Application of New Modified Bentonite in Decolorization of Simulated Printing and Dyeing Wastewater
2019-11-20
College of Biological and Environmental Engineering, Binzhou University, Binzhou, 256603, China
Abstract The problem of colority control of printing and dyeing wastewater has become a technical problem that plagues related companies. Bentonite is an adsorbent with excellent properties. Modification of bentonite with environmentally friendly substances can improve its decolorization performance. In this experiment, the simulated printing and dyeing wastewater was taken as the control object, and the bentonite was modified with environmentally friendly materials such as sodium carboxymethyl cellulose (CMC) and lignin to prepare a new modified bentonite; then the modified bentonite was used to adsorb the simulated wastewater to reduce the water colority and COD; finally the relevant design of the adsorption process was made. Results indicate that Mlignin∶MCMC∶Moriginal bentonite=1∶2∶97 had the optimum treatment effect, the optimum modification temperature was 30℃ and the modification time was 4 h; the optimum conditions for the adsorption process were: pH=5, temperature=30℃, reaction time=60 min, dosage=0.05 g of modified bentonite/mL simulated dye solution. The final removal rate of colority and COD reached 95.0% and 98.2%, respectively. Compared with the original bentonite, this new modified bentonite has greater adsorption capacity and thus has greater application value.
Key words Bentonite, Modification, Decolorization, Printing and dyeing wastewater
1 Introduction
In the wastewater of textile industry, printing and dyeing wastewater is the main source and difficult to treat, accounting for 80% of the total discharge of textile industry[1]. In printing and dyeing wastewater, the soluble substances are mainly organic pollutants, as well as toxic substances such as benzene, nitrogen, amine groups, which have complex structure and large color difference, accordingly difficult to decompose[2]. For printing and dyeing wastewater, the pollutants that people care mainly include colority and various harmful components in wastewater. The colority problem is a tough problem in wastewater treatment. Generally, the contribution of colored soluble substances to the colority indicator of industrial wastewater is up to 90%-95%, and the remaining comes from suspended solids and colloids[3].
At present, there are many methods for the treatment and decolorization of printing and dyeing wastewater, but most of methods have different degrees of limitations: such as chemical precipitation treatment of printing and dyeing wastewater will produce a large amount of sludge while processing and the sludge is difficult to treat; Fenton and hydrogen peroxide oxidation treatment have a good treatment effect on the colority and COD of wastewater, but the cost is high, and HO· will react with Fe2+and oxidize it to Fe3+, accordingly reducing the action efficiency of HO·[4]. Biological methods have low operating costs but could not realize complete decolorization of commercial dyes[5]. The adsorption method is simple and easy, and it is particularly important in the treatment of organic polluted wastewater including printing and dyeing wastewater. Therefore, it will have great potential market prospect and high research value to choose a kind of adsorbent which is cheap and easy to obtain, has good treatment effect and high recovery efficiency for decolorization of such wastewater. China is rich in bentonite reserves, and bentonite is an environmentally triendly adsorbent substance with excellent adsorption characteristics. Through modification, it is able to increase the specific surface area of bentonite molecules, enlarge the interlayer spacing, increase the cation exchange volume, change the surface properties, and accordingly significantly increase the adsorption characteristics of bentonite[6]. Lignin, cellulose, hemicellulose,etc. which are widely found in nature can be linked together to form a lignin- carbohydrate complex[7], so they are also good adsorbent materials. Therefore, in this study, we used lignin and cellulose materials to modify the bentonite, simulated the dyeing wastewater under laboratory conditions for decolorization experiments and obtained the optimum adsorption conditions. Besides, we carried out the process design of related coagulation adsorption sedimentation. This experiment is a relatively complete and innovative attempt to prepare and practice the adsorption materials.
2 Materials and methods
2.1 Instruments and chemicalsMain instruments used in this experiment included TU- 1901 Double Beam UV- Vis Spectrophotometer, THZ- 92CS Water Bath Constant Temperature Oscillator, and DJL10 COD Digestion System. The experimental bentonite was purchased from Shanghai Jianglai Biotechnology Co., Ltd., and sodium carboxymethyl cellulose and lignin were standard products purchased from Beijing Century Aoke Biotechnology Co., Ltd. The chromatographically pure methylene blue was used to simulate the dyeing wastewater, and all other reagents used were of analytical purity.
2.2 Plotting of methylene blue standard curveThrough the wavelength scanning, we obtained that the maximum absorption wavelength of the methylene blue dye solution was 655 nm. Then, a series of concentrations of methylene blue dyeing solution were prepared. Taking the absorbance as abscissa and the methylene blue concentration as ordinate, the standard curve was plotted:C=15.112 6×OD655-3.436 1,R2=0.995 8, whereCdenotes dyeing solution concentration (mg/L),OD655denotes absorbance of the dyeing solution at the wavelength of 655 nm.
2.3 Determination of decolorization rate and water sample CODThe decolorization rate was determined by spectrophotometry. The specific method was as follows: took the methylene blue solution with an initial concentration of 600 mg/L (C0), and carried out an oscillating adsorption experiment under different reaction conditions; after sedimentation, took a small amount of supernatant sample to determine the absorbance value of the solution. According to the measured absorbance value, combined with the standard curve, we calculated the concentration (C) of methylene blue in the water sample. Then, we calculated the decolorization rate of the solution using the following formula: Decolorization rate=(C0-C)/C0, whereC0denotes the concentration of methylene blue solution before adsorption reaction (mg/L), andCdenotes the concentration of methylene blue solution after adsorption reaction (mg/L). The determination of COD in water sample was based on the potassium dichromate method in the national standard.
2.4 Bentonite modification experimentWe first determined the optimum ratio of modified materials to the original soil: added sodium carboxymethyl cellulose, lignin and bentonite to a certain amount of distilled water, setting the ratio of the two modified materials as 1∶1 for the time being, the percentage of modified materials in total mass was 2%, 3%, 4%, 5%, 6%, 7%, 8%, and the modification temperature was 30℃. Under these conditions, the modification reaction was operated in a gas bath constant temperature oscillator for 8 h. Then, filtered the supernatant, sediment was dried in a constant temperature drying oven at 105℃, ground, and screened with a 100 mesh sieve for use. Next, took 100 mL of 600 mg/L methylene blue solution and placed in a 150- mL conical flask, and added 3 g of spare modified bentonite separately, and placed in a 30℃ gas bath constant temperature oscillator for 2 h for adsorption, and took small amount of the supernatant and measured the absorbance under a spectrophotometer and determined the optimum ratio of the modified material to the original bentonite according to the adsorption effect. Then, we determined the optimum ratio of the modified materials and the optimum modification time and temperature. The specific steps were as follows: on the basis of determining the optimum ratio of modified materials to original bentonite, adjusted the ratio of sodium carboxymethyl cellulose to lignin, so that MCMC∶Mlignin(mass ratio) was 0.2, 0.5, 1, 2 and 5, then determined the optimum ratio of the modified materials using the above adsorption experiment steps; on the basis of determining the optimum ratio, adjusted the modification reaction time to 2, 4, 6, 8, 10 and 12 h, and determined the optimum modification time according to the adsorption effect; on the basis of the above optimum modification conditions, adjusted the modification temperature to 15, 20, 25, 30, 35 and 40℃, determined the optimum modification temperature according to the calculated decolorization effect.
2.5 Adsorption experiment of modified bentoniteAccording to the preliminary experiment, it is found that the pH of the reaction system has the greatest influence on the adsorption effect. Therefore, we first determined the optimal pH value of the reaction system. The specific method was as follows: placed 100 mL of 600 mg/L methylene blue solution in a 150- mL conical flask, and added 3 g of alternate modified bentonite separately, adjusted the pH of the reaction system in the range of pH 1-14, and adsorbed in the gas bath constant temperature oscillator for 2 h at 30℃. After sedimentation, took a small amount of the supernatant, and measured the absorbance at the wavelength of 655 nm, and determined the optimum pH value of the adsorption experiment according to the decolorization effect. Then, we determined the optimum amount of modified soil, adsorption reaction time and reaction temperature. The specific method was as follows: on the basis of the optimum pH, added 100 mL of 600 mg/L methylene blue solution to a 150- mL conical flask, and added 1, 2, 3, 4, 5, 6 and 7 g alternate modified bentonite to adsorb, and determine the optimum dosage of the modified bentonite according to the decolorization effect; on the basis of determining the pH value and the dosage of the adsorbent, set the adsorption reaction time to 20, 40, 60, 90, 120, 150, 180 and 240 min, respectively, and determined the optimal adsorption time according to the adsorption effect; on the basis of the above optimal conditions, the adsorption reaction temperature was changed to 15, 20, 25, 30, 35 and 40℃, respectively, and carried out the adsorption in a gas bath constant temperature oscillator, and determined the optimum reaction temperature according to the adsorption effect.
3 Results and discussions
3.1 Formula and optimum modification conditions of new modified bentoniteThe modification of bentonite can be divided into inorganic, organic and organic- inorganic composite modification[8]. The commonly used organically modified materials are usually surfactants such as CTAC and CTMAB[9-10], which are more difficult to treat after wastewater treatment and become secondary pollutants. Cellulose and lignin belong to organically modified materials and are environmentally friendly. The introduction of different functional groups at the hydroxyl position of cellulose can improve the selectivity and adsorption capacity of cellulose[11-12]; lignin is one of the main components of the plant skeleton[13], containing a a large number of groups such as methoxy, hydroxy, carbonyl, carboxyl,etc., can adsorb polar organic substances and mesh colloidal particles dissolved in water and suspended particles, thereby functioning as a flocculating agent[14-15].
As shown in Fig.1A, addition of the modified material influences the discoloration effect. When the proportion of modified materials was 3%, the removal rate of colority was the highest (92.9%). This indicates that when the amount of the modified material is small, as they enter the original bentonite layer, the original bentonite layer spacing is increased, so the adsorbed surface area is increased. However, with the increase of the dosage of modified materials, the removal rate becomes smaller, indicating that the modified substances entering the original bentonite layer may be saturated, and the modified materials not entering the bentonite structure still have negative effects on the entire adsorption system. Thus, the modified bentonite, has no obvious effect on the removal of methylene blue, and also causes the turbidity of the solution to become large, accordingly affecting the absorbance. Previous studies have also shown that the interlayer spacing of organic bentonite and the removal rate of organic matter increase with the increase of the cationic surface active dose used in the modification, but when the addition amount is larger than the cation exchange capacity in the original bentonite, the interlayer spacing and the removal rate of organic matter reaches the maximum and basically constant[16]. As shown in Fig.1B, the mass ratio of the two substances, lignin and sodium carboxymethylcellulose, was sequentially adjusted under the condition that the optimum dosage of the modified material was determined to be 3%, and the variation range is from 0.2 to 5, the removal rate of methylene blue first increased and then decreased. When the ratio of lignin to sodium carboxymethyl cellulose was 1∶2, the maximum removal rate was 93.3%, indicating that the ratio of modified materials has effect on change of the original bentonite structure. As shown in Fig.1C, under the basis of determining the optimum ratio of the modified materials, the adsorption reaction test was carried out by changing the modification reaction time. It was found that the removal rate of the modified bentonite first increased and then decreased. When the modification time was 4 h, the removal rate reached the optimum value of 91.5%. This indicates that with the increase of the modification time, the amount of modified substances entering the original bentonite will increase, which will help to increase the layer spacing structure of the original bentonite. However, when the time is too long, the desorption phenomenon may occur, and the treatment effect may be weakened. As shown in Fig.1D, within the temperature variation range set in the experiment, the removal rate of colority also increases first and then decreases, indicating that the temperature has a certain influence on the modified material entering the original bentonite structure. At the temperature of 30℃, the removal rate reached an optimum value of 93.7%. In summary, the optimum ratio of new modified bentonite is Mlignin∶MCMC∶Moriginal bentonite=1∶2∶97, and the optimum modification reaction time and temperature are 4 h and 30℃, respectively.
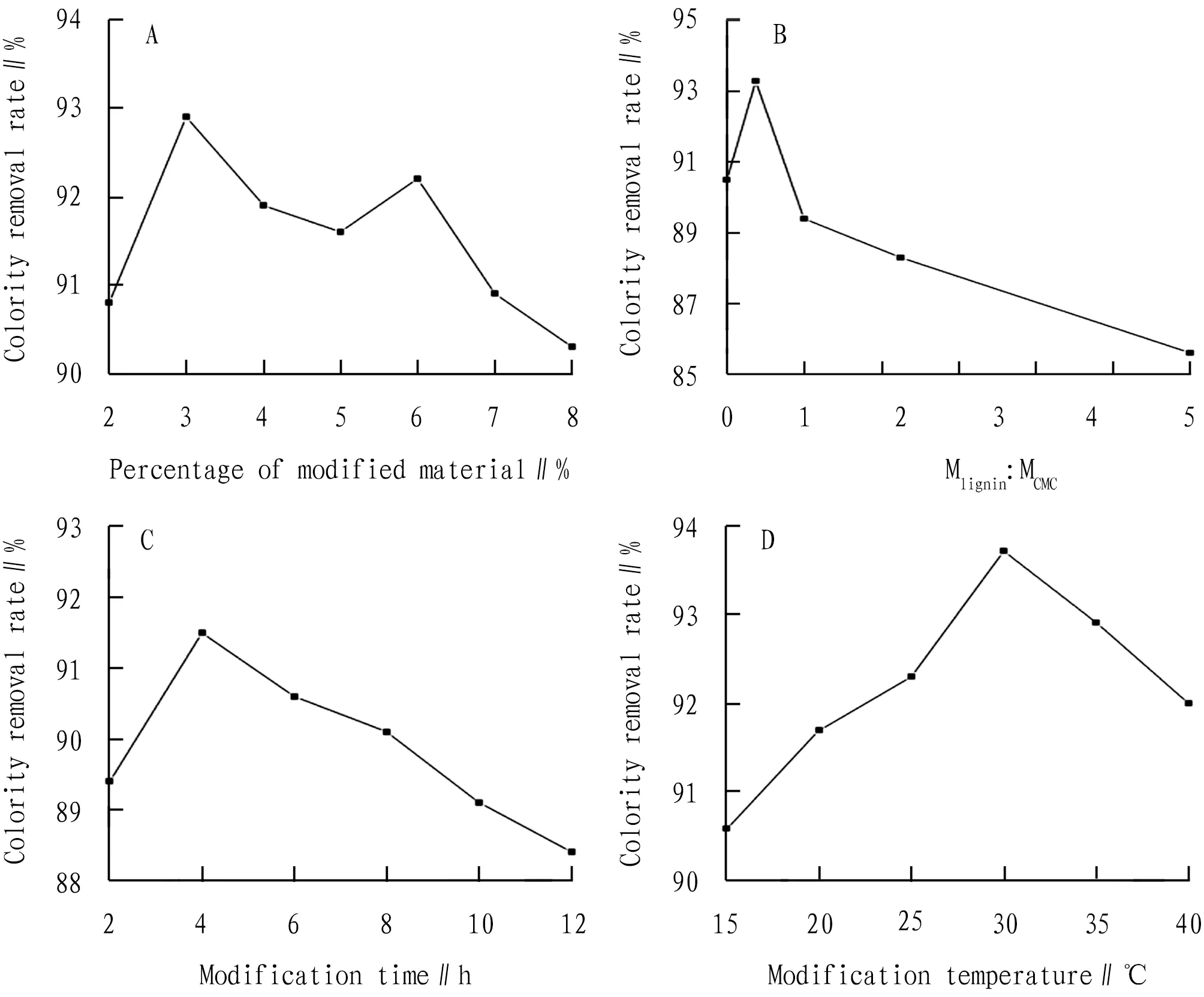
Fig.1 Optimum conditions for bentonite modification reaction
3.2 Determination of the optimal adsorption reaction conditionIn the past, some scholars studied the adsorption conditions of modified bentonite on printing and dyeing wastewater. For example, OZERetal.[17]used 1,6- diaminohexane modified natural bentonite to adsorb textile dye reactive blue 19 in aqueous solution, and studied the effects of pH, reaction time, initial dye concentration and temperature on the adsorption amount. Studies have shown that the amount of dye adsorption depends on pH, amount of adsorbent, reaction time and temperature. In this experiment, after preparing the new modified bentonite, we also carried out the adsorption experiment of simulated dyeing solution, and discussed the optimum adsorption reaction conditions. The results are shown in Fig.2.
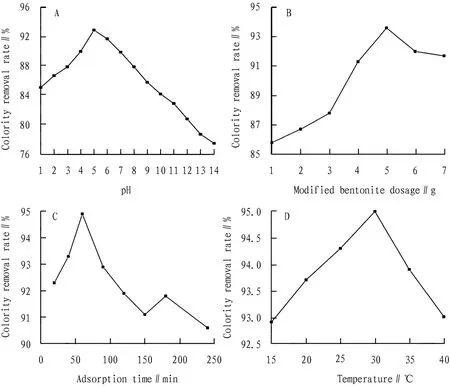
Fig.2 Optimum adsorption conditions for modified bentonite
The pH of the adsorption reaction system was adjusted in the range of pH 1 to 14. It was found that the removal rate of the simulated dye solution reached an optimum value of 92.9% at pH of 5. This indicates that the acidity and alkalinity of the reaction system have a great influence on the decolorization effect of the modified bentonite, and the acidic conditions are more favorable for the adsorption experiment, as shown in Fig.2A. As shown in Fig.2B, on the basis of determining the optimal adsorption pH value, we adjusted the dosage of the modified bentonite, and the bentonite sample dosage range was 0.01-0.07 g/mL simulated dyeing solution, and the removal rate of the colority was first increased then slightly decreased, and the optimum value was 93.6% when the dosage was 0.05 g/mL simulated dye solution. This indicates that when the dosage was lower than 0.05 g/mL, the removal rate increased with the increase of the dosage, and when the dosage was continuously increased, the adsorption capacity of the adsorbent became saturated, thus the removal rate decreased. As shown in Fig.2C, the absorbance of the supernatant was measured at the reaction time of the adsorption experiment at 20, 40, 60, 90, 120, 150, 180 and 240 min, respectively, and it was found that the optimum removal rate was 93.9% at 60 min. This indicates that the adsorption value of modified bentonite to simulated dyeing solution increased with the increase of adsorption time, but with the increase of adsorption time, the dye adsorbed by modified bentonite became saturated, and the removal rate of colority decreased. In recent years, the study of modified bentonite for the adsorption treatment of printing and dyeing wastewater has found that the main decolorization mechanism lies in physical adsorption, that is, hydrogen bond adsorption between hydroxyl and oxygen atoms on the surface of bentonite and organic compound molecules. Physical adsorption can be carried out at low temperatures, but since the adsorption of adsorbate and adsorbent is mainly van der Waals’ force, the adsorption poorly selective[18]. From the data of this experiment, after the adsorption reaches a certain time, there is desorption, which causes the turbidity of the water to increase. Therefore, after the adsorption is saturated, it should not oscillate again. Instead, it is necessary to settle the sedimentation and remove the sediment. As shown in Fig.2D, on the basis of determining the pH, the dosage and the reaction time, the reaction temperature of the adsorption experiment was adjusted in the range of 15-40℃. The removal rate of the simulated dye solution first increased and then decreased, reaching an optimum value of 95% at the temperature of 30℃. This indicates that the temperature has a certain influence on the adsorption characteristics of the modified bentonite. As mentioned above, the physical adsorption can be carried out at a low temperature, so the reaction temperature should not be too high.
3.3 COD and colority removal rate under optimal adsorption conditionsThe adsorption experiment was carried out on the basis of the optimum modification conditions and the optimum adsorption conditions. The concentration of the dye solution before the adsorption was 600 mg/L, the concentration of the dye solution after adsorption was 30.068 mg/L, and the colority removal rate was 95.0%, the COD value before adsorption was 12 000 mg/L, the COD value after adsorption was 216 mg/L, and the COD removal rate was 98.2%. This indicates that the new modified bentonite is also effective for removing high COD caused by dye molecules.
3.4 Comparison of colority removal rates of different types of soil samplesUnder optimum adsorption conditions, the decolorization effect of different types of soil samples is shown in Table 1. From Table 1, it can be seen that the modified bentonite, the original bentonite and the common soil are separately added under the same conditions, the removal rates vary greatly. In terms of the mechanism, it is possibly because the modified substance improves the environment inside the bentonite, the organic ions in the material replace the exchangeable ions between the montmorillonite layers, or react with the metal ions between the layers to form an organometallic complex, which enhances its lipophilicity and is easy to adsorb methylene blue molecules of organic pollutants; besides, it plays a role of pillaring on the internal structure of bentonite, which makes the spacing of bentonite layer larger, enlarges the space inside the layer, and forms organic bentonite with high interlayer spacing, and the specific surface area is further increased, accordingly increasing the adsorption capacity of methylene blue[19]. Therefore, the decolorization effect of the new modified bentonite prepared in this experiment is excellent.
Table 1 Decolorization effect of different samples under optimum adsorption conditions

Dye concentration before adsorption∥mg/LType of sorbent addedDosage∥gDye concentration after adsorption∥mg/LRemoval rate∥%600Modified bentonite530.06895.0600Original bentonite5187.56368.7600Ordinary soil5446.32525.6

Fig.3 Schematic diagram for the coagulation adsorption sedimentation tank(dm)
4 Conclusions
(i) It can be concluded from the experimental data that the new modified bentonite prepared this time has a good removal effect on the colority of printing and dyeing wastewater, and also has a good processing ability for COD. The added modified material can enter the structure of the original bentonite, thereby increasing the interlayer spacing and specific surface area of the original bentonite, accordingly improving the removal effect of the printing and dyeing wastewater. (ii) When the dosage of the modified material is 3%, the optimum removal rate is 92.9%; when the ratio of the modified material lignin to sodium carboxymethyl cellulose is 1∶2, the optimum adsorption rate is 93.3%; when the modification time was 4 h, the optimum adsorption rate is 91.5%; when the modification temperature is 30℃, the optimum removal rate reaches 95.0%. Thus, the optimum ratio of new modified bentonite is Mlignin∶MCMC∶Moriginal bentonite=1∶2∶97; the optimum modification temperature is 30℃, the modification time is 4 h. (iii) When the pH value is 5, the optimum removal rate is 92.9%; when the modified bentonite dosage is 0.05 g/mL dye solution, the optimum removal rate is 93.6%; when the adsorption time is 60 min, the optimum adsorption rate is 93.9%; when the adsorption temperature is 30℃, the optimum adsorption rate is 95.0%. Therefore, the optimum adsorption conditions are: modified bentonite dosage 0.05 g/mL dyeing solution, pH 5, temperature 30℃, and reaction time 60 min. (iv) For the specific mechanism of the modification of bentonite by lignin and sodium carboxymethylcellulose and the specific changes of the internal structure of the modified bentonite, it is still worthy of more in- depth research.
杂志排行
Asian Agricultural Research的其它文章
- Research on Intelligent Monitoring System Based on Raspberry Pi 3
- Cloning and Bioinformatics Analysis of tyeA Gene of Vibrio alginolyticus
- Protection and Renovation Strategies for Ancient Town Architectural Space Design from the Perspective of Place Spirit Theory: A Case Study of Hekou Ancient Town, Yanshan County of Jiangxi Province
- Coordinated Development Level of Economic Society and Ecological Environment in Binzhou City
- Teaching Reform of Building Construction Course under the Background of New Digital Technology
- Effects of Different Breeds, Number of Squabs and Temperature on Production Performance of Pigeons
