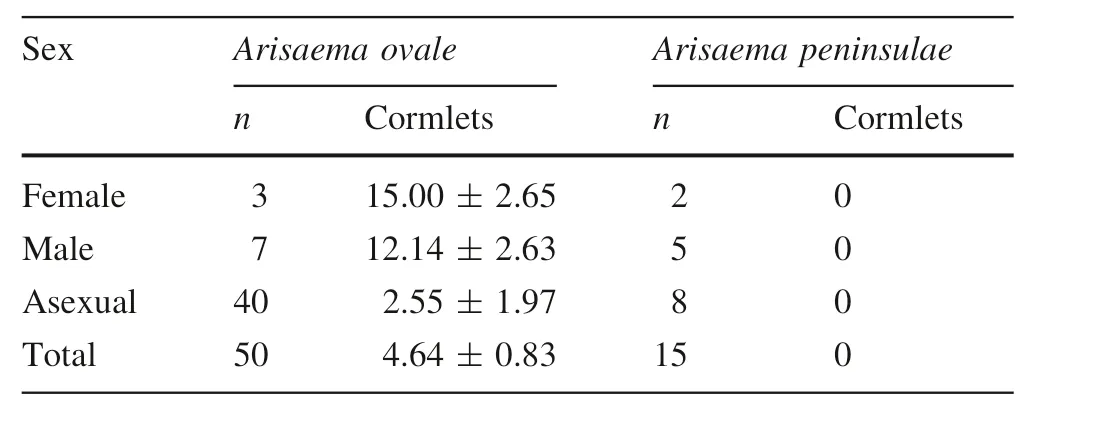
Table 1 The number of cormlets around a corm(mean±SE)of Arisaema ovale(n=50)and Arisaema peninsulae(n=15)collected outside the 0.36-ha plot

Table 2 Sex expression and pseudostem diameter(mean±SE)of Arisaema ovale(n=1087)and Arisaema peninsulae(n=84)in the 0.36-ha plot

Table 3 Results of the generalized linear model analysis
Discussion
Arisaema ovale exhibited clonal reproduction,while A.peninsulae did not(Fig.1c,d,Table 1).Cormlet production in A.ovale increased with the progression of sex expression from vegetative to flowering and from male to female or the increasing of plant size,because sexually mature and larger plants had more resources for clonal reproduction than did asexual and smaller plants.Takasu(1987)reported a similar relationship between cormlet production and size in A.thunbergii Blume subsp.urashima(H.Hara)H.Ohashi&J.Murata.
In the 0.36-ha plot,we observed 1087 A.ovale plants,but only 84 A.peninsulae plants(Table 2),and the ratio of asexual to flowering plants was significantly higher for A.ovale than for A.peninsulae(p <0.01,Fisher's exact test).A similar finding was reported for the populations of the clonal species A.thunbergii subsp.urashima and the nonclonal species A.peninsulae,A.serratum,and A.angustatum Franch. & Sav. (Takasu 1988). Moreover, both Arisaema species were relatively rare in this area prior to the onset of intensive Sika deer herbivory(Yoshimura 1965; Miyawaki 1984): Fujii (2010) reported that the number of flowering A.ovale plants more than doubled,while A.peninsulae numbers remained constant(below 38 individuals ha-1)after intensive grazing began.Therefore,these differences in population structures between the twoArisaema species were probably due to the presence/absence of clonal reproduction.These findings supported our first prediction that under intense grazing pressure,clonal,unpalatable species would have larger population size and higher abundance ratio of small individuals than would non-clonal,unpalatable species.
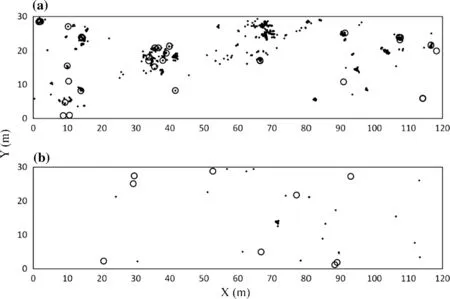
Fig.2 Distribution of Arisaema ovale(a)and Arisaema peninsulae(b)populations in the 0.36-ha plot.Small solid circles denote asexual plants and large open circles denote flowering plants

Fig.3 L(r)values of the population of Arisaema ovale(a),Arisaema peninsulae(b),and A.peninsulae without a patch of small plants(c)in the 0.36-ha plot.The solid line denotes the actual L(r)values for each plant,while broken lines denote 95%confidence envelopes for the random distribution of each plant. L(r) values outside the envelopes indicate significant deviation from random distribution
In neither population did we record any female plant(Table 2). The absence of females in the population appears to be uncommon, since Richardson and Clay(2001)investigated 81 populations of Arisaema spp.,and reported that only three populations lacked females.A.ovale and A.peninsulae are known to be unpalatable(Fujii 2010;Sakaguchi et al.2012);however,we observed that in this forest,large plants(i.e.,flowering plants)of both species were occasionally browsed by Sika deer(Matsumoto et al.,data not shown).Previous studies suggested that damage to the leaves or storage organs(i.e.,corms)of Arisaema reduced plant size,resulting in the degeneration of sex expression from flowering to vegetative and from female to male(Schaffner 1922;Maekawa 1924;Ruhren and Handel 2000).Thus,the sexual transition of the two Arisaema species on our study plot might have been inhibited owing to the prevention of resource accumulation due to intensive foraging by deer.This inhibition of sexual transition may have prevented sexual reproduction,leading to the absence of new seedlings in the A.ovale population.
The spatial distribution patterns of the two Arisaema species were similar(Fig.3).Contrary to our second prediction that A.ovale would show a more aggregated spatial distribution than A.peninsulae,both species showed highly aggregated distributions (Figs.2, 3a, b). However, the highly aggregated spatial distribution of A.peninsulae appeared to be due to only one patch of more than 50 small plants(pseudostem diameter ≤1.56 mm)(Figs.2b,3b).Arisaema spp.disperse seeds via frugivorous birds(Suzuki and Maeda 2014a,b;Kobayashi 2016)and/or dropping of infructescence(Bierzychudek 1982;Takasu 1987),and Arisaema infructescence contains more than 300 seeds(Bierzychudek 1982;Nishizawa et al.2005).Therefore,the dense patch of small A.peninsulae plants might have originated from a dropped infructescence.Without this single patch,A.peninsulae showed a weakly aggregated pattern of spatial distribution(Fig.3c).Concentrated seed dispersal may have led to the spatial aggregation of A.ovale;however,this effect appeared to be limited,because no seedling recruitment was observed in the A.ovale population for 2 years.
In this study,we demonstrated that the difference in clonal reproduction affected the population structure(Table 2)and patterns of spatial distribution(Figs.2,3)of the two Arisaema species in an intensively grazed riparian forest in Japan.Our results suggest that clonal reproduction enabled the rapid increase of the A.ovale population under intense grazing pressure.Additionally,both populations lacked female plants(Table 2),probably because of deer browsing of the larger plants(Matsumoto et al.,data not shown),indicating the limitations of sexual reproduction.Even in this situation,A.ovale could maintain its population via clonal reproduction;however,the genetic diversity of the population would gradually decrease(Kudoh et al.1999;Vandepitte et al.2010).Since populations with low genetic diversity are vulnerable to pathogens and/or environmental change(Schmid 1994;Reusch et al.2005),the large A.ovale population might not be stable in the future.
AcknowledgementsWe wish to thank Drs.T.Ise and N.Tokuchi,and the staffof the Ashiu Forest Research Station of the Kyoto University for their assistance with our study,K.Yamada and R.Tanaka for their help in the field.We are grateful to Dr.J.Murata for valuable advice on the taxonomy of Arisaema species,and Drs.K.Sakamoto and N.H.Miki for their helpful comments on this study.
杂志排行
Journal of Forestry Research的其它文章
- Protective and defensive roles of non-glandular trichomes against multiple stresses: structure-function coordination
- Assessment of early survival and growth of planted Scots pine(Pinus sylvestris)seedlings under extreme continental climate conditions of northern Mongolia
- Influencing in vitro clonal propagation of Chonemorpha fragrans(moon)Alston by culture media strength,plant growth regulators,carbon source and photo periodic incubation
- Variation analysis of growth traits of four poplar clones under different water and fertilizer management
- Nodule study in Albizia chinensis in relation to nitrogen metabolism,morphology and biomass
- Comparative transcriptome analyses reveal candidate genes regulating wood quality in Japanese larch(Larix kaempferi)
