Infrared Absorption Spectrum of Evaporated Water Molecules in Slit
2020-01-17CHENGGuangzhuangZHUCuifengZHANGMeitingLIChunYUANGuang
CHENG Guang-zhuang, ZHU Cui-feng, ZHANG Mei-ting, LI Chun, YUAN Guang
(College of Information Science and Engineering, Ocean University Of China, Qingdao 266100, China)
Abstract: The characteristics of polarized infrared spectroscopy of water molecules in the silicon wafer slits during capillary evaporation were explored in this article. Associated with the slit of the silicon wafer, the direction of the infrared polarized light—horizontal: the polarized direction is parallel to the direction of the silicon wafer slit; vertical: the polarized direction is perpendicular to the direction of the silicon wafer slit—was changed to measure the polarized infrared absorption of water molecules at 3 900-3 600 cm-1 (stretching vibration) and 1 800-1 400 cm-1 (bending vibration). The results indicated that compared with horizontally polarized light, water molecules, which is evaporated from the slit of the silicon wafers, had a strong absorption for vertically polarized light in the range of 3 900-3 600 cm-1 (stretching vibration) and 1 800-1 400 cm-1 (bending vibration), demonstrating that the direction of dipole moment of water molecules evaporated by capillary action tends to the normal direction of the silicon wafers slit.
Key words: evaporation of water molecules; capillary effect; polarized infrared spectrum
1 Introduction
Evaporation is one of the most universal phenomena that one can encountered in nature[1-3], in which, capillary evaporation is one of forms of it. Capillary evaporation is the process of liquid-vapor phase transformation in a tiny space, and it plays an essential role in several nature phenomena[4-6]such as the maintenance of humidity in the soil, the evaporation of water in plants, and the perspiration of mammals. Capillary evaporation, in addition, has shown a variety of promising applications including electronics cooling[7], evaporation of porous media[8], and micropump technology[9].
Plenty of scholars, in recent years, have devoted themselves to evaporation research. To explore the mechanism of water evaporation, molecular dynamics methods have been used in the existing research. Evaporation in a vacuum environment caused changes in the temperature and structure of the water clusters was indicated in Caleman’s study[10]. Discussing the factors affecting the evaporation of liquid drops, Mason had proposed that non-hydrogen bond collision between water molecules would cause the evaporation of water molecules[11]. Studies by Varilly and Nagata have shown that water molecules on the surface of liquid-gas can evaporate as long as they spontaneously acquire enough kinetic energy[12-13]. As for the exploration of capillary evaporation, the existing researches mainly focus on the morphology of liquid-gas interface, evaporation rate and evaporation of porous media. For instance, Li had conducted an experimental study on the evaporation kinetic limit of water in two-dimensional nanowires, and clarified the influence of closure, temperature and humidity on evaporation flux[14]. Merz’s investigation of capillary evaporation in soils had indicated that the dry layer on the soil surface has an inhibitory effect on capillary evaporation[15]. He had verified that the thin liquid film plays a decisive role in the evaporation rate[16].Ranjan had probed the effects of solid-liquid contact angle, wick porosity, solid-vapor superheat and liquid level in the wick pore on liquid meniscus evaporation under different capillary core conditions, and confirmed the hypothesis that liquid presents a static meniscus shape during evaporation[17].
The above researches show that capillary evaporation has been studied deeply, whereas the study on capillary evaporation of water molecules in the slit by polarized infrared spectroscopy has not been widely reported, so it is necessary to study and explore this problem. Polarization infrared spectroscopy, moreover, can be used for nondestructive detection of molecular vibration frequency, the absorption intensity and other information of substances. It is an acceptable tool for quantitative and qualitative analysis of molecular structure, with the advantages of analysis of high precision, fast detection speed, good repeatability and strong anti-interference ability[18-19]. The evaporation of water in silicon slits was studied by polarization infrared spectroscopy in this tractate.
2 Experimental Methods
Fig.1 depicts the schematic diagram of an infrared absorption device for capillary evaporation, wherein the illustration Ⅰ shows the relationship between transmittance of an empty device and the analyzer angle, and the horizontal and vertical polarization spectrum of empty device when the analyzer angle was rotated to 0°, as shown in illustration Ⅱ. The infrared light entered the vacuum chamber after passing through the polarizer, and was accepted by the HgCdTe detector of refrigeration after passing through the analyzer. Finally, the polarization infrared spectra were obtained by Fourier transform. The infrared windows materials of the vacuum chamber were made of zinc selenide (ZnSe) crystal, and the infrared spectrometer was Tensor27 (Bruker). Four clamped 10 mm×10 mm×0.25 mm double-sided polished monocrystalline silicon wafers were installed in the vacuum chamber, and deionized water was contained between the wafers. The sample pool was vacuumed by a mechanical pump, which caused evaporation of water within the slit of the wafer. The gaseous water molecules, immediately, entered the infrared measurement area.
Initially, the effect of the empty device (polaroid and empty sample cell) on the transmittance of polarized light was measured. With the air as the background, the polarizing angle was fixed (0°: the horizontally polarized light parallel to the slit of the silicon wafer; 90°: the polarized light perpendicular to the slit of the silicon wafer), and the analyzer angleθ(0°-90°) was rotated at intervals of 10° each time. The infrared spectra at different analyzer angles were measured, as shown in the illustration Ⅰ and Ⅱ in Fig.1. To understand the relationship between transmittance and the analyzer angle, the infrared absorption spectra without absorption peak position (2 250 cm-1) were selected. As shown in the illustration Ⅰ, after the infrared light passes through the empty device, its transmittance changes with the analyzer angle as the square curves of the cosine, which conforms to the laws of Marius, and the transmittance of the horizontal and vertical polarized light does not change significantly with the analyzer angle. Secondly, in order to eliminate the influence of residual moisture in the sample cell on the test results, the infrared absorption spectrum of the sealed empty device (polaroid and empty sample cell) was measured with time. With sealed air device (without vacuumed) as the background, in the first stage, the polarization spectrum of the sealed empty device (without vacuumed) was measured. We recorded it as 0 s, and the vacuum pump was turned on after 30 s. The sealed air device was continuously measured 10 times during the vacuuming process, each time interval of 30 s. Finally, the capillary evaporation of water molecules was measured. With empty device (without vacuumed) as the background, a silicon chip is installed that contains deionized water after clamping. In the first stage, the polarization spectrum of the device (without vacuumed) was measured, and it was recorded as 0 s, then the vacuum pump was turned on after 30 s, and the device containing deionized water was continuously measured 10 times in the vacuum process, each time interval of 30 s. The measurement spectrum range was 4 000-800 cm-1, the number of scans was 32, the resolution was 4 cm-1, and the scanning frequency was 20 Hz. For correcting baseline and smoothing of polarized infrared spectral, the software of OPUS was utilized.

Fig.1 Schematic diagram of the experimental setup. Ⅰ.Transmittance of the experimental setup as a function of the analyzer angle. Ⅱ.Horizontal and vertical polarization spectrum of the empty device when the analyzer angle is rotated to 0°.
3 Results and Discussion
During the air-tight device vacuumed, the changes of the infrared absorption of the water molecules stretching vibration was shown in Fig.2. Wherein Fig.2(a) and Fig.2(b) respectively show the relationship between the absorbance and time of all absorption peaks of water molecules in the stretching vibration zone at the horizontal and vertical incidence of polarized light. Illustration Ⅰ and Ⅲ respectively represent the horizontally and vertically polarized infrared spectroscopy of water molecules. When the direction of the polarized light is horizontal and vertical respectively, the relationship between the absorbance of the absorption peak in the stretching region of the water molecule and time was shown in illustration Ⅱ and Ⅳ. It is generally believed that the peak of absorption is caused by the difference in the rotation of water molecules. The constant values reached by the absorbances corresponding to different wave numbers may be different due to different modes of vibration and rotation[20]. In the stretching vibration zone, the infrared absorption peaks of water molecules are sharply reduced when the vacuum pump is turned on (30th second). With the increase of the vacuuming time, the infrared absorption tends to be constant, regardless of the direction of the polarized light, which indicates that the residual gas in the cavity has no selective absorption of polarized light.
As shown in Fig.2(a) and 2(b), the infrared absorption of water molecules in bending vibration (1 400-1 800 cm-1) is similar to the infrared absorption in the stretching vibration, which indicates that the infrared absorption is basically independent of the polarization of incident light.
In the stretching vibration region, water molecules, which are evaporated from the slit of the silicon wafers, absorb polarized infrared light as shown in Fig.3. When the direction of the polarized light is horizontal and vertical respectively, the absorbance of the partial absorption peak varies with the time from 3 900 cm-1to 3 600 cm-1(the stretching vibration), as shown in Fig.3(a) and 3(b). Where illustration Ⅰ and Ⅲ were the horizontally and vertically polarized infrared spectra of water molecules in the slit of a silicon wafer. Illustration Ⅱ and Ⅳ were the changes in absorbance of all absorption peaks in the stretching vibration region of the horizontal and vertical polarization spectra during the evaporation of water molecules in the slit of the silicon wafer, respectively.
The position of the absorption peak of the anhydrous molecules (2 250 cm-1) was selected for exploration. As shown in Fig.3(a) and 3(b), the absorbance at different times was always near the value of zero, indicating that the change in absorbance in the stretching vibration region of the gas phase water molecule was independent of the instrument.
When the vacuum pump was turned on (30th second), the absorbance was drastically reduced and eventually reached a stable value, indicating that there were no more vapor phase water molecules evaporating from the silicon wafers. when it comes to the trends of absorbance of polarized infrared light, in the stretching vibration region of water molecules, trends vary. Compared with horizontally polarized light, the water molecules have a transient increase or flattening tendency for the absorption of vertically polarized light (the direction of polarization parallel to the normal to the slit of the wafer, as shown in Fig.1), and then exhibited a relatively slow decay as the vacuuming time increases. The absorption of polarized infrared by water molecules may reflect the state of water molecules evaporated from the slits of the wafers.
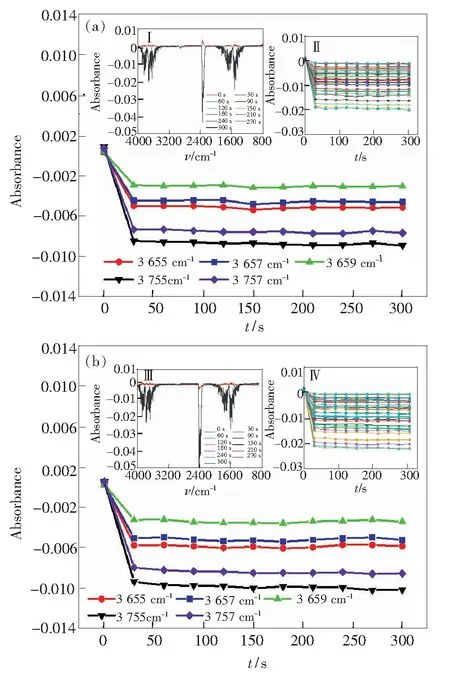
Fig.2 Polarized spectrum and the absorbanceversustime curves in stretching vibration zone(air-tight device). (a)When horizontally polarized light is selected, the absorbance of partial absorption peak is related to time in the stretching vibration region. Ⅰ.Horizontally polarized infrared spectrum.Ⅱ.The absorbance of all absorption peaks in stretching vibration zone varies with time when horizontally polarized light is selected. (b)When vertically polarized light is selected, the absorbance of partial absorption peak is related to time in the stretching vibration region. Ⅲ.Vertically polarized infrared spectroscopy. Ⅳ.The absorbance of all absorption peaks in stretching vibration zone varies with time when vertically polarized light is selected.

Fig.3 Polarized spectrum and the absorbanceversustime curves in the stretching vibration region during the evaporation process of water molecules in the silicon wafers slit. (a)When horizontally polarized light is selected, the absorbance of partial absorption peak is related to time in the stretching vibration region. Ⅰ.Horizontally polarized infrared spectrum. Ⅱ.The absorbance of all absorption peaks in stretching vibration zone varies with time when horizontally polarized light is selected. (b)When vertically polarized light is selected, the absorbance of partial absorption peak is related to time in the stretching vibration region. Ⅲ.Vertically polarized infrared spectroscopy. Ⅳ.The absorbance of all absorption peaks in the stretching vibration zone varies with time when vertically polarized light is selected.
In the bending vibration region, water molecules evaporated from the slit of the silicon wafers absorb polarized infrared light, as shown in Fig.4. When the direction of the polarized light is horizontal and vertical respectively, the absorbance of the partial absorption peak varies with the time from 1 800 cm-1to 1 400 cm-1(the bending vibration), as shown in Fig.4(a) and 4(b). The relation between the absorbance of gas phase water in the stretching vibration zone and the instrument has been explored,and it has been known that the changes of the absorbance of the gas phase water molecules have nothing to do with the instrument. In the analysis of the relationship between the absorbance of the gas phase water and the instrument, it has been known that the changes of the absorbance of the gas phase water molecules have nothing to do with the instrument. For the bending vibration of water molecules, the changes trend of absorbance corresponding to infrared light of different polarized light was similar to the infrared absorption in the stretching vibration region, as shown in Fig.4(a) and 4(b). Compared with horizontally polarized light, the absorption of vertical polarized light by water molecules also shows a brief increase or even flatlines briefly, and then shows a relatively slow decline with the increase of vacuum extraction time, which was consistent with the trend of change in the stretching vibration zone. In summary, the absorption of polarized infrared by water molecules may reflect the state of water molecules evaporated from the slit in the silicon wafers.
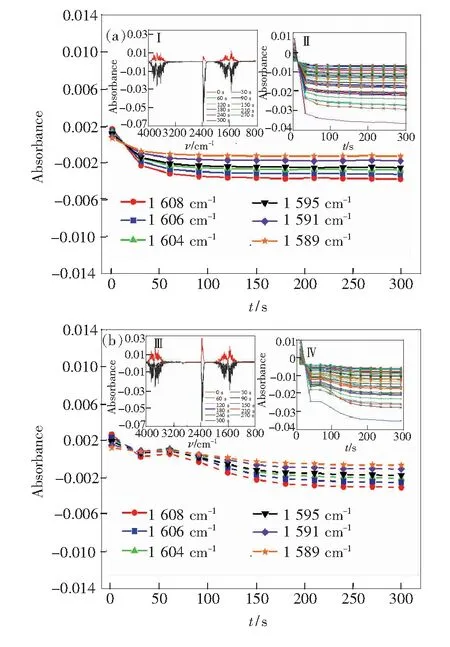
Fig.4 Polarized spectrum and the absorbanceversustime curves in the bending vibration region during the evaporation process of water molecules in the silicon wafers slit. (a)When horizontally polarized light is selected, the absorbance of partial absorption peak is related to time in the bending vibration region. Ⅰ.Horizontally polarized infrared spectrum. Ⅱ.The absorbance of all absorption peaks in bending vibration zone varies with time when horizontally polarized light is selected. (b)When vertically polarized light is selected, the absorbance of partial absorption peak is related to time in the bending vibration region. Ⅲ.Vertically polarized infrared spectroscopy. Ⅳ.The absorbance of all absorption peaks in the bending vibration zone varies with time when vertically polarized light is selected.
The main vibration modes of the water molecules are shown in Fig.5, whereais the symmetric stretching vibration andbis the bending vibration.When the dipole moment of the water molecules are coincided with the direction of the polarized infrared light, the infrared light will be strongly absorbed by the water molecules. Regardless of the vibration modes, the water molecules, as long as its dipole moment direction is consistent with the direction of the polarized infrared light, will have a significant absorption of the polarized infrared light, as shown in Fig.5. The measurement results showed that the water molecules, evaporated from the silicon wafer slit, had strong absorption of vertically polarized infrared light, demonstrating that the direction of the dipole moment of the water molecules tends to be the direction of the vertically polarized light. Moreover, the direction of the polarized light—horizontal: the polarized direction is parallel to the direction of the silicon wafer slit; vertical: the polarized direction is perpendicular to the direction of the silicon wafer slit—was associated with the direction of the slit of the silicon wafer. The direction of the dipole moment of the water molecules evaporating from the slit tended to be vertically polarized light, which means that the direction of the dipole moment was parallel to the normal direction of the slit. This may be because the direction of the slit has a limiting effect on the direction of the dipole moment of the water molecules. The insights gained from this research will greatly enhance our understanding of capillary evaporation, making it possible to develop bio-based and energy-related applications based on capillary evaporation.

Fig.5 Vibration modes of gas molecules of water
4 Conclusion
There are distinctions in the absorption of polarized light by water molecules evaporated from the slit. Compared with horizontally polarized light, water molecules evaporating from the slit of the silicon wafer have a stronger absorption for vertically polarized infrared light ranging from 3 900 cm-1to 3 600 cm-1(the stretching vibration) and 1 800 cm-1to 1 400 cm-1(the bending vibration), indicating that the vapor phase water molecules vaporized by capillary between silicon wafers are affected by the slit, and the direction of dipole moment tends to the normal direction of the slit in silicon wafers.
