New era for pancreatic endoscopic ultrasound:From imaging to molecular pathology of pancreatic cancer
2019-12-14LiviaArchibugiSabrinaGloriaGiuliaTestoniMiriamRedegalliMariaChiaraPetroneMicheleReniMassimoFalconiClaudioDoglioniGabrieleCapursoPaoloGiorgioArcidiacono
Livia Archibugi,Sabrina Gloria Giulia Testoni,Miriam Redegalli,Maria Chiara Petrone,Michele Reni,Massimo Falconi,Claudio Doglioni,Gabriele Capurso,Paolo Giorgio Arcidiacono
Livia Archibugi,Sabrina Gloria Giulia Testoni,Maria Chiara Petrone,Gabriele Capurso,Paolo Giorgio Arcidiacono,Pancreato-Biliary Endoscopy and EUS Division,Pancreas Translational and Clinical Research Center,IRCCS San Raffaele Scientific Institute,Milan 20132,Italy
Miriam Redegalli,Claudio Doglioni,Pathology Department,Pancreas Translational and Clinical Research Center,IRCCS San Raffaele Scientific Institute,Milan 20132,Italy
Michele Reni,Department of Medical Oncology,Pancreas Translational and Clinical Research Center,IRCCS San Raffaele Scientific Institute,Milan 20132,Italy
Massimo Falconi,Pancreatic Surgery Department,Pancreas Translational and Clinical Research Center,IRCCS San Raffaele Scientific Institute,Milan 20132,Italy
Abstract
Key words:Endoscopic ultrasound; Pancreatic cancer; Ribonucleic acid;Deoxyribonucleic acid; Mutation; Molecular; Organoid; Profiling; Personalized medicine
INTRODUCTION
Endoscopic ultrasound (EUS) was developed in the 1980s to improve ultrasound imaging of the pancreato-biliary system.The most significant technological development achieved by EUS along its history has been the linear probe,which allows a needle to be tracked in real time across the image plane into a target lesion and is the basis for all EUS-guided therapeutic procedures.Over the years,this technique has been implemented and is still ongoing today.Currently,EUS is considered an indispensable tool for the detection,characterization,and differential diagnosis of solid pancreatic lesions,including pancreatic ductal adenocarcinoma(PDAC)[1].
The reported sensitivity of EUS for detecting PDAC is between 94% and 100%[2,3].Compared with multidetector computed tomography (MDCT),EUS can detect about 14% of pancreatic cancers that were missed on MDCT[3].In particular,EUS performs better for the detection of tumors smaller than 20 mm,for which both magnetic resonance imaging (MRI) and computed tomography (CT) have higher miss rates[4-6].A meta-analysis evaluated the performance of EUS in those patients without an obvious mass on MDCT but with clinical suspicion for a pancreatic malignancy,and showed a higher sensitivity of EUS for detecting a pancreatic neoplasm[7].A direct comparison of imaging modalities in the modern era has shown that EUS identified pancreatic abnormalities in individuals considered to be at high risk for developing PDAC 43% of the time,compared with 33% and 11% for MRI and CT,respectively[8].
Besides its excellent performance in visualizing and diagnosing pancreatic lesions,EUS is mainly employed as part of the workup to obtain fine-needle aspiration (FNA)or fine-needle biopsy (FNB) material in patients suspected of having a primary tumor.Indeed,EUS-FNA has become the preferred method for acquiring tissue from pancreatic lesions,playing an essential role in the diagnostic algorithms in patients with a pancreatic mass.EUS-FNA is considered a safe procedure,and a large systematic review of more than 10,000 patients reported reassuringly low morbidity(0.98%) and mortality (0.02%) rates associated with EUS-FNA[9].To optimize tissue retrieval and in order to obtain core specimens,larger needles able to retrieve an FNB sample have been developed.To this end,a number of histology needles with changes in tip needle designs have been explored.However,one recent meta-analysis,which included prospective,randomized controlled trials and retrospective studies,showed that there was no significant difference between histology FNB and standard FNA needles in terms of diagnostic adequacy and accuracy,but FNB needle was superior for providing adequate histological tissue compared to FNA[10].
In the past few years,the advent of new combination chemotherapy regimens also used in the neoadjuvant setting have led to improvement in patients’ survival in all PDAC stages.In addition,knowledge on molecular changes associated with the occurrence and progression of PDAC has increased in parallel with the availability of data on the stratification of patients’ prognosis and possibly of response to various treatments[11].In this age of “personalized medicine”,the role of EUS for the management of PDAC is shifting from solely diagnosing the disease,staging it,and providing tissue for the diagnosis,towards acquiring material to obtain a detailed characterization of the tumor’s molecular signature,in order to select the most appropriate treatment.In the present editorial,we will discuss current knowledge regarding the use of EUS as a tool to obtain samples for molecular analyses and for the development of disease models.
Feasibility and pitfalls in obtaining pancreatic cancer DNA and RNA by EUS
Archival formalin-fixed,paraffin-embedded (FFPE) samples are a useful source of genomic DNA; nevertheless,most studies employing these samples derive from surgical specimens and are therefore representative of <20% of PDAC patients.Thus,the development of a reliable methodology for EUS-guided tissue acquisition (EUSTA),stabilization,and analysis is crucial for the development of molecular markers for clinical use.
Currently,the molecular analysis of samples acquired through EUS is performed non-routinely,either to help identify a PDAC when the cytology is not diagnostic or experimentally to predict prognosis and plan a specific therapy.EUS samples are often considered bearing a low content and low quality of representative material compared to surgical resection samples.A former study back in 2014 found that only 12.4% of 169 EUS-FNA cell block specimens obtained from malignant solid pancreatic masses have adequate cellularity for theranostic studies[12].
However,this area has greatly improved in the past few years.Hartleyet al[13]compared a “preresection” single FNA smear to two 5 µm curl of macrodissected FFPE taken from Whipple resections specimens.FNA smears resulted in an even better source of DNA,as,despite a similar nuclear area,FNA smears yielded greater DNA per nuclear area.KRAS codon 12 mutations were detected,in fact,in 77% of the samples compared to 57% of matched FFPE samples,with FNA retrieving a higher DNA yield compared to FFPE.
This also underlines that the way the sample is stocked might change the DNA extraction efficiency.In fact,Berryet al[14]proved how the KRAS mutation frequency in the same patients was significantly lower (45%) when using DNA extracted from EUS-FNA-derived FFPE blocks compared to EUS-FNA samples that were snap-frozen(80%).It is known,indeed,how formalin leads to protein-DNA cross-links and to degradation of nucleic acids.On the other hand,FNA samples are not formalin-fixed and retain whole nuclei.
Nevertheless,although EUS-FNA allows the extraction of DNA/RNA from the pancreatic sample,the absence of a pre-evaluation of this sample by a cytologist does not allow certainty regarding tumor cellularity in the sample.Some studies[15]have tried to overcome this limitation by performing parallel FNA and cytological evaluation of the same samples.Benesovaet al[15]extracted DNA and RNA from FNA-acquired tissue (put in RNALater) and FN cytological (FNC) air-dried smear,with a selected area trimmed out,of same patients undergoing EUS-FNA with a 22 G needle.The overall amount of isolated DNA/RNA from EUS-FNC samples was lower compared to EUS-FNA samples (10 ngvs147 ng,respectively,for DNA; 164vs642 ng,respectively,for RNA); however,the KRAS-mutant detection frequency in EUS-FNC samples was 90% compared to 78% in EUS-FNA samples.
Furthermore,a great disadvantage of FNA samples is the fact that the slides obtained by EUS-FNA are very few and must be destroyed in order to submit cellular material for DNA extraction.The quantity of DNA that can be extracted from a given tissue also depends on the shape of the tip and the size of the needle.A controversial topic is whether FNB might offer advantages over FNA for DNA or RNA extraction,which is a matter of debate.As Dreyer and colleagues[16]demonstrated,DNA and RNA analyses can be performed effectively on EUS-FNB samples,but the quantity of both DNA and RNA changes based on the type of needle adopted,resulting in the highest in their cohort when adopting SharkCore 22 G (2939 ng DNA yield and 481 ng RNA yield).However,their sampling collection and conservation methods (fresh frozen or FFPE) were not matched in the same patients and the quality of RNA was not reported.
Elhanafiet al[17]conducted a retrospective analysis of all patients undergoing FNA or FNB with genetic testing on pancreatic adenocarcinoma,both with 22 G needles(EUSN-3 Cook MedicalvsSharkCore Medtronic).A total of 145 samples were obtained with FNA and 22 with FNB,and were prepared with thin Prep-prepared slides (Hologic,Inc.,Bedford,MA,United States) and cell block specimens.A required minimum of 10% tumor cellularity was used as a limiting criterion to deem a sample sufficient,consistent with the prior literature.FNB samples were significantly more likely to have sufficient material for genomic testing compared to FNA samples(90.9%vs66.9%;P= 0.02).
Another possible pitfall is represented by the presence of heterogeneity in terms of presence of cancer cells,with EUS-TA only retrieving a part of the lesion that might not contain malignant cells or only a small percentage.Therefore,if a part of the biopsy where cancer cells are absent is employed for molecular analysis,false negatives might occur.This aspect has not been investigated widely.Berryet al[14],however,reported that only 2.5% (1 out of 40) of their pancreatic cancer samples that were positive for tumor cells at cytology and harbored KRAS mutations resulted in negative findings for tumor markers in the transcriptional profile.These results suggest that this might not be a major limitation to the use of FNB samples for transcriptional analyses.
An important point is also the evaluation of the total amount of tumoral cells,which should be analyzed to assess the probable retrievable amount of DNA to have reproducible results with a given technique.Fabbriet al[18]reported their experience with a threshold of about 5 ng good quality DNA necessary to detect KRAS mutations using a mutation-specific technique,and about 5-10 ng for next-generation sequencing.Furthermore,considering that a neoplastic cell holds 10 pg of DNA,they hypothesized a minimum cut-off number of lesional cells of 1000 to retrieve more than 10 ng of DNA,thus allowing to obtain an adequate specimen.
Another important factor is the lesional-to-non-lesional cell ratio (or lesional cell enrichment) and the type of technique used for sequencing.In fact,Sanger sequencing bears a low analytical sensitivity and requires at least 30%-40% of lesional cell enrichment to detect mutations,while next-generation sequencing or mutationspecific techniques are able to detect mutated KRAS alleles with 1%-5% tumor cell enrichment[18].
Indeed,a possible pitfall for DNA and RNA testing on pancreatic samples retrieved with EUS-FNA or FNB is the suboptimal content of pancreatic cancer cells and contamination with other non-malignant tissues,such as blood,gastric or duodenal wall cells,based on where the EUS-TA was performed,and also immune cells.Nevertheless,Berryet al[14]showed how the leukocyte marker cluster of differentiation 45 is scarcely expressed,as is also duodenal or gastric cell markers’ messenger RNA(commonly known as mRNAs).
Specifically referring to RNA,the main issue in its extraction from pancreatic tumor tissue is the high quantity of endogenous RNA ribonuclease (commonly known as RNase) that degrades RNA upon tissue acquisition.
Very few studies have been published on the best methodology for RNA extraction from mice and human pancreatic tissue but none of them have included a defined methodology for RNA extraction from tissue acquired through EUS.Nevertheless,as reported above,this has to be the goal in the near future,in order to provide our patients a defined path at first diagnosis.
Berryet al[14]extracted both RNA and DNA using a 22 G needle (ProCore Cook),snap freezing (in liquid nitrogen) the tissue after cytological rapid on-site evaluation(commonly known as ROSE) to confirm the diagnosis.They evaluated the quantity and quality of the DNA and RNA extracted using different methods,among which was the EUS-FNA pass snap-frozen in liquid nitrogen,and then homogenized and divided into smaller aliquots prior to processing.This technique allowed for retrieval of an average of 12.9 ± 3.2 µg of RNA and 4.8 ± 3.7 µg of DNA.In terms of quality,however,this method retrieved RNA with an RNA integrity number around 3,which is suboptimal.Interestingly,yields of genomic DNA were approximately 10-fold higher when an additional EUS-FNA pass was performed.
On the other hand,microRNAs (miRNAs) are a highly stable type of RNA,which is probably the reason why they have been studied as much or even more than mRNA of pancreatic cancer tissues on EUS-FNA; also,recent studies have elucidated how they could represent potential early biomarkers for pancreatic tumor detection and that they may also serve as prognostic factors[19].
SOMATIC MUTATIONAL ANALYSES FROM EUS-DERIVED PANCREATIC CANCER SAMPLES
Somatic mutations in EUS-acquired tissue have been investigated,especially for KRAS and particularly for cases in which the cytology could not be a determinant in the diagnosis.
Trisoliniet al[20]evaluated 89 pancreatic lesions having adequate cytology on EUSFNA samples by using a sequential approach for detecting KRAS mutations using mutant enriched-PCR (commonly known as ME-PCR).In all cases,DNA was extracted from cell-blocks and KRAS mutations were investigated by RT-qPCR followed by ME-PCR in non-amplifiable and negative cases.This “two-step”approach,proposed to evaluate KRAS mutations in indeterminate and negative cytology samples,simulates a realistic diagnostic workflow.Using this approach,the authors obtained a sensitivity of 90.2% and specificity of 100%.
Parket al[21]also reported a remarkable increase of the diagnostic yield of EUS-TA on pancreatic tumors when analyzing cytology and KRAS mutation in combination,evaluated on the sample flushed from the needle.
Elhanafiet al[17]performed somatic genomic testing using a 47-gene comprehensive solid tumor panel for the FNA/FNB rinse material or on the cell-block material of 25 PDAC patients.KRAS mutations were present in 88% of cases,while TP53 was in 68%and SMAD4 in 16%.Overall,tumor profiling identified two or more mutations in 84%of tested patients and three or more mutations in 56% of tested patients.There was only a slight divergence of survival between patients with wild-type TP53 and those with a mutated status,although that finding was not statistically significant.
Yoonet al[22]compared,for the same patients,baseline (from EUS-FNA FFPE) and after-treatment (from surgical specimen) somatic mutational profiles of 409 genes for seven patients.Results showed that after treatment,survival was worse in those harboring ARID1A mutations than those who harbored the wild-type,and that TP53 and KRAS mutations were not associated with survival.Also,KRAS mutations were present less frequently in specimens after treatment than at baseline,possibly representing a selection of less aggressive clones by chemotherapy.
A recent study by Dreyeret al[16]used genetic testing of a panel of 54 genes for 42 patients (including mostly PDAC cases,but also cases of other pancreatic lesions)using FNB samples and revealed mutations in KRAS (93%),GNAS (14%),TP53 (78%),CDKN2A (34%),and SMAD4 (32%),as well as in BRCA1(6%),ATM (12%),and BRAF(12%).
These studies collectively demonstrate that EUS-TA provides material that is adequate for mutational analyses of either single genes or of panels of genes.The optimal sample preparation in terms of needles,tissue conservation and handling is,however,unclear.Moreover,whether these genomic changes might change over time,over the course of the disease,and under treatments has been poorly investigated.
FEASIBILITY AND CLINICAL UTILITY OF PANCREATIC EUSFNA/FNB FOR OBTAINING RNA-BASED MOLECULARPROFILE
The PDAC microenvironment is characterized by a dense stromal compartment,comprising myofibroblast/cancer-associated fibroblastic cells,immune cells,and soluble factors,such as cytokines,chemokines,growth factors,and pro-angiogenic factors.This variable composition for different cellularity is a major limitation in the assessment of genetic mutations through tissue DNA-based analysis to allow classification of malignant and benign tissues,prediction of clinical outcomes,or selection of patient-specific treatments.In contrast,assessment of RNA expression level,protein level,or post-translational modifications could overcome the DNA analysis-related limitations.In fact,it is thought that PDAC heterogeneity is regulated at the epigenetic and transcriptomic levels and,therefore,clinical outcome and sensitivity to therapy could be associated with a given tumor phenotype.This would be important mostly for patients that are not eligible for surgery.Gene expression level can be measured by analyzing mRNA,the precursor of protein synthesis,using DNA microarray platforms,or RNA sequencing (RNAseq)[23].
Transcriptome analysis using cDNA microarrays has been shown to have a high yield for distinguishing benign from malignant lesions.Several studies have shown the feasibility of RNA extraction from EUS-FNA/FNB samples and the clinical utility to perform transcriptome analysis to improve the diagnostic accuracy of EUSFNA/FNB.Significant overexpression of keratin 7 (KRT7),lipocalin 2,and tissue-type plasminogen activator genes in PDAC,as shown in PDAC cell lines and specimens,was also observed in EUS-FNA samples[24].A molecular signature based on S100P(calcium binding protein P) and KRT7 expression was significantly associated with a better discriminatory capacity of PDAC from pseudotumoral inflammatory lesions[25].In EUS-FNA samples,the quantification of expression of other several biomarkers,such as calcium binding proteins S100A6 and S100A4[26],urokinase plasminogen activator receptor[27]combined with a 6-gene classifier (EpCAM2,Mal2,CEA5,CEA6,MSLN,and Trim29),and DNA mismatch excision repair gene MSH6[28],showed a high sensitivity and specificity for the diagnosis of PDAC and differential diagnosis with benign diseases.Other less investigated markers,but helpful to improve the diagnosis of PDAC on EUS-FNA samples,were revealed to be the transcription factor Snail,which mediates epithelial-mesenchymal transition and was significantly associated with invasive characteristics[29],as well as pancreatic duodenal homeobox-1(PDX-1)[30].
Other factors implicated in tumor invasiveness quantifiable in EUS-FNA samples are vascular endothelial growth factor (VEGF) and epidermal growth factor receptor(EGFR),involved in tumor angiogenesis,even if in one study RNA concentration and quality were relatively low in most samples.Both VEGF and EGFR were significantly overexpressed in PDACs and not significantly in pancreatic neuroendocrine tumors compared with normal pancreatic tissue.Moreover,EGFR expression was related to invasiveness in PDACs,whereas VEGF was inversely associated with tumor size[31].On the other hand,Costacheet al[32]found that mRNA expression of VEGF receptors VEGF-R1 and VEGF-R2 was significantly correlated with a shorter survival than VEGF-R-negative patients; as well,co-expression of VEGF-R1 and VEGF-R2 was found to be a poor prognostic factor in PDAC.Confirmation of the validity of EUSFNA samples as a source of tissue for molecular analysis was given by a study performed by Steget al[33],in which a significant concordance in the molecular profiles of hedgehog (HH)-pathway genes,potential mediators of pancreatic carcinogenesis,in matched snap-frozen,archival FFPE,and EUS-FNA samples,was observed.However,tissue heterogeneity and minimum content of cancer cells in PDAC EUSFNA samples represented major obstacles in molecular analysis[33,34],as confirmed by significantly different expression of HH signaling-associated markers compared to uninvolved pancreatic tissue.Laser capture microdissection on FFPE samples from EUS biopsies with cancer-cell enriched samples improved qRT-PCR analysis.Moreover,EUS-FNA biopsies could be used for multiple sampling to determine the modulation of gene expression with treatment,even if no significant changes in HH-pathway gene expression were observed in EUS-FNA samples obtained before and 2 wk after the administration of capecitabine and radiation[33].
The function of pancreatic intratumoral infiltrating immune cells is still not well understood.To the best of our knowledge,there is only one study that assessed the infiltrating immune cells in EUS-FNB samples,applying qRT-PCR analysis[35].Patients with a highly immunosuppressive profile tended to have a poor postoperative survival.A combination of CD15+ (neutrophils),CD206+ (tumor-associated macrophages),CD117+ (mast cells),and SMAD4 expression was independently associated with overall and recurrence-free survival.
A major limitation of cDNA microarrays is the amount of RNA required.RNAseq has the advantage of requiring only 100 ng of total RNA for reliable and reproducible transcriptome results.The application of this molecular technique on EUS-FNA samples is still rare and is currently under investigation.EUS-FNA was shown to provide sufficient material for targeted capture transcriptome RNAseq in the majority of specimens,using only a portion of a single FNA pass.RNAseq can be used to develop a classifier profile consisting of differentially expressed genes and separate benign from malignant pancreatic tissue in 83% of cases[23].Moreover,RNAseq was able to segregate patients into clinically relevant phenotypic subtypes (squamous and classical PDAC) in both pancreatic primary and liver metastatic lesions,showing the same subtype cluster in primary and metastatic disease[16].
There has also been increasing interest in miRNAs recently; these small chains of non-coding RNA are negatively involved in the post-transcriptional regulation of gene expression.Several studies have described an aberrant production of miRNAs during the development of precancerous lesions (pancreatic intraepithelial neoplasia and intraductal papillarymucinous neoplasms) and in pancreatic carcinogenesis,and defined several miRNA signatures associated with diagnosis,staging,progression,prognosis,and response to treatment.Few studies have attempted quantification of miRNAs on pancreatic EUS-FNA samples,and have mainly involved FFPE samples.MiRNAs could still be quantified,even in low amounts and highly degraded samples.
MiRNAs were first tested on EUS-FNA samples by Szafranskaet al[36].The authors found that the expression of a miR-196a/miR-217 classifier could discriminate PDAC from benign lesions with a sensitivity and specificity of 90% and 100%,respectively.Subsequently,the same group found a better 2-miRNA classifier (miR-135b/miR-24)for PDAC[37].The tissue miR-21/miR-155 classifier was a strong independent predictor of PC when up-regulated,with higher discriminating power compared to cytology and to the same classifier on plasma[38].Also,high levels of miR-21 in EUS-FNA samples of unresectable PDAC were associated to progression and reduced survival[39].Also,miR-10b was overexpressed in PDAC EUS-FNA material,and its reduced expression was associated with an improved response to neoadjuvant therapy,surgical resection,increased time to metastasis onset,and increased survival[40].In that study,evaluation of miRNA expression changes was performed using a combination of fluorescencein situhybridization/immunohistochemistry(IHC) assay in FFPE samples,on CK19-stained suspicious cells.In addition to wellestablished onco-miR-21 and -miR-10b,overexpression of other miRNAs (miR-221,miR-196a,miR-135b,miR-24,miR-130,miR-148a,and miR-93) in FFPE cell-blocks from EUS-FNA was also able to improve detection of PDAC and malignant pancreatic cysts from benign cases (accuracy up to 90.8%) when combined with standard cytology[41-44].As for other molecules,heterogeneity in miRNA profiles from EUS-FNA samples has been reported possibly due to the different techniques used to collect samples,isolate total RNA,and quantify miRNA levels.Contaminating elements may also provide significantly different miRNA expression.Thus,it has been hypothesized that analysis of cytological smears from EUS-FNA would be a better approach for the determination of miRNA levels as this would allow a precise evaluation of the fraction and representation of tumor cells.As mentioned before,Benesovaet al[15]showed how the overall amount of RNA extracted from air-dried cytological smears was lower compared to tissue acquired with EUS-FNA and put in RNALater,but gave reliable results with clinical validity (i.e.,prognostic role of miR-21).
FEASIBILITY AND CLINICAL UTILITY OF PANCREATIC ENDOSCOPIC ULTRASOUND-FINE NEEDLE ASPIRATION/FINE NEEDLE BIOPSY FOR ASSESSMENT OF TREATMENT EFFICACY
Selecting patients who are likely to respond positively to a specific treatment may help to improve the prognosis of patients with unresectable PDAC.Characterization of genes associated to tumor sensitivity or resistance to antitumor therapeutic factors using pre-treatment tumor tissue would help clinicians for the selection of appropriate treatment regimen and the development of individualized treatment.Few studies assessed potential biomarkers predictive of chemosensitivity and modifications of specific biomarkers during treatment on EUS-FNA/FNB samples.
Expression of HSP72 on EUS-FNA samples,evaluated through IHC,was significantly associated with that on resected specimens and was a helpful predictive marker for sensitivity to gemcitabine (GEM).GEM-resistance rate was significantly higher in patients with overexpression of p-HSP27,and the survival rate was significantly lower if p-HSP27 (Ser82) detection rate was > 51.6%[45].Another important molecular biomarker for prediction of GEM sensitivity in unresectable PDAC was S100A4 mRNA expression,analyzed in EUS-FNA samples through qRTPCR analysis.High expression of S100A4 mRNA was a predictor of GEM resistance,in contrast to low S100A mRNA expression levels in the effective patient group[46].Also,human equilibrative nucleoside transporter 1 (hENT1),a mediator of GEM uptake in human cells[47],deoxycitidine kinase (dCK),a GEM metabolism-related enzyme[48],and ribonucleoside reductase 1 (RRM1)[49]and ribonucleoside reductase 2(RRM2)[50],GEM resistance-related enzymes,have been investigated for their role as predictive biomarkers for GEM effect and sensitivity in unresectable PDAC patients.Expression levels of these genes in EUS-FNA samples were detectable,even if with discordant data.The predictive yield of hENT1 for GEM sensitivity and prognosis in unresectable pancreatic cancer was confirmed by Yamadaet al[51],who performed IHC assessment on preoperative EUS-FNB,according to their finding of concordant hENT1 expression with that found in resected specimens,thus providing important information on patients who could benefit from curative-intent resection.In fact,a significantly better prognosis was found in hENT1-positive patients than in those who were hENT1-negative.Also,a high level of RRM2 mRNA expression was correlated with a poor response rate to GEM and shorter overall survival[50].Analysis of mRNA expression of hENT1,dCK,RRM1,and RRM2 genes in microdissected cancer cells from EUS-FNA samples of patients receiving preoperative GEM-based chemoradiotherapy showed potential as a tool to perform individualized chemotherapy[52].In that study,laser capture microdissection of cancer cells revealed itself to be a molecular RNA-based method capable of overcoming the limitations presented by the large amount of blood and inflammatory cells and scarce cancer cells in most EUS-FNA samples.The same group measured EGFR mRNA levels in EUSFNA samples successfully by using laser microdissected neoplastic cells[53].High EGFR mRNA expression was found to be an independent prognostic factor in patients treated with GEM-based adjuvant chemotherapy,suggesting that quantification of EGFR mRNA expression levels could be a valuable tool to predict PDAC patient outcome,even when samples contained abundant contaminating cells.Yet another study attempted to assess the effect of capecitabine with concomitant radiotherapy (XRT) in patients with locally advanced pancreatic cancer.In these patients,mRNA expression of thymidine phosphorylase,dihydropyrimidine dehydrogenase,and tumor necrosis factor-α was quantitated in EUS-FNA samples obtained 1 wk before and 2 wk after chemo-XRT[54],but no significant difference was found in the mRNA expression levels between pre- and post-XRT.
EUS AS A TOOL TO GENERATE PATIENT-DERIVED XENOGRAFTS OR ORGANOIDS
PDAC aggressiveness is related to its genomic instability and heterogeneity.Patientderived xenograft (PDX) mouse models,also known as “avatar models”,may represent a promising tool to personalize pancreatic cancer treatments[55].The construction of PDX models from surgical specimens is extremely limited for PDAC patients,as only 10%-15% of them present with localized resectable tumors[56].Addressing this issue,Allaway and colleagues[57]succeeded in establishing PDXs from EUS-FNA biopsies of treatment-naïve primary pancreatic tumors.In addition,they performed genomic characterization of these models,revealing clinically relevant mutations (e.g.,KRAS,TP53,BRAF,and EGFR).Similarly,Berry and colleagues[14]established a preclinical PDX model from two patients diagnosed with pancreatic cancer,one expressing KRAS wild-type and the other KRAS mutant,to assess the sensitivity of these patients to the anti-EGFR inhibitor panitumumab.Even if PDX models can be useful for predicting drug response,their application in medicine is limited,as they are expensive and time- and resource-consuming,the engraftment rates can be as low as 20%,and tumor formation requires at least 12 wk[58].
Tumor organoids are three-dimension cultures of cancer cells and they can be derived from each patient,providing an individualized model.Compared to PDX models,tumor organoids are established more easily,having a success rate of 70%-80%[59].For these reasons,patient-derived organoids (PDOs) are now considered the best model to evaluate the molecular profile and chemosensitivity of different tumors in a rapid and high-throughput manner.
In 2017,Tiriac and colleagues[60]succeeded in creating organoids from PDAC specimens obtained by EUS-FNB sampling using a 22 G needle.They managed to establish organoids from the majority of patients (87%) within 2 wk from the EUS procedure and successfully propagated 66% of them for five passages.
An elegant work from Seino and colleagues[61]showed the possibility to take advantage of PDOs to deeply investigate the biological behavior of different pancreatic cancer subtypes.The investigators produced 39 lines of PDOs from surgical,FNA,and ascites specimens and performed genomic and transcriptomic analyses.As a result,they demonstrated that the mutational profile determined the requirements of organoids for niche-specific factors to survivein vitroandin vivo.
More recently,PDOs were obtained from primary tumors and metastases by Tiriac and colleagues[62]to study the phenotype of different lesions for the purpose of finding biomarkers for treatment response.Indeed,the investigators generated a total of 114 PDO cultures from 101 patients,with 72% of FNB samples and 78% of resected specimens propagated for at least five passages,respectively.First,they evaluated the genomic and transcriptomic landscapes of the PDO library by whole-exome sequencing and RNA sequencing.Then,they performed “pharmacotyping” on 66 PDAC PDOs to assess the sensitivity to chemotherapeutic agents commonly used to treat pancreatic cancer; the results highlighted the strong concordance between patient outcome and the chemosensitivity of PDOs.In addition,the possibility to repeat biopsies longitudinally gave the opportunity to follow the clinical course for a patient.The PDOs generated at diagnosis exhibited the same response to treatments as the primary tumor; at disease progression,the investigators isolated organoids,which showed resistance to the chemotherapeutic agents employed in the previous regimen.
To date,few groups are exploiting EUS-FNB to generate PDAC PDOs.However,these studies set a milestone by developing and optimizing a procedure for isolating and propagating PDOs from a minimal quantity of tissue acquired by EUS-FNB sampling.Of course,PDOs do not recapitulate the complexity of tumor microenvironment because they lack immune cells,blood vessels,and all the stromal components,which play fundamental roles in PDAC biology.Nevertheless,the unique opportunity to follow the evolution of tumors longitudinally,thanks to a minimally invasive sampling procedure,may open new routes to precision medicine.Seufferlein and Kleger[63]believe in “organoidomics” as a promising tool to improve the management of PDAC patients and to deepen our knowledge of pancreatic cancer.
CONCLUSION
In the past few years,a number of important changes in the care of PDAC patients have occurred:(1) It seems that the majority of patients benefit from combination chemotherapy[64],when the patients are fit and can tolerate it; (2) The possibility that both germline and somatic mutations can predict the response to certain treatments is being investigated and might offer important routes for treatment personalization[65];(3) Different molecular subtypes of PDAC exist with peculiar genomic and transcriptomic features and distinct clinical behavior[66]; and (4) Novel models that might help in investigating the molecular features and the chemosensitivity of the patients (avatar or organoids) almost in real time have been developed.In this scenario,the role of EUS as a tool to obtain tissue from the tumor at diagnosis in a scarcely invasive manner,possibly at multiple timepoints during the course of disease,is increasing.When reviewing the available literature on the topic,however,it is clear that the major problem regards the lack of standardization,optimization,and thus repeatability of the employed techniques (Table1),starting from the choice of needles and going to the handling of samples.A close collaboration among endoscopists,clinicians,pathologists,and basic scientists is necessary to fill these gaps.In addition,it is important that these techniques are employed in a translational research environment where physicians/scientists can develop research questions that are clinically relevant and of immediate utility for patients.
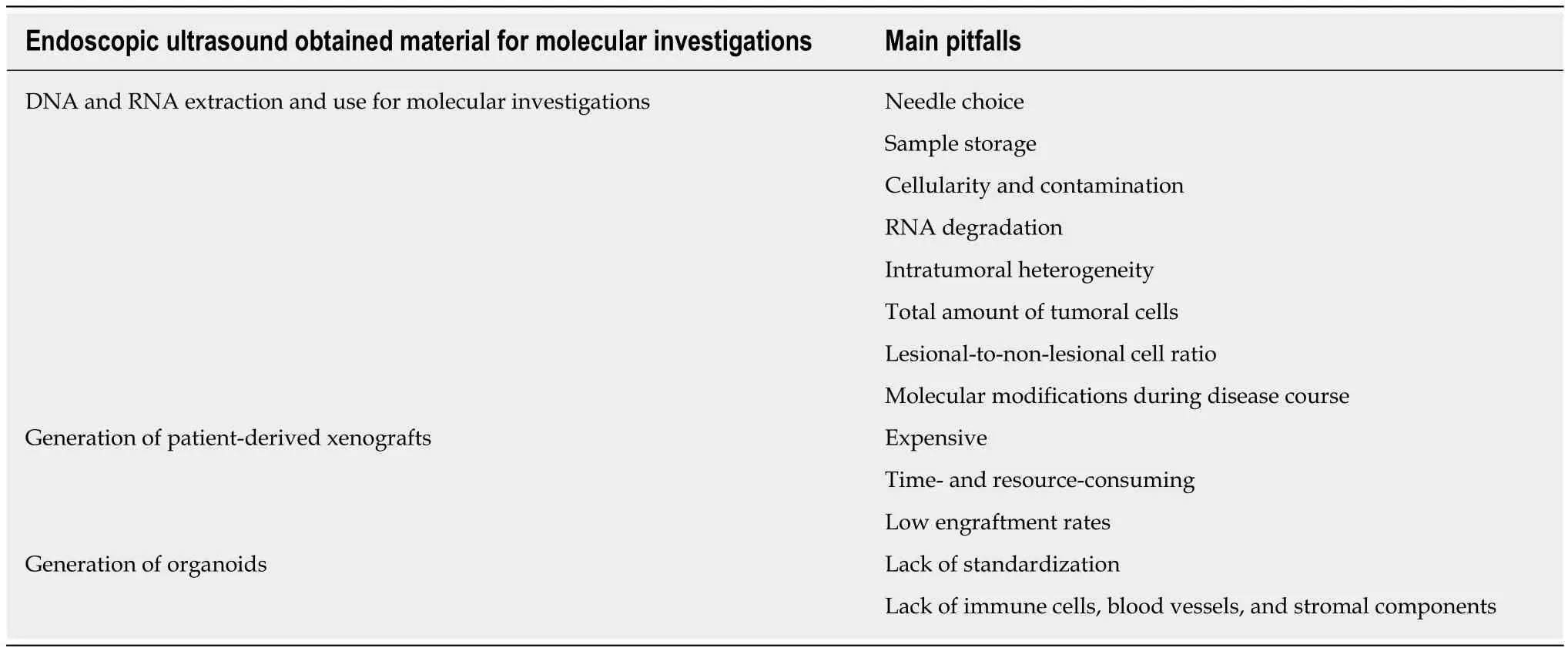
Table1 Main pitfalls towards the optimization and standardization of the use of endoscopic ultrasound-obtained material for molecular investigations
杂志排行
World Journal of Gastrointestinal Oncology的其它文章
- Efficacy of hybrid minimally invasive esophagectomy vs open esophagectomy for esophageal cancer:A meta-analysis
- Clinical significance of MLH1/MSH2 for stage ll/lll sporadic colorectal cancer
- Endoscopic full-thickness resection for treating small tumors originating from the muscularis propria in the gastric fundus:An improvement in technique over 15 years
- Validation and head-to-head comparison of four models for predicting malignancy of intraductal papillary mucinous neoplasm of the pancreas:A study based on endoscopic ultrasound findings
- Revisiting oral fluoropyrimidine with cetuximab in metastatic colorectal cancer:Real-world data in Chinese population
- Oral chemotherapy for second-line treatment in patients with gemcitabine-refractory advanced pancreatic cancer
