Ecological and Geographical Reasons for the Variation of Digestive Tract Length in Anurans
2019-12-27ChunlanMAIJianpingYUandWenboLIAO
Chunlan MAI,Jianping YU and Wenbo LIAO
1 Key Laboratory of Southwest China Wildlife Resources Conservation(Ministry of Education), China West Normal University, Nanchong 637009, Sichuan, China
2 Key Laboratory of Artificial Propagation and Utilization in Anurans of Nanchong City, China West Normal University,Nanchong 637009, Sichuan, China
3 Institute of Eco-adaptation in Amphibians and Reptiles, China West Normal University, Nanchong 637009, Sichuan,China
Abstract Changes of environmental conditions can shape organs size evolution in animal kingdoms.In particular,environmental changes lead to difference in food resources between different habitats,thereby affecting individual's energy intake and allocation.The digestive theory states that animals consuming food with low contents of digestible materials should result in increasing gut length.In this study,to test the hypothesis of digestive theory,we studied ecological and geographical reasons for variation in digestive tract length among 35 species of anurans distributing in different altitude and latitude.The results showed that ecological type significantly affected digestive tract length among species,with aquatic and terrestrial species having longer digestive tract than arboreal ones.Latitude was positively correlated with digestive tract length.However,altitude,as well as monthly mean temperature and precipitation,did not correlate with digestive tract length among species.Our findings suggest that aquatic and terrestrial species might forage less digestible materials than arboreal species,thereby displaying relatively longer digestive tract than arboreal species.
Keywords Anurans,digestive tract length,environmental change,ecological type
1.Introduction
Evolution in size of organ in organisms has caused concerns for evolutionary biologists and ecologists for decades(Piersma and Lilliendahl,1999;Hammondet al.,1999;Liaoet al.,2016a;Chenet al.,2016;Maiet al.,2017a,b;Tanneret al.,2017;Altonet al.,2017;Kotrschalet al.,2017;Liaoet al.,2018;Yanget al.,2018;Güneş and Danacıoğlu,2018;Mediniet al.,2018;Liuet al.,2018;Samuket al.,2018;Caiet al.,2019;Zhaoet al.,2019).In particular,energy store is an important factor affecting variation in the organ size of ectotherms in different environments(Lüpoldet al.,2017;Signoret al.,2017;Hammondet al.,2000;Jinet al.,2016a,b;Luoet al.,2017;Guet al.,2017;Iwai,2018;Josephet al.,2018;Huanget al.,2018;Tanget al.,2018;Mai and Liao,2019).For instance,to improve local adaptation,individuals living in more biotic conditions require more accumulated energy by increasing liver and muscle mass than individuals living in less biotic environments in anurans(Hesse,1924;Mülleret al.,2014;Zhonget al.,2017;Yanget al.,2017).
The digestive tract length varies with changing environments to meet variations in food availability(Sibly,1981).Actually,the digestive tract is linked to species adaptations in the process of evolution at different external environments in most vertebrates(Naya and Bozinovic,2004;Nayaet al.,2007;Nayaet al.,2008;Nayaet al.,2009;Louet al.,2013;Maet al.,2016;Wanget al.,2017).Consequently,adaptive radiation of the digestive tract to functional demands could provide for an important energy-saving mechanism.
The digestive theory states that animals consume food with low contents of digestible materials,which results in an increase in gut dimensions(Penry and Jumars,1987).The prediction of this theory has been successfully examined across a broad range of vertebrate taxa(Hansson,1985;Secor,2001;Hansson and Jaarola,1989;Nayaet al.,2009;Louet al.,2013;Maet al.,2016).
Ecological factors,such as ecological type,has been identified affecting local adaptation of animals(Gondaet al.,2009;Liaoet al.,2015;Shine,1989;Liaoet al.,2013;Zenget al.,2014;Liaoet al.,2014;Liaoet al.,2016b;Huanget al.,2019).For instance,digestive tract variation is confirmed to be correlated with habitat differences of species experienced(Nuňezet al.,1982).Anurans live in broad range of different habitats(Feiet al.,2010;Liaoet al.,2018)and the patterns of digestive tract variation associated with ecological type have been less studied than that of other animal groups(Hansson,1985;Hansson and Jaarola,1989;Sassiet al.,2007;Nayaet al.,2008).Only recently studies on the intra-specific variation in digestive tract length in anurans have elucidated the influence of seasonality and geographical location(Nayaet al.,2009;Louet al.,2013;Maet al.,2016;Wanget al.,2017).However,studies on the inter-specific variation in digestive tract length associated with ecological and geographical reasons among anurans are lacking.Here,we investigated the effects of ecological type on variation in digestive tract length across 35 species of anurans.We also investigated the effects of geographical location(e.g.,altitude,latitude,temperature and rainfall)on digestive tract length.
2.Materials and Methods
2.1.Data collectionA total of 328 adult males from 35 species were collected by hand at night using a flashlight during the breeding season between 2007 and 2018 from Hengduan Mountains in China(Table S1).All individuals then were taken back to lab and kept singly in wirenetting rectangular containers(20 cm×10 cm×15 cm;L×W×H)placed in a tank(90 cm×40 cm×40 cm;L×W×H)with a depth of 10 cm of fresh water.All individuals were killed by single-or double-pithing(Yuet al.,2018)and preserved in 4% phosphate buffered formalin for tissue fixation.After two to eight weeks of preservation,body size(snout-vent length:SVL)was measured to the nearest 0.01 mm with a caliper,and body mass was weighed to the nearest 0.1 mg with an electronic balance(Wuet al.,2016;Zenget al.,2016).Once the connecting mesenteries were cut,the entire digestive tract was aligned along a caliper with stretching it(Louet al.,2013).We measured digestive tract length to the nearest 0.01 mm with a caliper.
We classified ecological type for each species on a four-point scale:1=arboreal-occur mostly on trees,forage in trees and rarely come down to the ground;2=terrestrial-occur and forage mostly on ground;3=aquatic-semiaquatic-not entirely aquatic,uses both aquatic and terrestrial habitats;and 4=aquatic-occur and forage mostly in water.
2.2.PhylogenyWe constructed the new molecular phylogeny based on a matrix of three nuclear and three mitochondrial genes with good coverage across our 35 species(≥13 species for each gene).The mitochondrial gene consisted of the large and small subunits of the mitochondrial ribosome genes(12S/16S)and the cytochrome b(CYTB).The nuclear genes consisted of the recombination-activating gene 1(RAG1),the tyrosinase(TYR)and the rhodopsin(RHOD).We provided genbank accession numbers for the gene sequences used to generate the phylogeny(Table S2).The sequences were aligned using the MUSCLE function in MEGA v.6.0.6(Tamuraet al.,2013)and each gene of the best nucleotide substitution model was determined using the Akaike Information Criterion in jModelTest v.2.1.2(Darribaet al.,2012).The best substitution model was GTR+G for 12S and TYR,HKY+G for RAG1 and RHOD,and GTR+I+G for CYTB and 16S,respectively.
Using these models,we constructed the phylogenies based on BEAUti and BEAST v.1.8.3(Drummondet al.,2012),with unlinked substitution models,no calibration points,a relaxed uncorrelated lognormal clock and a Yule speciation process due to a lack of fossil dates.We allowed the Markov Chain Monte Carlo(MCMC)simulation to run for 50 million generations,and then sampled a tree every 2000thgeneration.The effective sample size(ESS)values for each of the tree statistics showed the satisfying convergence of the Bayesian chain and adequate model mixing in the program Tracer v.1.6.0(Rambaut,2014).We then used TreeAnnotator v.1.8.3(Drummondet al.,2012)to generate a maximum clade credibility tree with mean node heights and a 20% burnin before ending the analysis.
2.3.Statistical analysesWe performed all analyses in the statistical software R v.3.3.1(R Development Core Team,2016).We used phylogenetic generalized leastsquared(PGLS)regressions(Freckleton,2002)to account for statistical non-independence of data points because of shared ancestry of species in the R package based on the molecular phylogeny.To evaluate the phylogenetic relationship of the covariance in the residuals,we estimated the phylogenetic scaling parameter Pagel's λ of these regressions using maximum-likelihood methods.We used likelihood ratio tests to establish whether the models with the maximum-likelihood value of λ differed from models with values of λ=0 or λ=1,respectively.λ close to 0 indicated phylogenetic independence and λ close to 1 indicated a strong phylogenetic association of the traits.Since digestive tract was subject to a wide range of selective pressures that acted simultaneously,we assessed the relationships between digestive tract and ecological type based on Markov Chain Monte Carlo GLMMs,implemented in the MCMCglmm R package v2.20(Hadfield,2010)with body size added as a covariate to account for allometric effects.In all cases,we used inverse-Wishart priors(V=1,v=0.002).Each model was run for 5 100 000 iterations with a 100 000 burn-in and a thinning interval of 5000.After running the models,we examined the autocorrelation of samples to make sure that it was<0.1.We presented parameter estimates from models as the posterior mode and the 95% lower and upper credible intervals(CIs)of the posterior samples.Significance values(pMCMC)were the proportion of samples from all the iterations that are greater or less than 0.We used PGLS treating digestive tract length as the response variable,altitude and latitude as the predicted variables,and body size and sample size as covariates to test the effects of altitude and latitude on digestive tract length.In the mean time,we tested the effects of temperature and precipitation on digestive tract length.
3.Results
MCMCglmm revealed that ecological type significantly affected variation in the digestive tract length across 35 species of anurans(Table 1).Digestive tract length was positively correlated with body size(Table 1).Aquatic and terrestrial species had relatively larger digestive tract than arboreal species after controlling body size effect(Figure 1).When ecological type could be split into simply‘terrestrial'(for terrestrial and arboreal)and‘aquatic'(for aquatic and semi-aquatic),we found that terrestrial species tended to have relatively longer digestive tract than aquatic species(Post.mean=0.064,CI 95%=-0.050-0.175,P=0.253).
PGLS revealed that interspecifical variation in digestive tract length was positively correlated with latitude,but not altitude(Table 2).Meanwhile,average monthly temperature and precipitation was not correlated with variation in relative digestive tract length(Table 2).

Figure 1 The difference in digestive tract length among ecological types among 35 species of anurans.Relative digestive tract size was estimated using the residuals from observed digestive tract length minus predicted digestive tract length based on the regression of digestive tract length on body size when controlling body size effect.
4.Discussion
Our study demonstrates a marked effect of ecological type on relative digestive tract size across 35 species of anurans.The digestive tract length is positively correlated with latitude,but not altitude,average monthly temperature and precipitation.Below we discuss what may underlie the ecological and geographical reasons for the variation of digestive tract length in anurans.
Differences in environmental conditions was related to variations in organ morphology in organisms(Hammondet al.,1999;Zhonget al.,2017;Liaoet al.,2011;Liaoet al.,2013;Pascoalet al.,2017;Martinezet al.,2018;Mølleret al.,2018;Samuket al.,2018).In particular,variation in digestive tract length associated with food changes can adapt different environmental types(Hammondet al.,1999).A comparison for digestive tract between individuals from different ecological types is important for understanding how physiological traits are affected by environmental conditions,and consequently,how they evolve(Chown and Nicolson,2004).Actually,environmental difference often determines food availability;in frogs mostly via affecting the abundance of digestible foods(Wen and Zhang,2010;Shiet al.,2011).In this study,we found that ecological types significantly affected the digestive tract length,with arboreal species exhibiting shorter digestive tract than aquatic species.Often individuals foraging more indigestible materials result in increasing area and length of digestive tract to digest and absorb more nutrients(Penry and Jumars,1987;Sassiet al.,2007).For anurans,there are evidences that individuals consuming higher proportion of indigestible materials(e.g.,high-fiber food)have larger intestines than individuals consuming arthropods(Nayaet al.,2009).Hence,we inferred that arboreal species probably consumed more digestible materials than aquatic species,which can explain consequently the effect of ecological type on variation in relatively digestive tract length among species.

Table 1 The effect of ecological type on digestive tract length when controlling body size among 35 species of anurans using MCMCglmm.
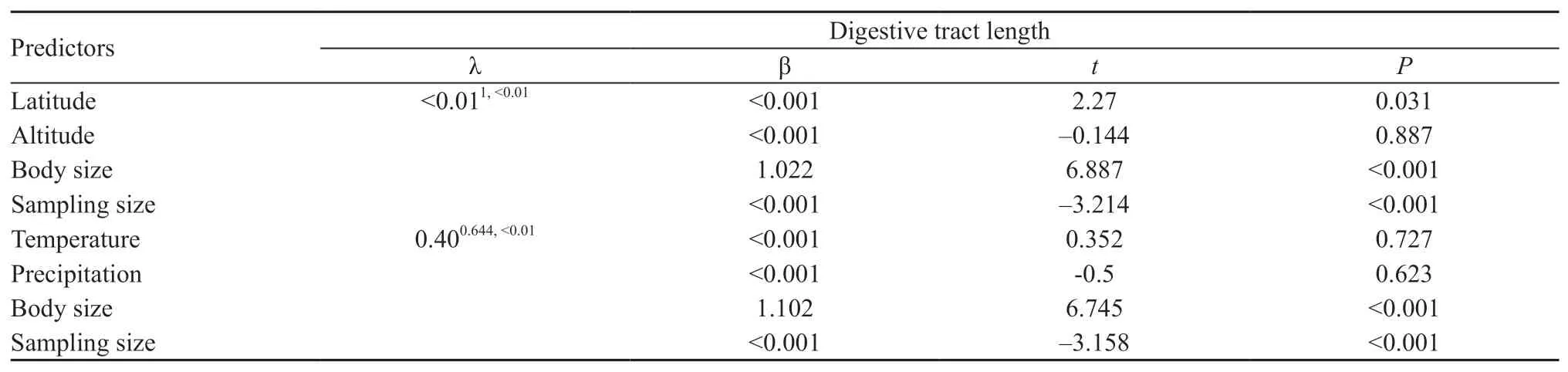
Table 2 The relationships between digestive tract length and altitude and latitude,as well as temperature and precipitation when controlling body size among 35 species of anurans using PGLS.Phylogenetic scaling parameters(superscripts following λ denote P-values of likelihood ratio tests against models with λ=0 and λ=1,respectively).
A positive correlation between digestive tract length and body size has been reported in most anurans species(Nayaet al.,2009;Louet al.,2013;Maet al.,2016;Wanget al.,2017).Energy requirements can lead to the variation in digestive tract per unit body mass(Pulliainen,1976).Hence,larger species should need more energy than smaller species,and thus possessing longer digestive tracts(Louet al.,2013).We found that there was positive relationship between digestive tract length and body size,suggesting that longer digestive tract evolution for larger species was possible to correlate with energy requirements.
Previous studies have shown that individuals living in lower altitude/latitude with higher temperature and less precipitation possess relatively shorter guts than those living in higher altitude/latitude with lower temperature due to more digestible materials(Nayaet al.,2009;Louet al.,2013;Maet al.,2016;Wanget al.,2017).In particular,increased composition of the digestible foods(e.g.,animal-based foods)and the decreased indigestible foods(e.g.,low-quality,high-fiber food)in low altitude may result in decreasing digestive tract length among populations in frogs(Nayaet al.,2009;Maet al.,2016).Here,we did not find an increase in digestive tract length with increased altitude.In the contrast,our findings of the latitudinal increase in digestive tract length are consistent with the three frog speciesexamined for altitudinal variation in the digestive tract(Nayaet al.,2009;Louet al.,2013;Wanget al.,2017).Hence,more digestible materials in low latitude can promote short digestive tract length in anurans.Moreover,we found that variation in digestive tract length was not correlated with both temperature and precipitation across 35 species.Future study need collect more species to address these relationships.
Taken together,differences in environmental conditions shape variation in digestive tract length to meet differential selective forces or constraints.We observe significant effect of ecological type on digestive tract length,terrestrial species tending to have relatively longer digestive tract than aquatic species among 35 species of anurans.Although digestive tract length is positively correlated with latitude,it is not correlated with altitude,temperature and precipitation.
AcknowledgementsWe thank Long Jin,Shangling Lou,Maojun Zhong,Li Zhao and Cheng Chen to help the data collected in fieldwork.The publication of this article was funded by the National Natural Sciences Foundation of China(31 772451;31970393),the Science and Technology Youth Innovation Team of Sichuan Province(19CXTD0022),the Key Cultivation Foundation of China West Normal University(17A006)and Talent Project of China West Normal University(17YC335).The field studies do not involve endangered or protected species.All the methods used in this study related to capture and handling of the animals was approved by the Institutional Animal Care and Use Committee(IACUC)at China West Normal University.
Appendix
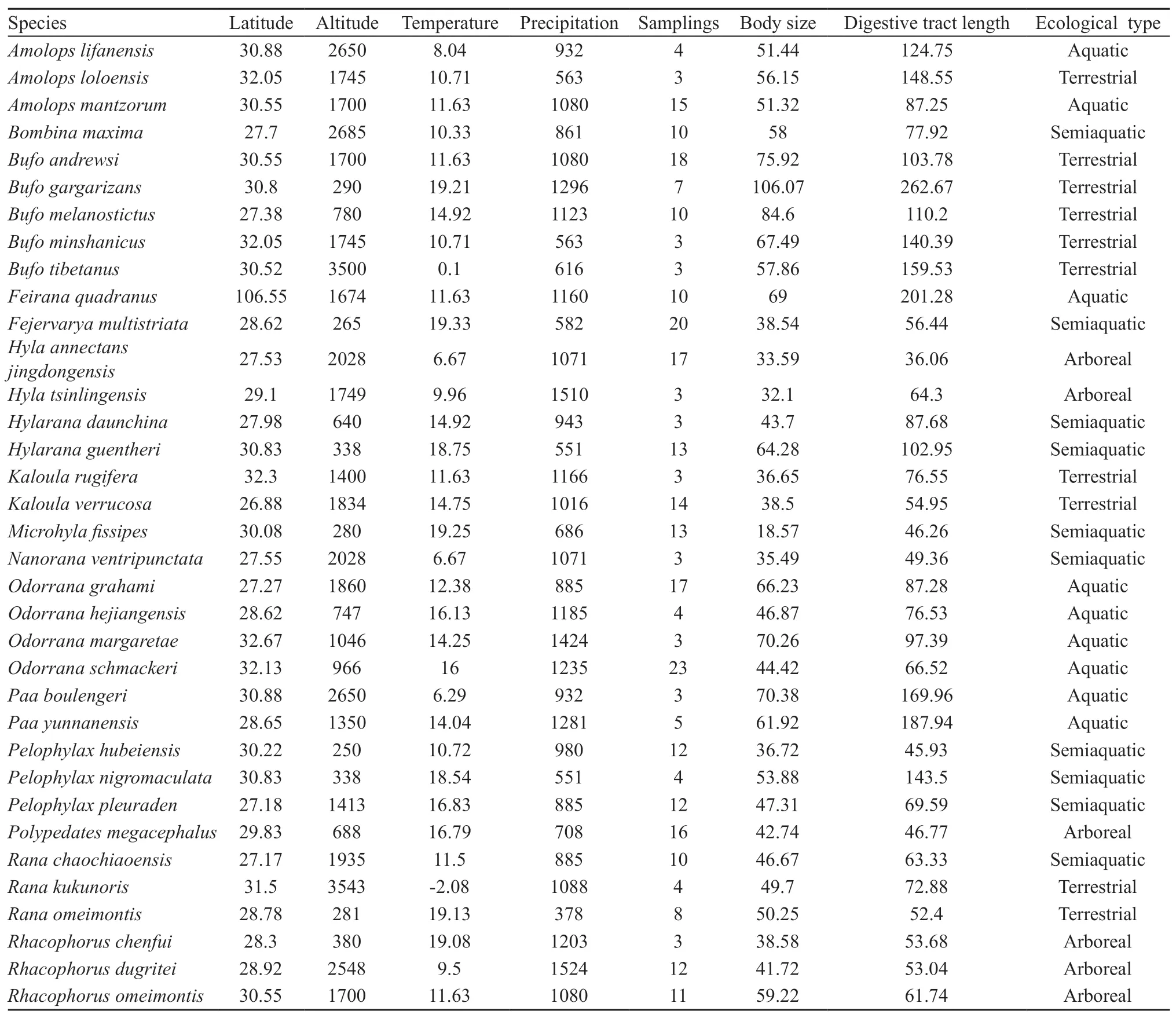
Table S1 Species,latitude(°),altitude(m),temperature(°C),precipitation(mm),sample size,body size(mm),digestive tract length(mm)and ecological type.

Table S2 Genbank accession numbers for the gene sequences used to generate the phylogeny.
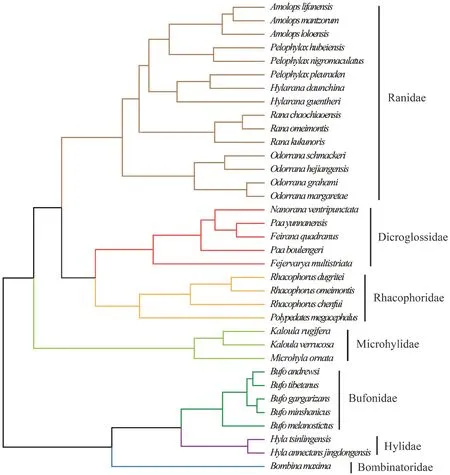
Figure S1 The phylogenetic tree of the 35 species of anurans in the comparative analysis.
杂志排行
Asian Herpetological Research的其它文章
- Correlation between Climatic Factors and Genetic Diversity ofPhrynocephalus forsythii
- Home Range Size and Overlap of the Small Nocturnal Schlegel's Japanese Gecko(Gekko japonicus),Introduced into a City Park in Korea
- Mating Ethogram of a Video-aided Study of Mating and Parturition in Captive Chinese Crocodile Lizards(Shinisaurus crocodilurus)
- Geographical Distribution and Morphological Variability of the Rapid Racerunner,Eremias velox(Pallas,1771)(Reptilia,Lacertidae)in the Eastern Periphery of Its Range
- Description of a New Species of Amolops(Anura:Ranidae)from Tibet,China
- Molecular Cloning,Characterization and Sequence Analysis of KCNQ4 in Large Odorous Frog,Odorrana graminea
