Geographical Distribution and Morphological Variability of the Rapid Racerunner,Eremias velox(Pallas,1771)(Reptilia,Lacertidae)in the Eastern Periphery of Its Range
2019-12-27MarinaCHIRIKOVATatjanaDUJSEBAYEVAJinlongLIUandXianguangGUO
Marina A.CHIRIKOVA,Tatjana N.DUJSEBAYEVA,Jinlong LIU and Xianguang GUO
1 Institute of Zoology, Ministry of Education and Sciences, Almaty 050060, Kazakhstan
2 Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China
Abstract Phenotypic traits are usually correlated with the environment where organism occurs.In this study,the distribution of Eremias velox in the eastern periphery of its range was specified,and its morphological variation was analyzed.Linear dimensions,pholidosis,coloration and pattern features were compared among 135 specimens from nine populations inhabiting the Balkhash,Ili and Alakol basins,Junggar and Turpan depressions in the territory of Southeast Kazakhstan and Xinjiang,Northwest China.The populations from the Junggar Depression(Kuytun,Shihezi and Urumqi)were characterized by higher mean values of linear characters,the number of scales across the middle of the body and gular,and were similar in the dominant coloration patterns.Small size,dark coloration and almost complete dominance of the striped-type coloration pattern among the specimens from the Alakol Lake islands seem to have an adaptive significance associated with the isolation of the population and type of their habitats.The subspecies Eremias velox roborowskii(endemic to the Turpan Depression)is elevated to species level,as supported by morphological divergence congruent with molecular and geographical data,including its peculiar type of coloration pattern,significantly lower amount of femoral pores and a higher percentage of specimens with one enlarged preanal scale(72.7%). These results together confirm a high degree of variability in morphology for E.veloх in the eastern periphery of its range,reflecting a complex orography and the existence of multiple geographical barriers in this territory.
Keywords Eremias velox, Kazakhstan,Xinjiang,habitat,pholidosis,coloration,intraspecies differentiation
1.Introduction
Variation is a central concept in biology(Hallgrímsson and Hall,2005).Phenotypic variation is the raw material for natural selection,and its unit is the individual,through which fitness is optimized via effects on survival and reproduction.Selective influences and stochastic processes like drift accumulate across individuals,yielding variation at the population level(Lande,1976).Phenotypic traits are usually correlated with the environment where organism occurs,through effects on physiological,functional and ecological performance(Arnold,1983).Across populations,phenotypic plasticity and local adaptation are major frameworks for studying associations between the phenotype and the environment,and for elucidating the mechanisms underlying intraspecific phenotypic diversity(Ghalamboret al.,2007;Mullenet al.,2009).
The Rapid Racerunner,Eremias veloxsensu lato,is one of the most widespread species of the genusEremias.Its range extends from Northern Iran and Afghanistan in the south to the southern boundary of the steppe in Kazakhstan in the north;and from East of Ciscaucasia in the west to Northwest China in the east(Sindaco and Jeremčenko,2008).The eastern periphery of the species range lies in Southeast Kazakhstan and Xinjiang within the Balkhash-Alakol,Ili,Junggar and Turpan depressions,China(Figure 1;Brushko,1995;Zhao,1999;Chirikova,2007).
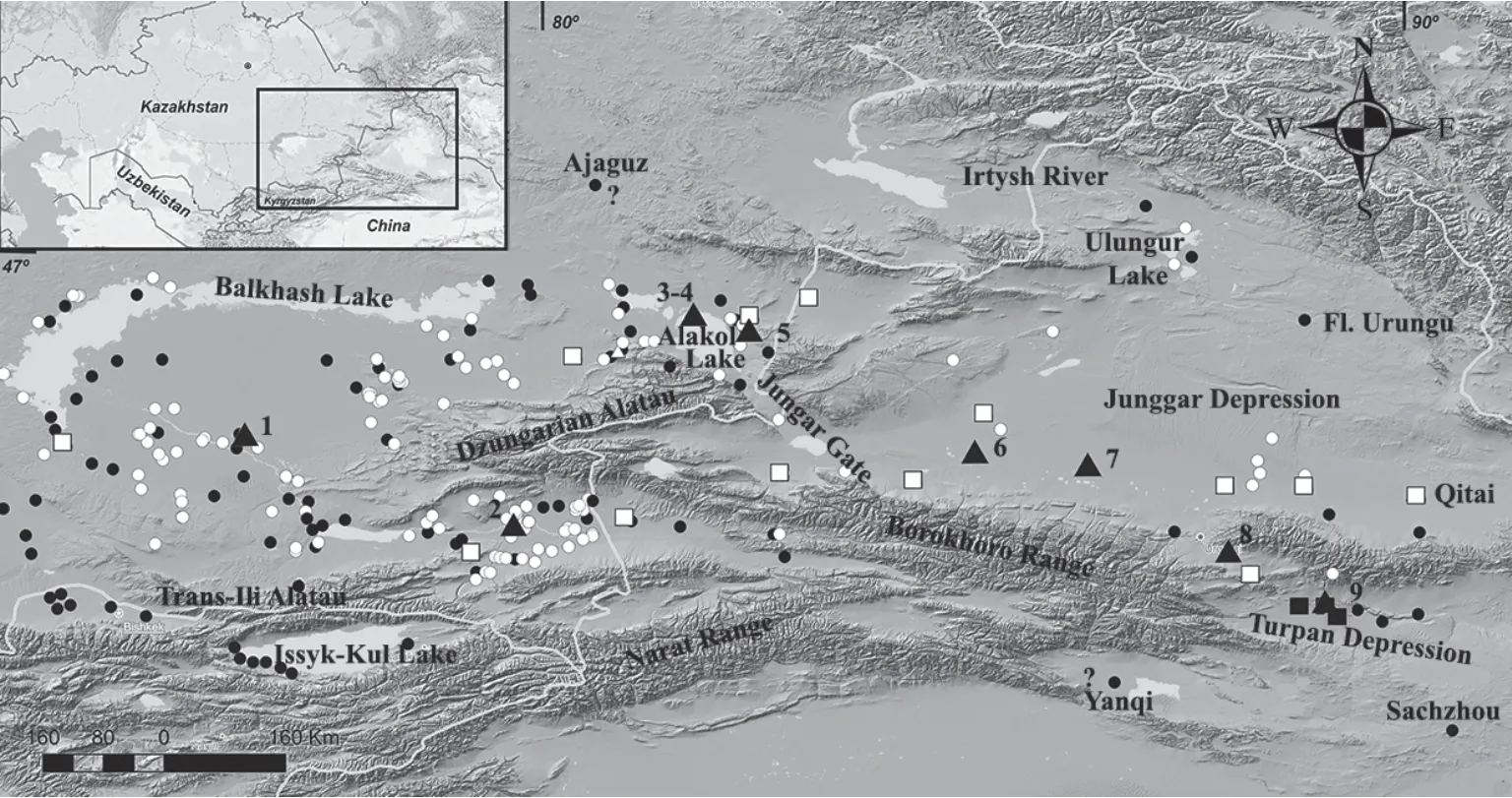
Figure 1 Distribution of the Rapid Racerunner in the eastern periphery of the range.Black triangles:samples studied(1.The right bank of the Ili River,20 km to the east of Bakanas village;2.Sands at the confluence of the Charyn River in the Ili River(Charyn);3.Alakol Lake,Kishkene-Araltobe island,1970;4.Alakol Lake,Kishkene-Araltobe island,2015;5.25 km to the southeast of Makanchi village,Barmakkum Sands;6.Kuytun;7.Shihezi;8.Urumqi city suburb;9.Turpan Depression);Black circles:literature evidence;White circles:new information(2000-2015);White squares:localities,the samples which are grouped in a clade with a nominative subspecies according to the results of molecular genetic analysis(Liu et al.,2014);Black squares:localities,samples which formed a separate clade and were identified as Eremias velox roborowskii(Liu et al.,2014).
Having an extensive range,the Rapid Racerunner displays a high morphological variability.Historically,based on the morphological characters,four subspecies are recognized within the species(Szczerbak,1974;Eremchenko and Panfilov,1999a;Sindaco and Jeremčenko,2008):E.v.velox(Kazakhstan,Central Asia republics,Dzungaria,Northern Iran);E.v.caucasia(Russia:to the south from the lower Volga to the state border,Azerbaijan and Eastern Georgia);E.v.roborowskii(China:foothills of western Nanshan,Hami desert,Turpan Depression);andE.v.borkini(Kyrgyzstan:Issyk-Kul Depression).Nevertheless,it should be noted that Liuet al.(2019)has challenged our understanding of the taxonomy and distribution of different subspecies inE.veloxsensu lato on the basis of phylogeographical analysis of mtDNA sequences.Thus,one of the relevant issues of the intraspecific taxonomy ofE.veloxsensu lato still remains regarding the taxonomical relation of the populations inhabiting Southeast Kazakhstan and neighboring Xinjiang,China.
TheE.v.roborowskiisubspecies was described by Bedriaga(“1905”1907)on the basis of the specific coloration patterns among specimens from the“Ssatschsheu”(=Dunhuang,Gansu Province)and“Luk-Tschun”(=Lükqün,Turpan Depression,Xinjiang Uygur Autonomous Region)localities.Later,Boulenger(1921)putE.v.roborowskiiinto the nominative subspecies;but Szczerbak(1974),after repeating the study of lizards from the same areas,confirmed the validity of the subspecies.Since the holotype was not specified in the original description,Szczerbak distinguished the lectotype and described the series again.According to Szczerbak's data,E.v.roborowskiidiffered from the nominate subspecies in a higher number of gulars and coloration patterns.Exploring the Chinese populations,Szczerbak(1975)noted that there was a significant difference in the number of femoral pores between the samples from the Ssatschsheu and Luk-Tschun,and those from Junggar Depression.However,Wanget al.(2014),after an examination of the external morphological characters of the Rapid Racerunners from five localities in Xinjiang(Ili,Turpan and Junggar depressions),did not find any significant differences between them and came to the conclusion that it was necessary to clarify the intraspecific differentiation ofE.velox.
Molecular genetic analysis of the genusEremiasby Wanet al.(2007)pointed out the existence of two subspecies in Xinjiang,i.e.,the nominative androborowskii;and Rastegar-Pouyaniet al.(2012)speculated thatroborowskiicould be observed not only in Xinjiang but also in the contiguous territory of Kazakhstan.In the latter case,however,the authors themselves recognized the selective nature of theroborowskiiclade,which included individuals from populations geographically remote from each other,i.e.,from the Aral Sea to East Kazakhstan.Rastegar-Pouyaniet al.(2012)associated these results with the gene flow betweenroborowskiiand the nominative subspecies.A more recent molecular genetic study using material from the type territory showed that Southeast Kazakhstan is inhabited only by the nominative subspecies;while in XinjiangE.v.roborowskiican also be found alongside the nominative(Liuet al.,2014).
Such a disagreement on the taxonomical position ofE.veloxpopulations from Southeast Kazakhstan and neighboring Xinjiang motivated us to conduct a detailed study of lizard morphological variation,to elucidate its variability and to clarify the taxonomical position of the certain populations.First,we implemented an analysis of geographical distribution of this species in the eastern part of its range to reveal the isolated populations and to check whether there is some exchange between them.Second,we evaluated the variability of the morphological characters for these populations,considering a sexual dimorphism,an inter-character correlation and substrate preferences of the lizards.We also examined a possible association of phenotypic variation with geographical isolation,with special attention to the most remote and isolated population from the Turpan Depression in Xinjiang.
2.Materials and Methods
2.1.DistributionA map with the occurrences ofE.veloxwas constructed in the ArcMap environment to analyze the distribution of the Rapid Racerunner(Figure 1).The basis of the map was literary materials(Szczerbak,1974;Yao,1983;Zhao,1985;Wang and Autumn,1990;Brushko,1995;Daiet al.,2004;Chirikova,2007;Wanet al.,2007;Liuet al.,2014;Wanget al.,2014),supplemented by information from the collections of the Chengdu Institute of Biology(CIB)and the Institute of Zoology of the Republic of Kazakhstan,as well as data personally collected during field survey in 2013-2015.The geographical coordinates of the localities of lizard finds were determined with GPS(recent data)or restored by topographical maps(old data).
2.2.MorphologyFor exploring morphological variability ofE.veloxin the eastern periphery of its range,we sampled a total of nine populations in the intermountain and foothill depressions of Xinjiang and Southeast Kazakhstan(Figure 1):1.the right bank of the Ili River,20 km to the east of Bakanas village(12 males)(Bakanas);2.sands at the confluence of the Charyn River in the Ili River(Charyn)(6 males,14 females);3.Alakol Lake,Kishkene-Araltobe island,1970(15 males,6 females);4.Alakol Lake,Kishkene-Araltobe island,2015(11 males,3 females);5.25 km to the southeast of Makanchi village,Barmakkum Sands(Makanchi)(11 males,6 females);6.Kuytun(6 males);7.Shihezi(8 males,6 females);8.Urumqi city suburb(8 males,4 females);9.Turpan Depression(19 males)(see Appendix 1).
To characterize morphological diversity within and divergence across populations,we considered 12 morphological characters,which have been extensively used for the systematics of the genusEremias(e.g.,Szczerbak,1974):1.L.(Longitudo corporis)-snoutvent length;2.L.cd.(Longitudo caudalis)-tail length(from cloaca to the tip of the tail);3.L.c.(Longitudo capitis)-head length;4.Lt.c.(Latitudo capitis)-head width;5.P.p.(Pes posterior)-hind limb length(from the glenoacetobular cavity to the base of the claw of the 4th finger);6.Sq.(Squamae)-the number of scales around midbody;7.Ventr.(Ventralia)-the number of transverse rows of thoracic and abdominal scales;8.G.(Gularia)-the number of gular scales along mid-line of throat;9.P.fm.(Pori femoralis)-the number of femoral pores;10.Sq.cd(Squamae caudalis)-the number of scales around the 9-10th tail ring;11.the number of enlarged preanal scales;12.the characters of the head pholidosis,with a special attention to presence/absence of the granules around the supraoculars,which demonstrating a geographic variability(Chirikova,2004).
Different coloration patterns were identified on the basis of the location of the spots,their sizes,as well as the stripes on the upper back and size and the location of pigmented spots on the sides of the trunk.For comparison between the populations,the male individuals were used.To calculate the similarity of populations by phenes(coloration patterns in our case),the index proposed by Zhivotovsky(1982)was used,which has been successfully applied for phenotypic comparison in other vertebrate groups(e.g.,Korablevet al.,2011;Korablevet al.,2014).
r=√p1q1+√p2q2+√pmqm,whereris the population similarity index,andpis the frequency of the phene in the first population,andqis the frequency of the phene in the second population,andmis the number of phenes.
2.3.Statistical analysisStatistical analyses were performed using the STATISTICA 10 software(Statsoft,1999-2019).The critical value of statistical significance when checking for null hypothesis was set at 0.05.If the significance value of the statistical criterion for the measure was exceeded,the null hypothesis was applied.The normality of the distribution of quantitative characters in the separate comparison groups was checked using the statistical criteria of the Kolmogorov-Smirnov and Shapiro-Wilk tests.Both tests are capable of revealing significant differences between the pattern of character distribution and the normal distribution model wherein Shapiro-Wilk test is especially powerful to check the normal distribution in the limited samples(Kendall and Stuart,1973;Afifi and Azen,1982).The hypothesis of normality of the distribution is accepted if the criterion gives a value of more than 0.05 in a particular subgroup of observations.Arithmetical means and mean squares(standard)of errors of mean,as well as the coefficient of variation,are calculated for all the quantitative characters in the comparison groups.The mean values and confidence intervals are presented in graphical form.Descriptive statistics are presented in the text asX±SE,whereXis the mean,and theSEis standard error of mean.To compare the central characters of the groups,parametric and nonparametric methods were used:ANOVA analysis of variance,including by means of the Kruskal-Wallis test and Wilcoxon signed-rank test,median test and the Van-der Waerden test(Kendall and Stuart,1973;Afifi and Azen,1982;Kimet al.,1989).To assess the correlation between a pair of quantitative indicators,Pearson and Spearman correlation coefficients were used.A scatterplot was used for the graphical presentation.
A cluster exploratory analysis was undertaken,which main aim was to identify latent groups of observation(Ledermann and Lloyd,1984;Kimet al.,1989).The cluster analysis is to split a set of observed objects and characters into different groups or clusters so that the members of each group are similar to one another.Given the differences in the scales of the analyzed characters,all the quantitative characters were transformed into one standard form before conducting the cluster analysis.As a result,all the characters in the new scales had zero mean and standard deviation equaled to one.A hierarchical clustering algorithm was used to conduct a cluster analysis with these new variables.Hierarchical cluster analysis with a dendrogram allowed the number of allocated clusters to be visually determined.In both cases,the Ward's algorithm was used(Kimet al.,1989)and the Euclidean metric was applied(Ledermann and Lloyd,1984;Kimet al.,1989).
3.Results
3.1.Distribution and habitatsThe Rapid Racerunner is widespread in Southeast Kazakhstan,North and East Xinjiang(Figure 1).During field studies,we recorded encounteringE.veloxin the Balkhash,Alakol and Ili depressions,Sogetinsk plain,Junggar Gate,Junggar Depression as well as the southern foothills of Bogda Shan range at the periphery of the Turpan Depression.
The dominant type of biotope for the Rapid Racerunner in Southeast Kazakhstan,North and East Xinjiang can be categorized as fixed sands and the surrounding clay plains.This is present in the Alakol Depression(Makanchi)in the pit-and-mound sands withArtemisiasp.,Astragalussp.and gramineous plants;in the Ili Depression(Charyn)-at pit-and-mound sands with bushesHaloxilonsp.andCalligonumsp.and at salt marshes in the lowlands.
In the Shihezi area(in the Junggar Depression),the Rapid Racerunner inhabits small areas of fixed sands withHaloxylonsp.,Ceratoidessp.andArtemisiasp.bushes and at the clay salt marshes in the lower parts between the barchans;and in Kuytun-at the ridged barchans and in the lower parts between the barchans withHaloxylonsp.andArtemisiasp.In the Turpan Depression,the biotopes of the Racerunners are represented by the sandyloessic and gravel-loessic plains(type territory:Shanshan County),low barchans with fine gravel andCalligonumsp.,Alhagisp.,Tamarixsp.andHaloxylonsp.for the dominants,at the foot of the mountains-Gobi-type gravel deserts.The racerunners' habitats in Xinjiang were fragmented drastically due to industrial activity.
On the islands of Lake Alakol,the Racerunner inhabits rubble-loam plains and sabulous-rocky slopes of low hills with vegetation ofArtemisiasp.,Atraphaxissp.,Salsolasp.and gramineous plants in different combinations(Kubykin,1975;our data).The ground has a darker coloring because of the typical desert ground conditions forming its rubble-rocky soil.
3.2.Morphological analysisA test of the hypothesis of normality showed that most of the morphological characters had a normal distribution.The results of the test are given in Table 1.The Shapiro-Wilk test,as the most reliable statistic criterion for small number of observations,revealed the normal distribution for L.,L.cd.and P.fm.for all the samples.The Shapiro-Wilk test rejected the hypothesis of normal distribution for L.c.(No.6),Lt.c.(No.9),L.p.(Nos.8,9),Sq.(No.4),Ventr.(Nos.2,3,5),G.(No.8),Sq.cd.(No.6).Since for the other characters the normal distribution was not detected for all samples,we used both parametric and nonparametric methods of analysis.
3.2.1.Sexual dimorphismSignificant differences(P=0.0002,P=0.0001)between males and females were identified for the linear biometric characters such as the head length and width,the hind limb length.The diagram showing the sexual dimorphism of linear and pholidosis characters is presented in Figure 2.Males had higher values for the mentioned characters.In addition,males had a greater relative length of hind limbs(P=0.01)and tail(P=0.001).
The scalation(pholidosis)characters in our samples did not exhibit significant sexual differences.Previously,when describing theE.v.veloxandE.v.caucasiasubspecies,Szczerbak(1974)noted sexual dimorphism for Ventr.and Sq.cd.and in his later work(Scerbak[=Szczerbak],1981)for Sq.and P.fm.Sexual dimorphism was observed in the coloration patterns on the upper and side parts of the torso.Over 80% of females had lightcolored stripes on their back.We have not observed specimens with pigmented spots on the back.The upper row of white pigmented spots,which on some specimens merged into stripes,was vaguely seen on the sides of the torso.The bottom row of colored pigmented spots was weakly pronounced and could be seen reaching only the middle of the side.The coloration pattern on the backs of the males significantly varied and usually took the shape of spots.The rows of pronounced white and colorful spots on the sides of the torso reached the base of the thighs.
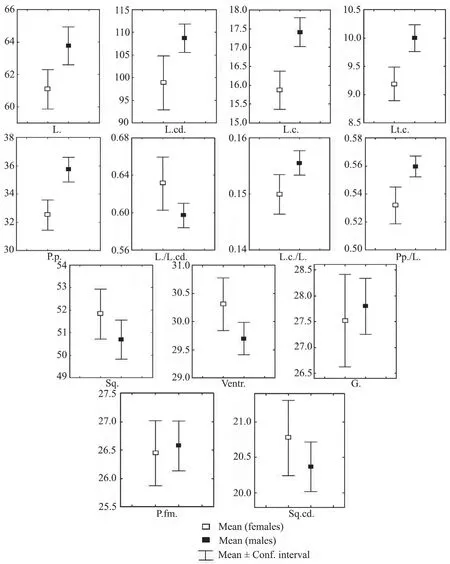
Figure 2 Sexual dimorphism in linear and pholidosis characters of Eremias velox.L.:snout-vent length;L.cd.:tail length;L.c.:head length;Lt.c.:head width;P.p.:hind limb length;Sq.:the number of scales around midbody;Ventr.:the number of transverse rows of thoracic and abdominal scales;G.:the number of gular scales along mid-line of throat;P.fm.:the number of femoral pores;Sq.cd:the number of scales around the 9-10th tail ring.
3.2.2.Correlation of charactersA positive correlation among all linear biometric characters was observed in both sexes.The values of the Spearman and Pearson coefficients are listed in Table 2.The correlation among the scalation characters was either very weak or absent.Characters correlation analysis in each population confirmed the presence of positive correlation between linear biometric characters in all the populations.Analysis of the scalation characters demonstrated a weak correlation between Sq.and G.(on both coefficients)and Sq.and Sq.cd.(on Spearman).As an example,we provided the scatterplot with relationship between L.and L.c.and between Sq.and G.in Figure 3.
3.2.3.Variability of linear biometric charactersGiven pronounced sexual dimorphism across the linear biometric characters,we have conducted comparison only among males.We have provided differences in the size of specimens from different populations.However,these differences did not reach a statistically significant level(P>0.05).Specimens from Bakanas(No.1)demonstrated high average values on linear biometric characters,as well as specimens from Xinjiang(Nos.6-9)(Figure 4A,C,D).
3.2.4.Variability of scalation charactersAlthough our material did not exhibit statistically significant sexual differences in pholidosis,we took into account data from previous researchers(Szczerbak,1974;Scerbak[=Szczerbak],1981;Shammakov,1981)and considered only males.
The majority of the scalation characters from Southeast Kazakhstan and Xinjiang was considered to be overlapping with each other;but there were some differences.The comparison showed that the Xinjiang racerunners(excluding the Turpan samples)had a higher count of Sq.(54.8-55.7)in comparison with others(46.5-52.1).Specimens from the Turpan(No.9)had statisticallylower counts for this trait:49.36(P=0.0001:Figure 4E).A higher count of G.(28.2-29.6),in comparison with populations in Kazakhstan(25.0-27.57)excluding Charyn,was observed among all populations in Xinjiang.However,this difference was statistically significant only when conducting pair-wise comparison of specimens from Shihezi and Makanchi(Р=0.0008),and Turpan and Makanchi(P=0.002)(Figure 4F).In the Kazakhstan populations,the highest count for gular scales was observed among specimens from Charyn,but only when conducting pair-wise comparison with the Makanchi specimens(P=0.002).Additional analysis of six specimens from the Horgos population(geographically located between the populations from Charyn and Xinjiang),did not reveal a high average count for G.;but there were individuals with the maximum G.count[26-33(27±1.11)].The Xinjiang populations(Nos.6-8)differed,with higher counts of mean Sq.cd.(27.6-29)compared to the rest of the populations(25-26.8),although the difference was not statistically significant.Specimens from the Lake Alakol islands(Nos.3-4)and Turpan were characterized by low average values of femoral pores(20.06,20.0 and 19.05,respectively;Figure 4G).
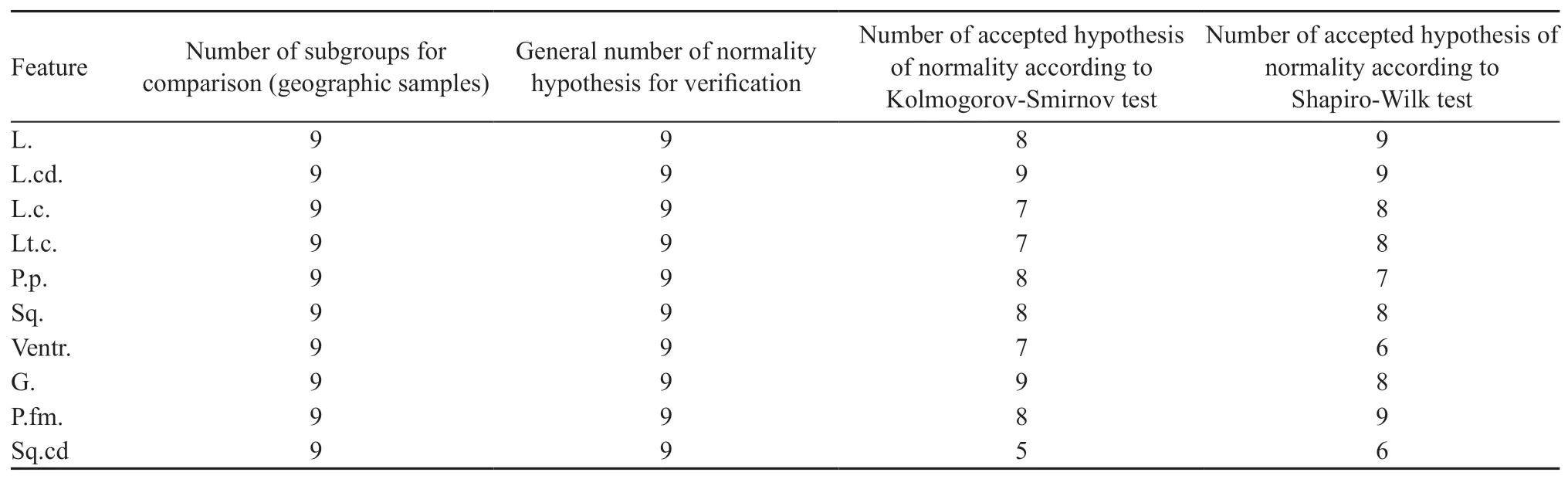
Table 1 Test of normality of distribution of the morphological characters studied.
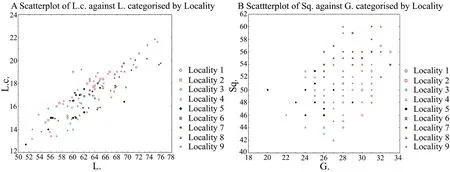
Figure 3 Example of correlation of linear(A)and pholidosis(B)characters in the studied samples of Eremias velox.L.:snout-vent length;L.c.:head length;Sq.:the number of scales around midbody;G.:the number of gular scales along mid-line of throat.
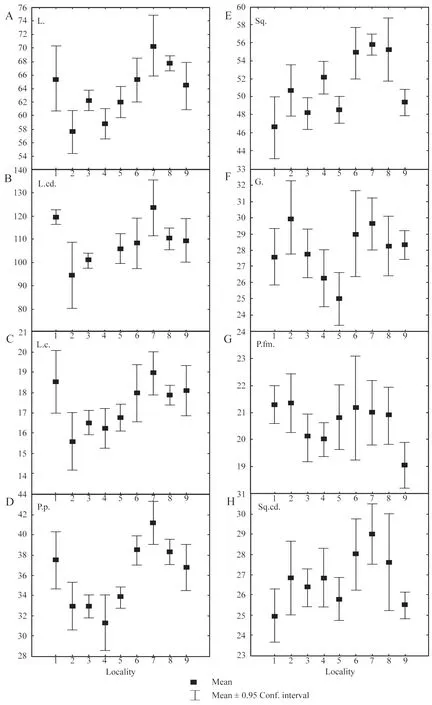
Figure 4 Variability of linear(A-D)and pholidosis(E-H)characters of male Eremias velox.L.:snout-vent length;L.cd.:tail length;L.c.:head length;P.p.:hind limb length;Sq.:the number of scales around midbody;G.:the number of gular scales along mid-line of throat;P.fm.:the number of femoral pores;Sq.cd:the number of scales around the 9-10th tail ring.
Analysis of the discrete scalation characters of the head and preanal area showed differences between specimens on two characters-the shield pattern in the preanal area and the presence or absence of granules around supraocular(Table 3,Figure 5).72.7% of the Turpan racerunners had one enlarged preanal scale.Specimens from most populations had an incomplete row of granules scales between the supraocular and frontal scales.The largest number of specimens with the full row of granules was observed in the Bakanas population(No.1);the prefrontal scales of specimens from Turpan(No.9)and the Lake Alakol islands(No.3)were not separated by the granules(43.75%;Table 3).The remaining percentage of specimens,whose frontal scale was not separated by the granules,amounted to 0-22.2%.
Cluster analysis of five scalation characters(Sq.,G.,P.fm.,Sq.cd,Ventr.)showed that there was no clear differentiation between the studied populations(Figure 6).All clusters comprised specimens of various populations that were presented in different proportions.However,Xinjiang populations made up more than half of the first cluster:66% if including the Turpan specimens,while 54% if excluding the Turpan samples.The second cluster was represented by two subclusters.In addition to the above,specimens from the Lake Alakol islands(38.6%)made up the majority of the first subcluster(I);and in the second subcluster(II)there were more specimens from the Turpan Depression(35.7%)(Figure 6).
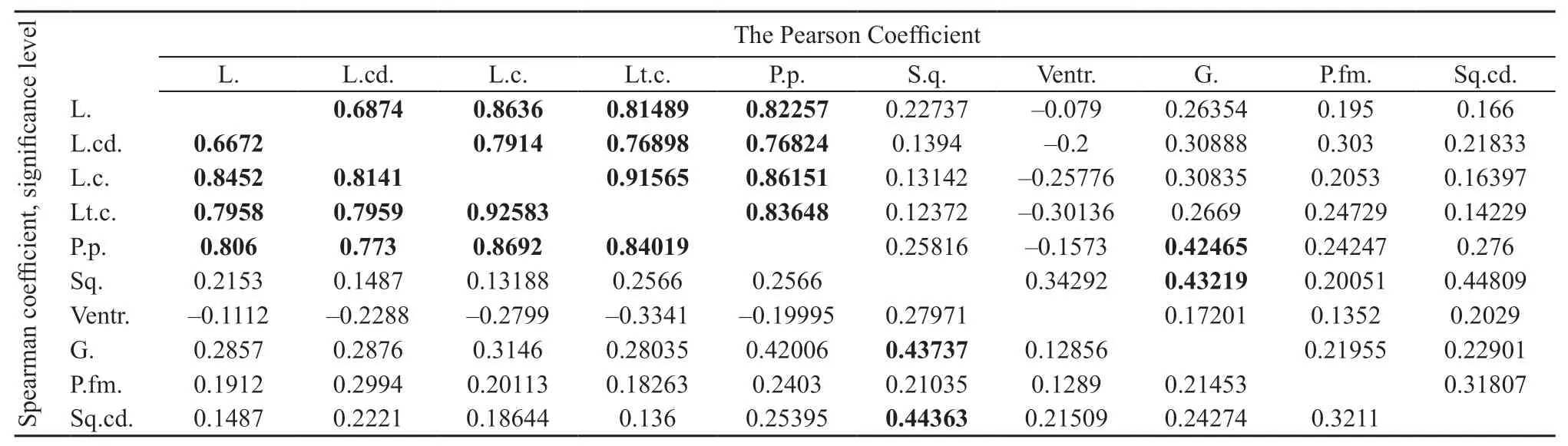
Table 2 Correlation of morphological characters of Eremias velox(characters with a correlation equaling to P<0.0001 are given in bold)
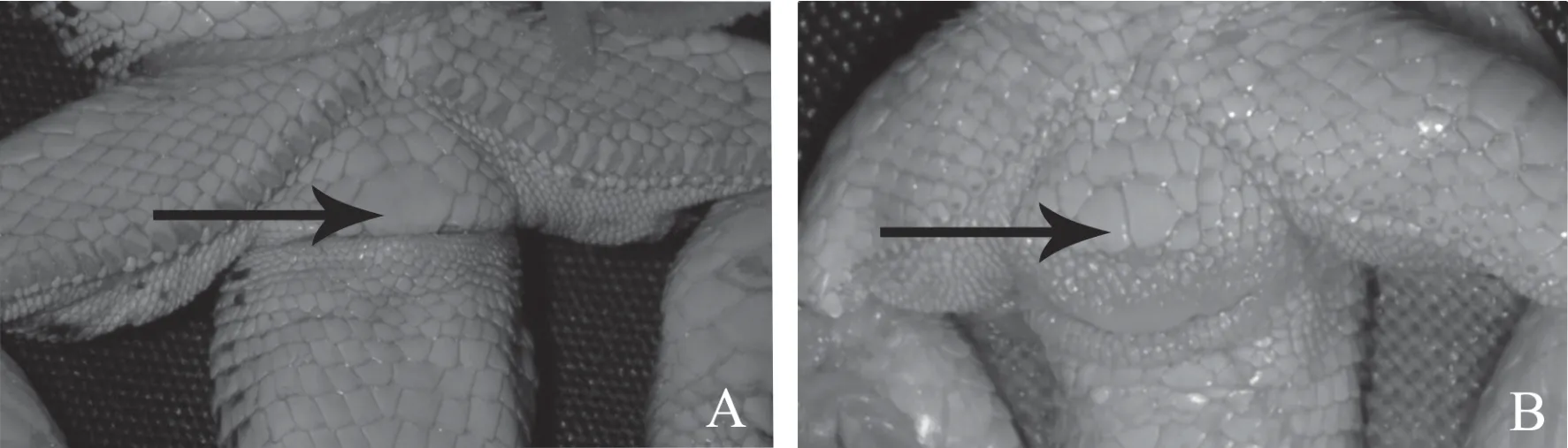
Figure 5 Preanal area shield pattern variations among the Rapid Racerunners from the eastern periphery of the range:A:one enlarged preanal scale;B:two enlarged preanal scales.
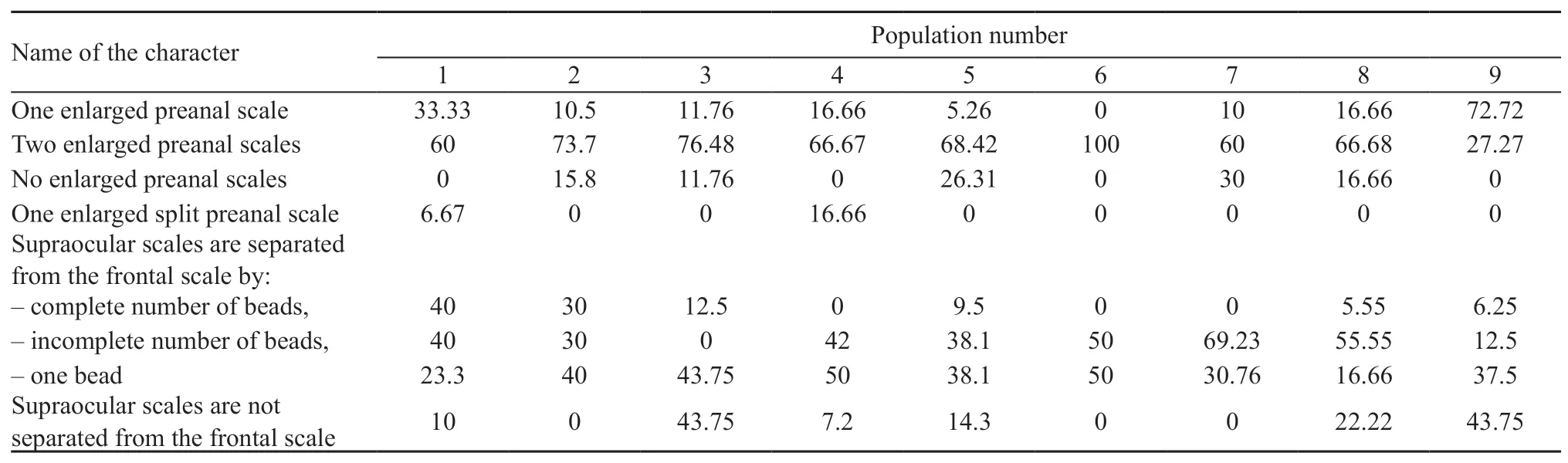
Table 3 Variability of pholidosis of the head and preanal area for male Eremias velox.See Materials and Methods for the population number.
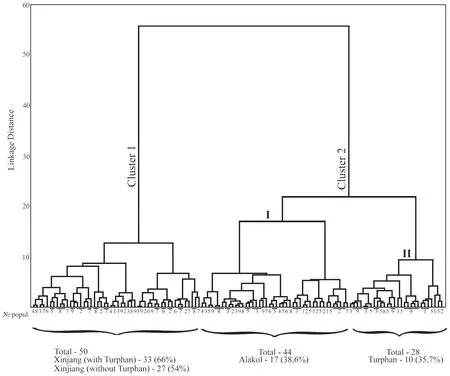
Figure 6 Cluster analysis of five pholidosis characters of Eremias velox.
3.2.5.Variability of coloration patternColoration and its pattern play an important role in studies of the intraspecific taxonomy ofE.velox.The back-coloration pattern of the nominate subspecies is characterized by the presence of variations such as one or three longitudinal dark stripes of contrasting colors,longitudinal intermittent stripes or rows of spots,and dark dots and spots.The sides of the torso often have two rows of pigmented spots,but it is not always the case.The pigmented spots of the bottom row are blue,green or light grey.
In the specimens studied,we identified 12 types of dorsal coloration patterns(Figure 7;Table 4):(1)a broad dark-brown streak between two light-colored stripes on the back;(2)large dark spots between two light-colored stripes on the back;(3)small dark spots on light-colored stripes on the back;(4)dark spots located both on the light-colored stripes on the back and between them,as well as dark spots from the edges of the pigmented spots on the sides reaching the light stripes on the back;(5)dark spots,often elongated in the transverse direction,located across the back;(6)numerous small spots and dashes on the back;(7)dark spots grouped along the light stripes;(8)light stripes split into elongated spots;(9)two rows of pronounced pigmented spots;(10)light stripes absent and the coloration in the form of occasional small specks or dots;(11)similar to type 2,but all dark elements of coloration poorly pronounced;(12)very rare dots between the light stripes.
As shown in Table 4,11 types of coloration pattern were observed among males,and nine types were noted among females.At the same time,except for a quarter of females from Urumqi(No.8),in all other populations,only males had the types of coloration patterns without stripes or with weakly pronounced stripes:spotted,speckled and dotted patterns(Figure 7:4,5,6 and 9;Table 4).
A relatively high diversity in coloration pattern was noted among most of the males,only except for some populations from the Lake Alakol islands,Shihezi and Turpan,in the studied populations,as well as across the populations(Table 4).The males from the Lake Alakol islands,Shihezi and Turpan in contrast demonstrated high uniformity of the pattern at intra-population level.The females of Alakol islands and Makanchi samples shared high uniformity of patterns(Table 4).Male individuals from the Alakol islands,collected in different periods,showed a high similarity between each other(r=0.9)and,to a lesser extent,with the geographically nearest population of Makanchi(r=0.74)(Table 5).The Turpan individuals differed with a peculiar pattern,which was not registered in any other studied populations.
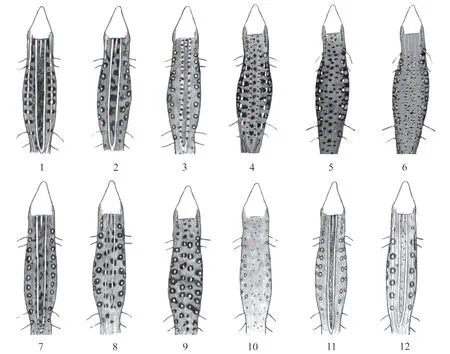
Figure 7 Variation of the coloration patterns on the upper torso of Eremias velox in the eastern periphery of the species range.
Specimens with a pattern having pigmented spots along the middle of the back dominated among the males of four populations(Bakanas,Charyn,Kuytun and Shihezi:Nos.1,2,6 and 7,respectively),and other types of pattern were dominant in the remaining populations(Figure 7:9;Table 4).The similarity index(r)for the coloration patterns among the populations compared ranged from 0.57 to 0.82(Table 5).The bottom row of blue and green spots was well pronounced only up to the middle of the body among the majority of adult specimens from the populations.The main background of the body was gray or grayish-brown.
In the populations from the Lake Alakol islands(Nos.3-4),collected at different times,specimens of both sexes were characterized by a dark-grey background to the upper torso and a striped pattern(Figure 7:1).Among some males and females,the top row of side spots on the torso took a shape of a light-colored stripe,the appearance of which varied between different individuals.There were no spots on the pileus.
Specimens from the Turpan Depression had a peculiar type of coloration pattern.The upper part of the back for the males was covered with many spots;and most spots were randomly scattered and elongated in the transverse direction(Figure 7:5 and 6;Table 4).The coloration pattern on the sides had white spots surrounded by large black spots on the upper row and large blue spots on the bottom row,which almost reached the hind limbs.Our calculations indicated that the largeness of the blue spots was determined by the scale counts that make them up,compared to specimens from other sites.Thus,for the Turpan specimens,the blue spots on the middle of the torso were composed of at least 10 scales,while the count for the specimens from other sites was 2-8 scales.And the spots in the forelimbs area covered 20-40 and 12-20 scales,respectively.The Turpan specimens were distinguished by rust-brown coloration of the upper torso and absence of bright distinct spots on the pileus.
4.Discussion
4.1.DistributionOur field data showed thatE.veloxhas more dense spatial distribution within the eastern periphery of the species range than it was recognized before(Figure 1).The most northernE.veloxfound in the eastern periphery of the range was known from the collections of A.A.Kushakevich(ZISP No.6837)in the Sergiopole suburbs(now Ayaguz town)(Nikolsky,1905).Our survey of the territory led us to assume that this finding does not belong to the city suburbs with a steppetype landscape,but to the valley of the southern course of the river with the same name,where on the left bank Karakum sands are located.Thus,the northern boundary of theE.veloxdistribution in the eastern periphery of the range in the Kazakhstani territory lies within the northern border of the Balkhash and Alakol basins(Figure 1).
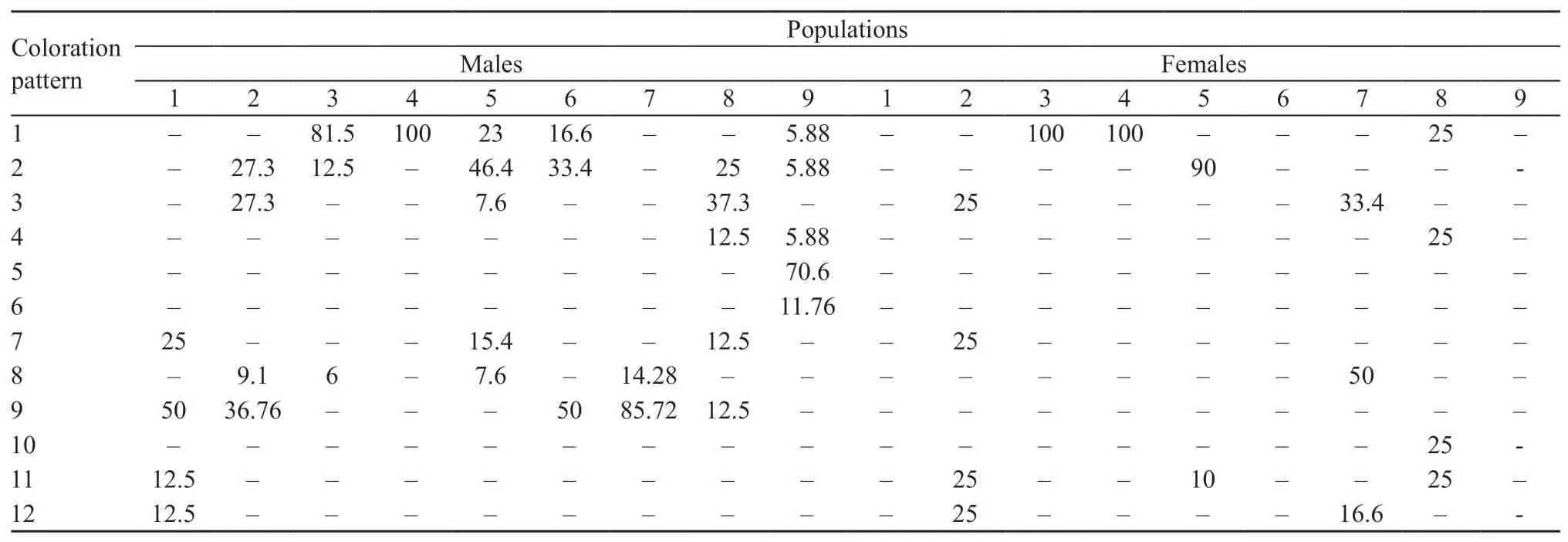
Table 4 Occurrence of different types of coloration patterns of Eremias velox in the studied populations(in %).See Materials and Methods for the sample number.Types of coloration patterns refer to Figure 7.
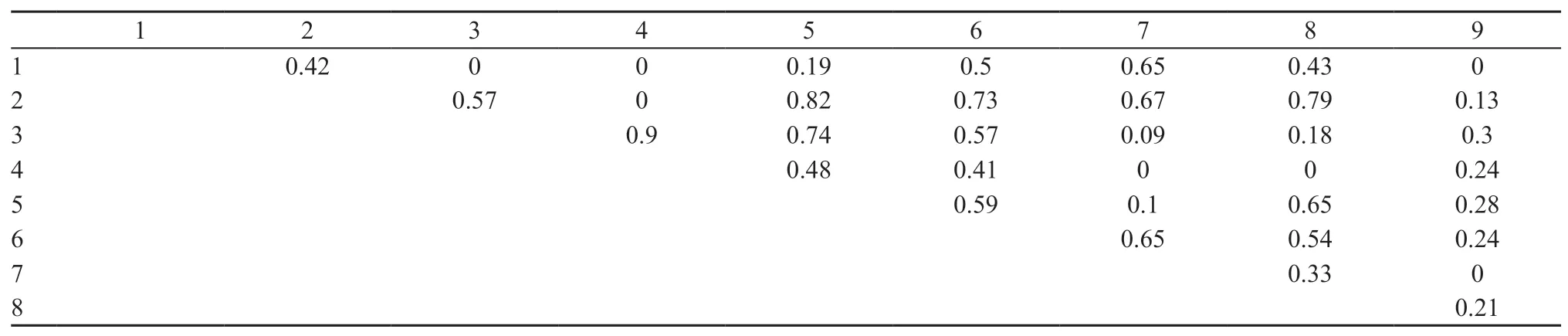
Table 5 Similarity of samples by coloration pattern(males).
The most reliable northern,eastern and southern records ofE.veloxin the eastern periphery of the species range,except forE.v.borkiniin the Issyk-Kul Depression,lie in Xinjiang(Figure 1).They come from Buerjin at the Black Irtysh River(Shiet al.,2002;Wanet al.,2007);the Ulungur Lake Basin(first finds in 1878-1879[Potanin,ZISP No.5173 and Przhewalsky,ZISP No.6548:Szczerbak,1974]confirmed by us in 2014);Qitai County(Wanet al.,2007;Liuet al.,2014)and the Turpan Depression,where,since the first discoveries in 1895(Roborowski and Kozlov,ZISP Nos.9130,9132:Szczerbak,1974),the presence of a resident population was registered repeatedly(Wang and Autumn,1990;Shiet al.,2002;Liuet al.,2014;Wanget al.,2014).The northern boundary of the distribution ofE.veloxin the eastern periphery of the range generally coincides with the northern border of the desert landscape zone,occupying the Southeast Kazakhstan and Xinjiang on 46°40'-47°40' N(Zaychikov,1964;Medeu,2010).
It is necessary to dwell on some dubious records of racerunners found far from the described areas of Xinjiang and identified asE.velox.The Ssatschsheu(=Dunhuang)locality(1894,Roborowsky and Kozlov,ZISP No.9198:Nikolsky,1905)is probably an error forE.veloxbecause numerous new racerunner collections from the Dunhuang region have produced onlyEremias vermiculata.By reasoning similar to that in Maceyet al.(1997)onTeratoscincus roborowskii,the only record labeled from Dunhuang is probably the result of mixing of museum labels during examination of specimens collected by the Russian Geographic Society Expedition to Central Asia in 1893-1895 under the leadership of V.I.Roborowski(Kozlov,1899).The route of this expedition(Figure 6 in Maceyet al.,1997)passed through the Turpan Depression in September 1893 and September 1895.The expedition stayed in Ssatschsheu(Dunhuang)during the spring of 1894.We suggest that the specimen ZISP No.9138 could have been collected together with ZISP No.9130 and ZISP No.9132 by V.I.Roborowski and P.K.Kozlov during the last part of their expedition between the Hami Oasis and Turpan Depression.It should be mentioned that Yao(1983)documented the Rapid Racerunner in the vicinity of Dunhuang in Gansu Province,China.It is most probable that some individuals ofE.vermiculatawere incorrectly assigned toE.veloxin Yao(1983).Zhao's(1985)finding ofE.veloxin the Tarim Basin(Lop,Hotan and Yecheng),according to Eremchenko and Panfilov(1999b),appeared to result from his inability to distinguish betweenE.veloxandEremias buechneri.Surprisingly,the specimen(voucher number CIB3558)from Yanqi County in Xinjiang was recorded asE.veloxin Daiet al.(2006).After carefully revisiting that specimen(CIB3558),we confirmed its identity to beEremias multiocellatacomplex rather thanE.velox.
According to the updated data,the eastern periphery ofE.veloxrange is limited to the northern borders of the Balkhash and Alakol basins and the valley of the Black Irtysh River in the north;the eastern borders of the Junggar and Turpan depressions in the east;and the northern foothills of the Tian Shan-Borohoro,Ketmen and Trans-Ili Alatau in the south.
In our opinion,at least four geographically isolated populations with different levels of isolation can be distinguished within the area described.One of them inhabits the Turpan Depression,which has undergone a deepening isolation since Neogene(Murzayev,1966).This population is separated from the species' main range by extreme eastern spurs of the Eastern Tien Shan-Borokhoro,Iren-Khabyrga,Jarges and Bogda-Shan(or Bogda-Ula)ridges.The second isolated population inhabits the Junggar Depression surrounded by the ridges of different mountain systems and formed in Late Pliocene and Pleistocene(Mamedov and Novikov,2015).There are some ways of possible contact between the populations of the Junggar Depression,Balkhash and Alakol basins.There are the Junggar Gate and the intermountain depression between the Urkashar and Birliktau mountains.The third isolated population inhabits one of the islands of Alakol Lake.It has very recent appearance because the island itself was formed no more than 150 years ago(Kurdukov,1951).The fourth exists in Issyk-Kul Lake Basin.It is separated from main species range by the ridges of the Northern Tien-Shan,which has acquired its highland appearance in Middle Pliocene(Burtman,2012).
4.2.HabitatsAn analysis of the biotopes of the Rapid Racerunner showed that the fixed sands dominated among the preferable habitats ofE.veloxin the eastern periphery of its species range,which generally confirmed Szczerbak's(1974)observations.Nevertheless,the species does not avoid the clay and debris substrates as was noted for the Ili Basin(Korelov,1948;Ananjeva,1976;our data)and Alakol Lake islands(Kubykin,1975;our data),as well as gravel-loessic substrate which is typical for the Turpan Depression(our data).
4.3.Morphological variabilityWe found a sexual dimorphism in the linear biometric characters ofE.veloxwhich was earlier shown by Ananjeva(1995,2003)for Bakanas population.The males from the European populations also have a larger head size and longer tails and limbs(Scerbak[=Szczerbak],1981).Scalation characters in our material did not demonstrate significant sexual differences.Previously,when describing theE.v.veloxandE.v.caucasiasubspecies,Szczerbak(1974)noted sexual dimorphism for Ventr.and Sq.cd.and in his later work(Scerbak[=Szczerbak],1981)for Sq.and P.fm.The males in the Turkmen populations demonstrated dominance across all main diagnostic pholidosis characters(Shammakov,1981).However,none of the authors specified the level of statistical significance of the differences.
Intraspecific morphological variability may be common in species inhabiting heterogeneous environments(Ghalamboret al.,2007),especially if the dispersal capability is relatively limited and the gene flow between populations is low(Slatkin,1987).Our results indicate certain similarities and differences in the phynotype of the Rapid Racerunner populations inhabiting the intermountain and foothill basins in Southeast Kazakhstan and Xinjiang.The samples from Xinjiang(excluding the Turpan Depression)were characterized by aboveaverage values for linear biometric characters,the number of scales across the torso,the scales around the 9-10th tail rings,as well as the guttural scales.The length of the head,torso and hind limbs of specimens from the Xinjiang populations confirm the general picture of cline variability for the linear biometric characters ofE.velox.Most of the populations studied had differences in the dominant type of coloration patterns and scalation characters,not statistically supported in all cases.The noted originality in the morphology of the Xinjiang populations can be explained to some extent by the longduration and distinct geographical isolation from the surrounding territories of Xinjiang(Murzayev,1966).The Turpan and Alakol island lizards were characterized by the biggest differences in morphological features.The specimens of most populations(mainly the males)differed by high diversity of the dorsum coloration.The specimens in Alakol island and Shihezi populations were visibly uniform in dorsum coloration but such patterns were also found in other populations(Table 4).More than 70% of the Turpan specimens were recognized by uniform unique coloration pattern.
It is known that the coloration and the pattern of the Racerunners of theEremiasspecies(Kerzina,1950;Szczerbaket al.,1993;Orlova and Terbish,1997),includingE.velox(Elpatjevsky,1903;Dinesman,1953;Szczerbak,1974),correlate with the characteristics of the substrate.On the other hand,the character(i.e.,percentage ratio of coloration patterns)is widely used in the intraspecific taxonomy ofE.velox(Szczerbak,1974;Shcherbak[=Szczerbak],1975;Eremchenko and Panfilov,1999a).As we know,the dominant type of biotope of the Racerunners from the studied populations was fixed sands with the surrounding clay plains.The racerunners from the Alakol islands,inhabiting the rubbleloam plains and sabulous-rocky slopes of low hills with a predominance of dark rocks,covered with desert dust,stand out in this regard.The racerunners had noticeably darker coloration,having the smallest size and most welldefined striped pattern on the backs of individuals of both sexes,compared to individuals from other populations.A similar striped pattern on the back was also noted by us among males from the Makanchi,Kuytun and Turpan populations;but the percentage of the specimens was low(Figure 7:1;Table 4).
Along with some scalation characters(the low number of femoral pores and a higher percentage of individuals with an enlarged preanal scale),the particular traits of the Turpan specimens are manifested in their coloration pattern.At one time,this provided a reason for Bedriaga(1912)to identify,and later to Szczerbak(1974)to restore,the subspecies ofE.v.roborowski.The Turpan Racerunners have a well-pronounced transverse-spotted coloration pattern of the back and very big blue spots on the sides.As far as we know from the literature,such a coloration pattern is extremely rare forE.velox:it was observed only among single cases from Turkmenistan,Uzbekistan and Kazakhstan(Szczerbak,1974;Chirikova,2004;our data).An unusual rust-brown coloration pattern of the upper torso of the Turpan Racerunners might be determined by a common substrate of the Turpan Depression represented by reddish concentrations of loess.
When exploring the morphological characters ofE.veloxfrom the Turpan Depression,Wanget al.(2014)used a difference coefficient(Mayret al.,1953)and showed that none of the pholidosis characters reached the adopted indicator of subspecies differences.Nevertheless,Mayr and Ashlock(1991)indicated that interpopulational comparison should be carried out by taking into account a controversial picture of the morphological variability of the characters and the degree of isolation of the studied species.The Turpan Depression,which started its formation in the Cretaceous period,became significantly deeper and more isolated with the growth of the surrounding ridges in the Neogene(Murzayev,1966).According to the results of molecular-genetic study(Liuet al.,2014),the haplotypes of the Rapid Racerunner from the Turpan Depression constitute an independent basal clade,which is quite far from the haplotypes of the Junggar,Ili and Alakol depressions and the southern Balkhash region.An example,confirming the originality of the inhabitants of the isolated Turpan,is an independent evolving lineage(species)of the Roborowsk's Plate-tailed GeckoTeratoscincus roborowskii(Maceyet al.,1997;1999;2005).
Zoologists have long recognized that populations of a species occurring in different habitats may exhibit alternative forms(Mayr,1942).Such morphological differences among habitats may confer selective advantages to individuals inhabiting physically different environments.In our opinion,the geographical isolation ofE.veloxin the Turpan Depression,the second lowest point in the world(-150 m)being separated before the Pleistocene(Wang,1985),together with the morphological peculiarities and genetic distinctiveness of this form provide a solid basis to support its separate taxonomic status.Liuet al.(2014),using the concatenated cytband 12S rRNA gene sequences,found a significant mean genetic divergence(uncorrectedp-distance)of 9.3% betweenE.v.veloxandE.v.roborowskii.Since this work was submitted for publication,Liuet al.(2019)reported a phylogeogrpahical study complemented with ecological niche modeling onE.veloxin arid Central Asia,which further provided the molecular and ecological niche bases to recognize the Turpan populations as a distinct genetic lineage.The split betweenE.v.veloxandE.v.roborowskiiis strongly supported.These two taxa show a deep divergence estimated approximately 3 million years ago based on mitochondrial DNA data(Liuet al.,2019).The two subspecies are separated by the Tianshan Mountains(Liuet al.,2014;2019),which could act as a long-term barrier to gene flow and therefore promote speciation.The Tianshan Mountains as primary driver for isolation have been identified also for other reptiles(Maceyet al.,2005;Shi and Zhao,2011).Thus,considering the concordant molecular,ecological and morphological divergence,we suggest treatingE.v.veloxandE.v.roborowskiias distinct species,elevating the latter to species status.A full revision of the species complex,however,will necessitate inclusion ofE.v.borkini,a recently described subspecies(Eremchenko and Panfilov,1999a)not present in our study.As it is morphologically distinct from bothveloxandroborowskii,and separated from them by large mountains,it might also be characterized by a relevant genetic divergence.To decipher the evolutionary mechanisms that drive diversification inE.veloxcomplex,further study is necessary to investigate how phenotypic variation responds to genetic and environmental variability in an explicit geographical framework.
AcknowledgmentsWe thank V.LEONOV for his help in the statistical analysis of the data;Nikolai N.BEREZOVIKOV,Ilya I.KABAK and Nadir Sh.MAMILOV for additional information on peculiarities of landscapes,climate and relief of some of the regions studied,and for their critical comments,which significantly improved this manuscript.We are grateful to Valentina F.ORLOVA and Konstantin D.MILTO for opportunity to work with herpetological collections;Natalia B.ANANJEVA for their critical remarks during the manuscript preparation;Andrey V.BARABANOV for consultations on the first description ofE.v.roborowskiiand A.AUBAKIROVA and R.SIM for their help with English translation and correction.This study was supported by the Ministry of Education and Sciences of Kazakhstan(Grant No.1850/GF4),the Strategic Priority Research Program of the Chinese Academy of Sciences(XDPA20050201),the Ministry of Science and Technology of the People's Republic of China(Grant No.2015DFR30790),and the National Natural Science Foundation of China(Grant No.31672270).
杂志排行
Asian Herpetological Research的其它文章
- Molecular Cloning,Characterization and Sequence Analysis of KCNQ4 in Large Odorous Frog,Odorrana graminea
- Description of a New Species of Amolops(Anura:Ranidae)from Tibet,China
- Ecological and Geographical Reasons for the Variation of Digestive Tract Length in Anurans
- Mating Ethogram of a Video-aided Study of Mating and Parturition in Captive Chinese Crocodile Lizards(Shinisaurus crocodilurus)
- Home Range Size and Overlap of the Small Nocturnal Schlegel's Japanese Gecko(Gekko japonicus),Introduced into a City Park in Korea
- Correlation between Climatic Factors and Genetic Diversity ofPhrynocephalus forsythii
