Prebiotic UG1601 mitigates constipation-related events in association with gut microbiota: A randomized placebo-controlled intervention study
2019-11-19JaeRyangChuSaemYiKangSungEunKimSolJiLeeYoungChulLeeMiKyungSung
Jae Ryang Chu, Saem-Yi Kang, Sung-Eun Kim, Sol-Ji Lee, Young-Chul Lee, Mi-Kyung Sung
Abstract BACKGROUND Constipation is a common functional gastrointestinal disorder and its etiology is multifactorial. Growing evidence suggests that intestinal dysbiosis is associated with the development of constipation. Prebiotics are subjected to bacterial fermentation in the gut to produce short-chain fatty acids (SCFAs), which can help relieve constipation symptoms. The prebiotic UG1601 consists of inulin,lactitol, and aloe vera gel, which are known laxatives, but randomized, controlled clinical trials that examine the effects of this supplement on gut microbiota composition are lacking.AIM To assess the efficacy of the prebiotic UG1601 in suppressing constipation-related adverse events in subjects with mild constipation.METHODS Adults with a stool frequency of less than thrice a week were randomized to receive either prebiotics or a placebo supplement for 4 wk. All participants provided their fecal and blood samples at baseline and at the end of intervention.Gastrointestinal symptoms and stool frequency were evaluated. The concentrations of serum endotoxemia markers and fecal SCFAs were determined.The relative abundance of SCFA-producing bacteria and the gut microbial community in the responders and non-responders in the prebiotics supplementation group were evaluated.RESULTS There were no significant differences in gastrointestinal symptoms between groups, although the prebiotic group showed greater symptom improvement.However, after prebiotic usage, serum cluster of differentiation (CD) 14 and lipopolysaccharide (LPS) concentrations were significantly decreased (CD14, P =0.012; LPS, P < 0.001). The change in LPS concentration was significantly larger in the prebiotic group than in the placebo group (P < 0.001). Fecal SCFAs concentrations did not differ between groups, while the relative abundance of Roseburia hominis, a major butyrate producer, was significantly increased in the prebiotic group (P = 0.045). The abundances of the phylum Firmicutes and the family Lachnospiraceae (phylum Firmicutes, class Clostridia) (P = 0.009) were decreased in the responders within the prebiotic group. In addition, the proportions of the phylum Firmicutes, the class Clostridia, and the order Clostridiales were inversely correlated with several fecal SCFAs (P < 0.05).CONCLUSION Alterations in gut microbiota composition, including a decrease in the phylum Firmicutes and an increase in butyrate-producing bacteria, following prebiotic UG1601 supplementation might help alleviate symptom scores and endotoxemia.
Key words: Prebiotics; Constipation; Gut microbiota; Endotoxemia; Short-chain fatty acids https://cris.nih.go.kr. The registration identification number is KCT0002422.
INTRODUCTION
Constipation is one of the most common functional disorders, with an estimated prevalence of 8%-20% in population-based studies[1-6]. In general, patients with constipation complain of one or more symptoms, including hardened stools,infrequent evacuation, uncomfortable sense of incomplete evacuation, and excessive time spent for successful defecation[7]. In addition to its polysymptomatic nature, the etiology of constipation is multifactorial. Diet quality and quantity are the most common determinants for transient constipation, while secondary constipation can result from neurological disorders, medication, and/or muscular dystrophy[7,8].Although constipation does not require immediate medical attention, the quality of life in patients with constipation is an important issue.
The gut microbiome comprises a variety of beneficial and harmful bacteria that actively interact with the host[9]. Intestinal dysbiosis is associated with the development of constipation[10]. Prebiotics, comprising one or more indigestible carbohydrates such as inulin, fructo-oligosaccharide, or galacto-oligosaccharide, act as a good energy source for the growth of select favorable bacteria[11]. In addition,prebiotics contribute to changes in the gut microbial community by playing a role in reducing harmful bacteria and increasing the abundance of beneficial bacteria[12-14].Upon reaching the colon, prebiotics undergo bacterial fermentation to produce shortchain fatty acids (SCFAs). SCFAs, especially butyrate, can change stool consistency and lead to relief from pain or discomfort during defecation[15,16]. SCFAs also influence changes in gut motility by stimulating the contraction of colonic smooth muscles,thereby relieving the symptoms of constipation[17,18].Many studies have demonstrated the effects of specific prebiotics on constipation, stool consistency, colonic transit time,and fecal evacuation[13,19-22]. Although the use of prebiotics has been suggested as an alternative for alleviating constipation, the number of randomized, placebo-controlled intervention trials with possible mechanistic explanations are limited. Also, recent development in metagenome analyses can facilitate the understanding of gut microbiota composition and their consequent effects on systemic homeostasis.Therefore, in this randomized placebo-controlled intervention study, we investigated the efficacy of supplementation with the prebiotic UG1601, which consists of inulin,lactitol, and aloe vera gel, all known laxatives, to relieve the symptoms of constipation associated with the gut microbiota in Korean adults.
MATERIALS AND METHODS
Study subjects
Adults (aged 18-60 years) were recruited to the study through advertisements on the university website and on-campus posters. The inclusion criteria were (1) A stool frequency of less than three times a week; (2) Sensation of incomplete evacuation more than 25% of the evacuation time; and (3) Straining with defecation more than 25% of the time. We selected subjects who experienced one or more of these item not less than 3 mo and not more than 6 mo[23,24]. Potential subjects were excluded if they (1)Experienced gastrointestinal diseases or other chronic diseases; (2) Had undergone a major surgery; (3) Used prebiotics, probiotics, or synbiotics within a month; and (4)Received antibiotic treatment in the last 3 mo. Volunteers who are smokers, pregnant,or lactating were also excluded. Written informed consent was obtained from all participants. This study was performed according to the ethical recommendations of the Declaration of Helsinki and approved by the Institutional Review Board of Sookmyung Women's University (SMWU-1601-BR-087-01). This study was also registered with the Clinical Research Information Service (https://cris.nih.go.kr) (No.KCT0002422).
Study materials
The prebiotic UG1601 was provided by Unigen, Inc. (Cheonan, Korea). UG1601 is white-colored powder, composed of inulin (61.5%), lactitol (34.6%), and aloe vera gel(3.9%). Chemical structures of inulin and lactitol and a schematic representation of aloe vera pulp structure are presented in Figure S1. Maltodextrin powder with a texture, color, and odor identical to that of prebiotics was used as the placebo.Prebiotics and placebo powder were prepackaged in a pouch bag and consecutively numbered using the randomization table.
Study design
A randomized, double-blinded, placebo-controlled and parallel study design was used in accordance with the Consolidated Standards of Reporting Trials (CONSORT)guidelines[25]. Sample size was determined using G*Power Analysis program(G*Power 3.1, The G*Power Team, Belgium). All subjects were randomly divided into the placebo (n= 20) and prebiotic groups (n= 20) following a simple randomization procedure using SAS uniform function. A randomization list was blinded until analyses were completed. To allocate the participants, a computer-generated list of random numbers was used. Study subjects, care providers, and individuals assessing outcomes were blinded. A third party who was not associated with this study generated the random allocation sequence, enrolled participants, and assigned participants to interventions. The subjects were asked to consume 13 g/d of either the prebiotic UG1601 or placebo dissolved in water for 4 wk and send a photograph of the empty packet every day after consumption. Stool frequency and gastrointestinal symptoms were recorded at baseline, week 4, and 2 wk after the intervention. All subjects completed a three-day dietary record (2 weekdays and 1 weekend day) to assess their typical diets using the 24-h recall method. Dietary intake of energy,carbohydrates, proteins, fats, and fiber was calculated by the Computer Aided Nutritional analysis program (CAN-Pro 5.0, Korean Nutrition Society, Seoul, Korea).Blood and fecal samples were collected at the beginning and end of the experimental period. Fecal samples were collected in the morning. All samples were self-collected in sterile conical tube and transferred immediately to research staff. All samples were stored at -80 °C until analysis.
Stool frequency score determination
To assess the efficacy of UG1601 on constipation improvement, stool frequency was scored according to a six-point scale from 0 to 5 on the basis of the number of evacuations per week (stool frequency per week < 1 = a score of 0; 1 ≤ stool frequency per week < 2 = 1; 2 ≤ stool frequency per week < 3 = 2; 3 ≤ stool frequency per week <4 = 3; 4 ≤ stool frequency per week < 5 = 4; and stool frequency per week ≥ 5 = 5).
Assessment of gastrointestinal symptoms
We investigated the effect of UG1601 on change in gastrointestinal symptoms and the existence of side effects. Gastrointestinal symptoms, including stool consistency,feeling of incomplete evacuation, time required for evacuation, and flatulence, were assessed based on data provided by participants. Each symptom was categorized into three responses (worsened, improved, or unchanged) depending on any postinterventional improvement in their gastrointestinal symptoms.
Measurement of serum endotoxemia markers
We measured serum lipopolysaccharide (LPS) and their receptor, cluster of differentiation (CD) 14 concentration as measures of bacterial translocation due to increased membrane permeability. LPS is also known to be associated with gut motility. Serum CD 14 concentration was determined using a Quantikine®ELISA kit(R&D Systems, Minneapolis, MN, United States) according to the manufacturer's instructions. Serum LPS concentration was measured using a commercial ELISA kit(MyBioSource, San Diego, CA, United States).
Fecal SCFAs concentration measurement
Fecal SCFAs concentration was measured to determine the association with changes in microbiome composition. Quantification of fecal SCFA concentration was analyzed by gas chromatography-mass spectrometry using an Agilent 7890B GC with an MSD 5977A mass spectrophotometer (Agilent, Santa Clara, CA, United States) and the ion source was electron impact. The carrier gas helium was injected at a rate of 1 mL/min through a DB-5MS column (Agilent, Santa Clara, CA, United States). Before analysis,frozen feces were thawed, and diethyl ether was added, followed by acidification with HCl. The supernatant was extracted at two different stages, and derivatization was performed at 37 °C for 1 h after the addition of MTBSTFA. Acetate, propionate,and butyrate concentrations in the fecal samples were calculated using a standard curve. All experiments and analyses were performed at the Korea Basic Science Institute Western Seoul Center (Seoul, Korea).
Fecal DNA extraction
Fecal DNA was extracted using a QIAamp®DNA Stool Mini Kit (QIAGEN, Hilden,Germany), according to the manufacturer's instructions. Briefly, the frozen fecal samples were thoroughly homogenized. Homogenized samples were heated in a water bath at 95 °C and centrifuged at 20000 ×gat 25 °C for 1 min. After centrifugation, the supernatant was mixed with proteinase K and transferred to the column for washing and separating the DNA. The concentration of the extracted DNA was adjusted to 1 ng/μL and stored at -20 °C until analysis.
Quantification of the relative abundance of bacteria by real-time quantitative polymerase chain reaction
To quantify the relative abundance of SCFA-producing bacteria and prebioticsensitive bacteria, we chose eleven representative bacteria; acetate-producing bacteria[Bifidobacterium longum,Bifidobacterium adolescentis(B. adolescentis), andBifidobacterium catenulatum(B. catenulatum)]; propionate-producing bacteria [Prevotella ruminicola(P.ruminicola),Propionibacterium acidipropionici(P. acidipropionici), andPropionibacterium freudenreichii (P. freudenreichii)]; butyrate-producing bacteria [Faecalibacterium prausnitzii(F. prausnitzii),Clostridium leptum(C. leptum), andRoseburia hominis(R.hominis)]; and prebiotic-sensitive bacteria [Bifidobacterium lactis(B. lactis) andLactobacillus acidophilus(L. acidophilus)]. Real-time quantitative polymerase chain reaction (PCR) was performed on a 7500 Fast Real-Time PCR system (Applied Biosystems, Foster City, CA, United States) using a qPCRBIOSyGreen Mix Lo-Rox(PCR Biosystems Ltd., London, United Kingdom). Each primer sequence targeted the 16s rRNA region of the bacteria (Table S1). Bacterial abundance was expressed as a relative value by using the calculation formula: Log 10 [threshold cycle (Ct) of specific bacteria/Ct of total bacteria][26].
Microbial community analysis
The microbial community of the responders and non-responders in the prebiotic group was analyzed using 16s rRNA pyrosequencing. Twelve subjects in the prebiotic group were selected and grouped as responders and non-responders. The“responders” were defined as subjects whose time required for evacuation had decreased and serum CD 14 concentration had decreased by > 10% at the end of the study. The “non-responders” were defined as subjects whose time required for evacuation was unchanged by prebiotic supplementation, while the serum CD 14 concentration had increased by > 10% at the end of the study. For microbial content analysis, the extracted metagenomic DNA was amplified using primers targeting the V3 and V4 regions of the 16S rRNA gene. Amplification, sequencing, and data analysis were performed by ChunLab, Inc. (Seoul, South Korea).
Statistical analysis
Normality test and Levene's test were performed for the collected data. Differences between the two groups were evaluated using Student'st-test or Mann-Whitney U test. Changes in the values during the treatment period as well as the changes in values at baseline and week 4 were compared by pairedt-test or Wilcoxon signed rank test. Correlation analysis was evaluated using Pearson correlation coefficient or Biserial correlation coefficient. All statistical analyses were performed using IBM SPSS 23.0 for Windows (IBM Corporation, Armonk, NY, United States).
RESULTS
Demographic characteristics of study subjects
Of the 42 subjects who agreed to participate in this study, 40 met the inclusion criteria.The participant screening protocol is illustrated in Figure 1. Ten men [median age, 25 years; median body mass index (BMI), 23.43] and thirty women (median age, 24 years;median BMI, 21.33) completed the study. The two groups showed similar demographic characteristics (Table 1). Compliance of subjects was evaluated based on daily intake; the compliance of the placebo and prebiotic groups was 98% and 99%,respectively. Dietary intake of energy, carbohydrates, proteins, fats, and fiber did not significantly differ within each group (baselinevsweek 4) or between the groups at baseline or week 4 (Table S2).
Stool frequency score and changes in the gastrointestinal symptoms
As shown in Table 2, the stool frequency score significantly increased in both prebiotic (P< 0.001) and placebo (P= 0.002) groups after 4 wk of intervention.Although the prebiotic group showed a greater improvement in gastrointestinal symptoms, no significant changes were noted in gastrointestinal symptoms (Table 3).
Changes in the gastrointestinal symptoms two weeks post intervention
Stool frequency, stool consistency, regularity of evacuation, time required for evacuation, and flatulence 2 wk after the intervention did not significantly differ between the two groups. Additionally, the time required for symptom change did not significantly differ between the two groups (7.00 ± 1.73vs6.33 ± 1.89, Table S3).
Serum concentrations of CD 14 and LPS
The placebo and prebiotic groups had similar basal CD 14 concentrations (1.59 ± 0.07 and 1.51 ± 0.05 μg/mL, respectively). After 4 wk of intervention, the serum CD 14 concentration decreased by 7.84% and 6.62% in the prebiotic and placebo group,respectively, compared to the baseline concentration (prebiotic,P= 0.012; placebo,P=0.130) (Figures 2A and 2C). However, the change in CD 14 concentration (from baseline to week 4) in the prebiotic group did not significantly differ from that observed in the placebo group (-0.06 ± 0.09vs-0.10 ± 0.06,P= 0.670). Serum LPS concentration decreased in both groups (placebo, 122.48 ± 4.52vs119.59 ± 5.49 ng/mL; prebiotic, 130.78 ± 6.36vs109.27 ± 4.51 ng/mL), and the prebiotic group alone showed a significant change after 4 wk of intervention (P< 0.001) (Figures 2B and 2D).The change in LPS concentration was significantly larger in the prebiotic group compared to that in the placebo group (-2.89 ± 3.53vs-21.51 ± 3.29,P< 0.001).
Fecal SCFAs concentration
The concentrations of the three major SCFAs, namely, acetate, propionate, and butyrate, in the fecal samples at baseline did not differ between the two groups. There was also no significant difference within or between the groups after 4 wk of intervention (acetate, 90.12 ± 11.53 ng/mgvs103.45 ± 11.26 ng/mg; propionate, 62.33± 7.85vs74.14 ± 7.60 ng/mg; butyrate, 75.62 ± 6.65vs75.23 ± 10.79 ng/mg) (Table S4).The concentrations of SCFAs at week 4 were not significantly different from baseline concentrations (Table S4).
Relative abundance of SCFA-producing bacteria
The relative abundance of acetate-producing bacteria decreased, and that ofB.adolescentisin the prebiotic group had significantly decreased at the end of the intervention (P= 0.040; Figure 3A). The relative abundance ofB. catenulatumin the prebiotic group increased by 3.45% at week 4, although this change was not significant (Figure 3A). The relative abundance of the propionate-producing bacteria,namely,P. ruminicola, P. freudenreichii, andP. acidipropionicidid not significantly change (Figure 3B). The relative abundance of the butyrate-producing bacteria,namely,F. prausnitziiandC. leptum, slightly increased at week 4, compared to that at baseline (F. prausnitzii, 1.53%;C. leptum, 4.39%) in the prebiotic group, although these values were not significantly different within or between groups (Figure 3C).However, the relative abundance ofR. hominisincreased by 15.93% at week 4; this difference was significantly larger than the difference noted in the placebo group (P=0.045; Figure 3C). The relative abundance of the prebiotic-sensitive bacteria, namely,B. lactisandL. acidophilus, had not significantly changed at the end of the intervention in either group (Figure 3D).
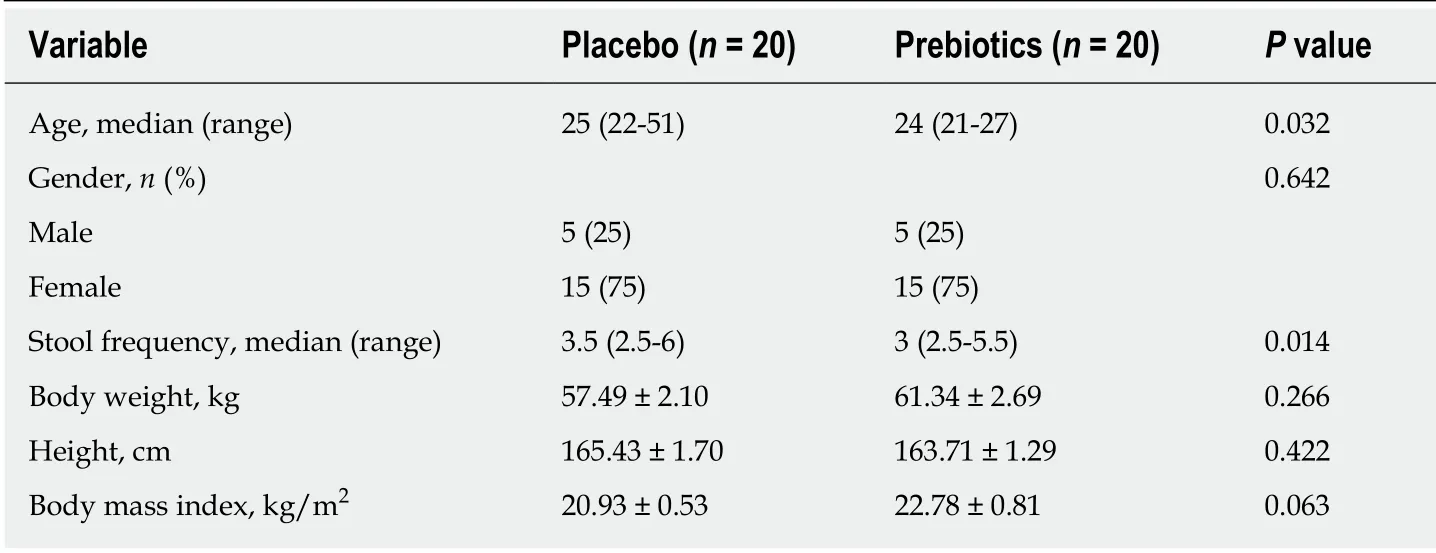
Table 1 Demographic characteristics of study participants at baseline
Microbial community analysis between responders and non-responders treated with prebiotics
Since we could not observe clear improvements in constipation-related symptoms,except stool frequency (shown in Table 2), upon prebiotic supplementation, the differences in the genome-wide microbial composition between responders and nonresponders were compared to determine differences between the two groups, if any.The “responders” are defined as subjects whose serum CD 14 concentration had decreased by > 10%, with improvement in the time required for evacuation (n= 6).The “non-responders” were defined as subjects whose serum CD 14 concentration had increased by > 10% (n= 6) without any changes in the time required for evacuation. To compare the changes in microbial diversity among responders and non-responders, we used three different measures of microbial diversity, including operational taxonomic units, Chao1, and Shannon diversity indices. No statistically significant differences were noted within and between the groups for diversity indices(Table S5).
To compare the changes in the microbial community in responders and nonresponders, we performed 16s rRNA pyrosequencing targeting the V3-V4 hypervariable region. The individual microbiome profiles representing the most abundant 12 phyla at baseline and week 4 are presented in Figure 4A. Especially, after 4 wk of prebiotics treatment, the proportion of the phylum Firmicutes was lower and the proportions of the phyla Proteobacteria and Actinobacteria were higher in the responder group than in the non-responder group, although these differences were not significantly different (Figure 4B). We further analyzed the changes in the relative abundance of subordinate taxa (from baseline to week 4) (Figure 4C and D). In the responders of the prebiotic group, the phylum Firmicutes (P= 0.031), the class Clostridia (P= 0.058), the order Clostridiales (P= 0.058), and the family Lachnospiraceae (phylum Firmicutes, class Clostridia) (P= 0.009) were decreased after 4 wk of intervention compared to the non-responders (Figure 4D). Changes at the subordinate species level indicated that the relative abundances ofPrevotella stercorea(P. stercorea),Bacteroides plebeius(B. plebeius), andBacteroides stercoris(B.stercoris) tended to increase in the responder group, while it decreased in the nonresponder group after intervention. In the non-responder group, the relative abundance ofBacteroide s fragilis(B. fragilis) showed a decreasing trend compared to the level at the baseline (Figure 4D).
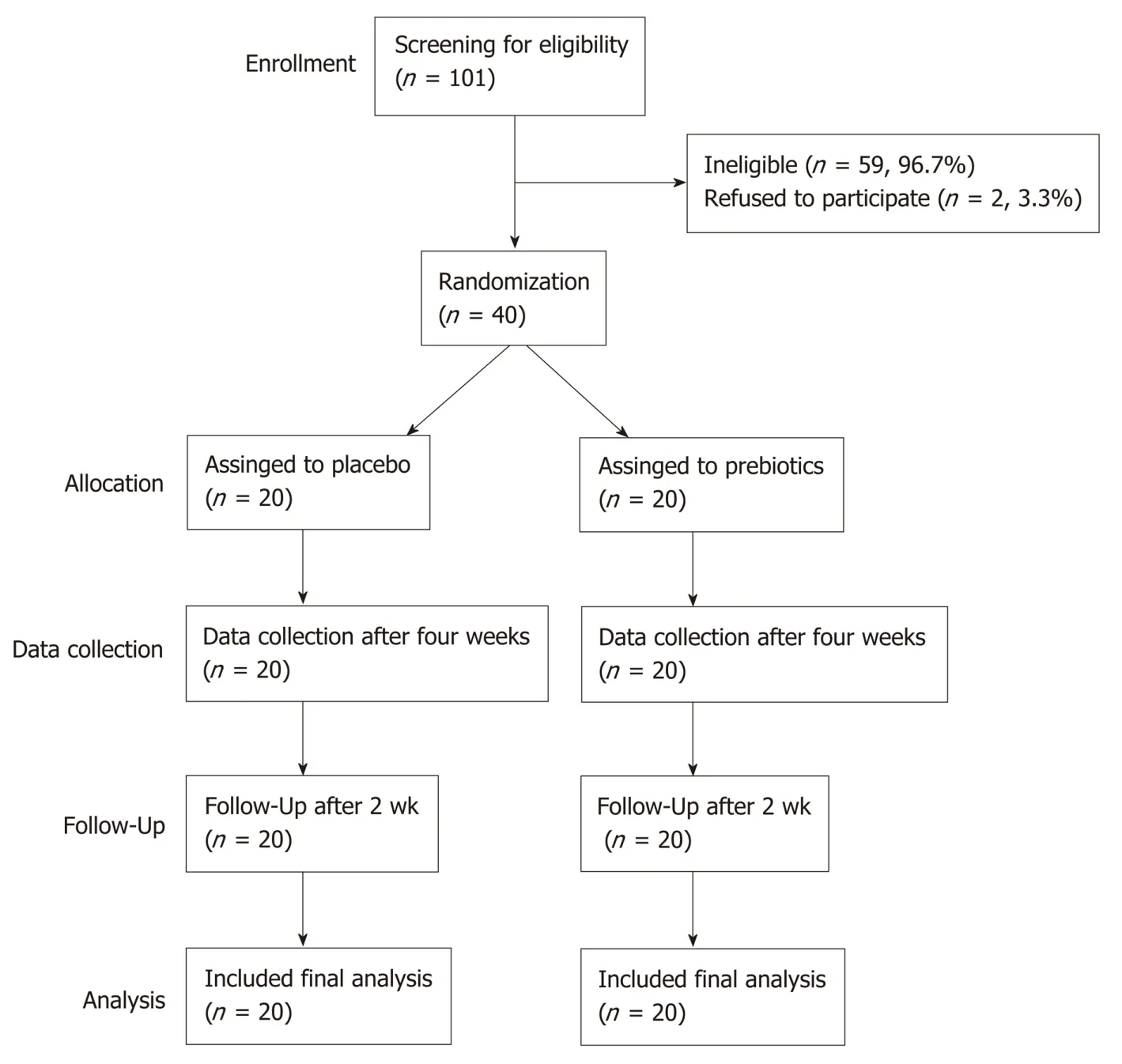
Figure 1 CONSORT diagram illustrating participant recruitment, follow-up, and analysis.
Correlations of gut microbiota with serum endotoxemia markers and fecal SCFAs
To investigate associations between gut microbiota and factors related with constipation, we examined correlations between relative abundances of bacterial groups with clinical factors, serum endotoxemia markers, and fecal SCFA concentrations. The relative abundance of the phylum Firmicutes was negatively associated with fecal concentrations of acetate (R2= -0.6,P= 0.040), propionate (R2= -0.72,P= 0.008), and butyrate (R2= -0.62,P= 0.031). Similarly, the proportions of the class Clostridia, the order Clostridiales, and the family Ruminococcaceae showed a negative relationship with acetate (Clostridia, R2= -0.65,P= 0.023; Clostridiales, R2= -0.65,P= 0.023; Ruminococcaceae, R2= -0.78,P= 0.003) and propionate (Clostridia, R2= -0.7,P= 0. 011; Clostridiales, R2= -0.7,P= 0.011; Ruminococcaceae, R2= -0.67,P=0.017) (Figure 4E). In addition, a positive correlation was found between the proportion of the phylum Proteobacteria and butyrate (R2= 0.66,P= 0.020). The relative abundance of the class negativicutes was positively associated with serum CD 14 concentration (R2= 0.61,P= 0.037) and negatively correlated with propionate (R2=-0.81,P= 0.002) and butyrate (R2= -0.62,P= 0.032) (Figure 4E).
DISCUSSION
In the present study, we used a prebiotic supplement containing inulin, lactitol, and aloe vera gel, which are non-digestible carbohydrates with different known characteristics that are positively associated with relief from constipation. The materials used in this study are known laxatives, about which only few controlled clinical studies are available[27-29], but their effects on microbial composition have not been investigated. This clinical trial showed that 4 wk of intervention with the prebiotic UG1601 in patients with mild constipation resulted in decreased serum concentrations of the bacterial endotoxin LPS and its receptor CD 14. UG1601 supplementation induced an increase in the abundance of the fecal butyrateproducing bacteriumR. hominisand improved stool frequency.
A recent study reported that intestinal dysbiosis leads to the development of constipation. Antibiotic-treated mice receiving fecal microbiota from constipated individuals showed abnormal defecation, which was associated with a serotonintransporter involved in regulating gastrointestinal motility[10]. SCFAs are major bacterial fermentation products that have been suggested to stimulate the mucosal receptor or colonic smooth muscle to increase motility and to maintain gut integrity by regulating epithelial cell proliferation[17,18,30]. Butyrate produced by gut bacteria such asR. hominiscontribute to gut integrity through the replacement of damaged colon cells and by regulating the expression of tight junction proteins or mucus production[31]. Increased gut integrity blocked the leakage of LPS into the circulatory system and the activation of TLR signaling, which accelerated the release of proinflammatory cytokines[32]. In particular, the bacterial endotoxin LPS regulates gastrointestinal motility by increasing the intestinal transit time and causing sphincteric dysfunction[33]. Moreover, a recent study found that serum endotoxin activity was positively associated with constipation in patients undergoing chronic hemodialysis[34]. Thus, we hypothesized that increasing the SCFA content by prebiotic supplementation would reduce endotoxemia associated with dysbiosis-induced gut barrier damage, thereby leading to relief from constipation symptoms. In this study,we did not observe significant differences in fecal SCFAs concentrations after prebiotic supplementation. A recent systemic review on dietary intervention trials to compare SCFA production in obese subjects did find consistent and significant differences in fecal SCFAs regardless of changes in body weight[35]. Possible explanations for this inconsistency included small sample sizes, heterogeneity of study participants, and lack of standardized fecal SCFA measurements. Our study also included limited number of study subjects which might be too small to overcome the inter-individual variations. Also, significant proportions of SCFAs produced in the intestine escape fecal excretion due to enterohepatic circulation[35]limiting representativeness of fecal SCFAs as total SCFAs produced in the intestine. However,our results indicated that levels of the endotoxemia markers decreased within 4 wk of prebiotic intervention, and that the relative proportion of several SCFA-producing bacteria might be related to a decrease in the levels of these markers.
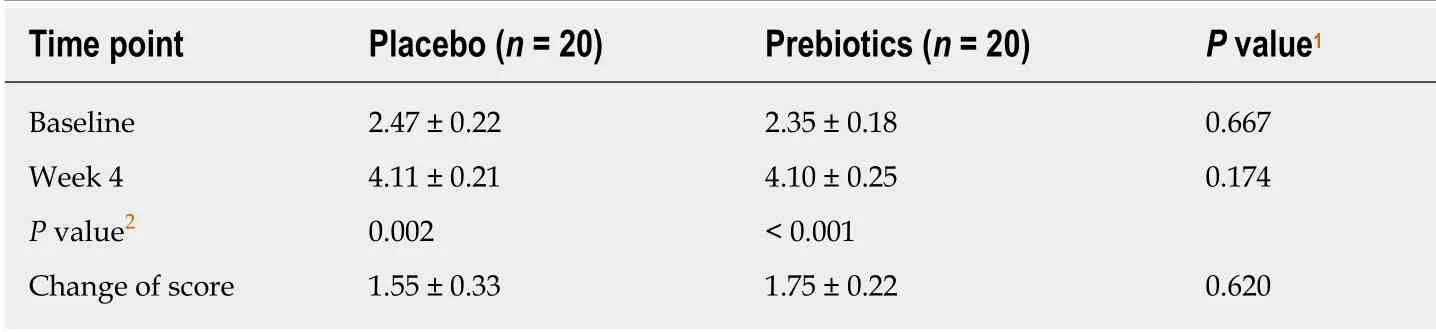
Table 2 Stool frequency score of 40 mildly constipated subjects treated with either placebo or prebiotics at baseline and 4 wk of intervention
Case-control studies have demonstrated differences in gut microbiota composition between patients with constipation and healthy control subjects. A previous study found that patients with constipation exhibited a decrease in the abundance of bifidobacterium, lactobacillus, clostridium, bacteroides, andStreptococcus faecalis, and increases in the abundance of potentially harmful bacteria such asEscherichia coliandStaphylococcusaureus, compared to levels in healthy control subjects[36]. Another study reported that patients with constipation had a significantly lower proportion of Bacteroidetes phylum, while the proportion of Firmicutes, Actinobacteria, and Proteobacteria phyla was higher compared to that in the control group[37]. We assumed that the differences between the responders and non-responders within the prebiotic group would be derived from the differences in the composition of the gut microbiota in association with endotoxemia and gut motility. Indeed, we found that the abundances of the phylum Firmicutes, the class Clostridia, and the order Clostridiales were reduced in the responders after 4 wk of intervention, representing the inverse associations with several fecal SCFAs. Increased abundance of the phylum Firmicutesis a major characteristic of patients suffering from constipation[11], and the Bacteroidetes : Firmicutes ratio is positively correlated with stool consistency[38].
Contrary to our expectations, the abundance of Proteobacteria showed an increasing trend in the responder group. This increase was previously shown to be related to various metabolic diseases, including diabetes, obesity, or inflammatory bowel disease[39]. However, a study showed that the LPS derived fromthis speciesactivated Toll-like receptor signaling and acetylcholine response. Increased acetylcholine response restores spontaneous contraction frequency, suggesting that Proteobacteria might contribute to normal gut motility[40]. In addition, the abundanceof Bacteroidetes (P. stercorea, B. plebeius, andB. stercoris), which are known to ferment carbohydrates and affect colonic transit time[41], tended to be higher in the responder group of this study. In particular, the abundance ofB. fragilistended to decrease after prebiotic supplementation in the non-responder group.B. fragilisis a well-known species that can directly induce the conversion of CD4 + T cells into Foxp3 + Tregcells by using their own polysaccharide A. Increased Tregcapacity promotes the production of the anti-inflammatory cytokines, mediating increased mucosal surface tolerance and decreased intestinal inflammation[42]. Thus, it appears that a decreased proportion ofB. fragilismight be associated with the increased circulating CD 14 levels observed in the non-responder group. Furthermore, we found that the proportion of the class Negativicutes, known to harbor outer membranes containing LPS, was positively associated with serum CD 14 concentration and negatively correlated with levels of several fecal SCFAs. Taken together, our data suggest that alterations in gut microbiota composition would be associated with differences in response to prebiotic supplementation.
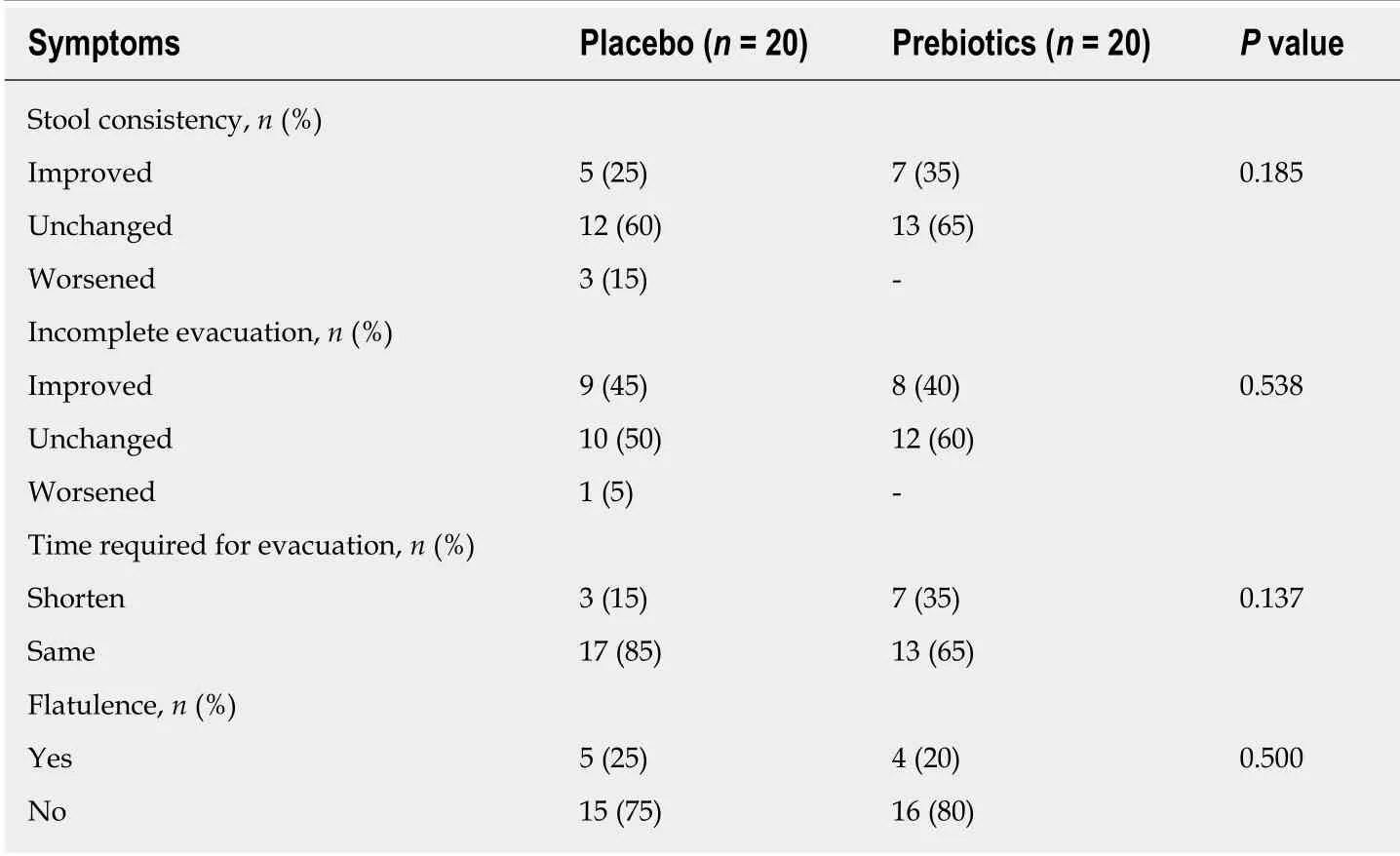
Table 3 Changes in the abdominal and fecal symptoms of 40 mildly constipated subjects after 4 wk of intervention, based on self-reporting
The present study was a randomized, double-blinded, placebo-controlled trial with a high rate of compliance. This is one of the few clinical studies on the association of gut microbiota composition and constipation[12,43,44]. However, our study has several limitations. The lack of significant improvement in constipation symptoms could be attributed to the small sample size of this intervention study. In addition, the participants were mostly young females with mild constipation, who were otherwise healthy. Given that sex- and gender-specific differences have been reported in patients with constipation[45,46], further clinical studies are required to provide evidence on sex- and gender-associated disparities in the effects of prebiotics on relieving the symptoms of constipation. Moreover, the dose of each substance in the prebiotic UG1601 might not be sufficient to improve constipation symptoms.Considering previous studies reporting the beneficial effects of each substance in prebiotic supplements[47-56], adequately powered clinical trials that take into account the dose of bioactives within each substance are needed to develop prebiotics that are effective in relieving constipation-related symptoms.
Although we did not measure hematochezia or serum concentrations of calcium,ferritin, potassium and magnesium, these are also important indicators to determine the degree of stool softness[57-59]. Thus, the inclusion of these clinical and biochemical factors may be helpful for diagnosing and managing constipation in clinical settings.
In conclusion, supplementation with the prebiotic UG1601 in subjects with mild constipation improved stool frequency and suppressed endotoxemia, as determined by the concentrations of LPS and CD 14. Alterations in the microbial composition including a decrease in the abundance of the phylum Firmicutes and an increase in the abundance of butyrate-producing bacteria may reduce the available LPS content which can regulate intestinal motility and lower endotoxemia.
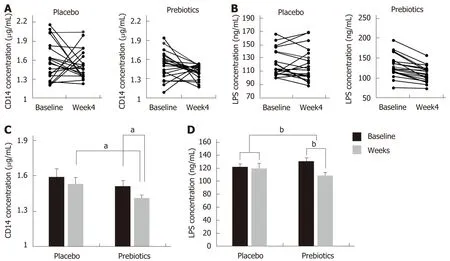
Figure 2 Changes in the serum cluster of differentiation 14 and lipopolysaccharide concentrations after 4 wk of intervention. A and C: Changes in the serum cluster of differentiation (CD) 14 after 4 wk of intervention; B and D: Changes in the serum cluster of lipopolysaccharide (LPS) concentrations after 4 wk of intervention. After 4 wk of intervention, the concentrations of serum CD 14 and LPS were significantly reduced in the prebiotics group. Bar charts show the mean ±standard error of the mean. aP < 0.05; bP < 0.001. Significantly different from the values at placebo or baseline, using the Student’s t-test or paired t-test. CD: Cluster of differentiation; LPS: Lipopolysaccharide.
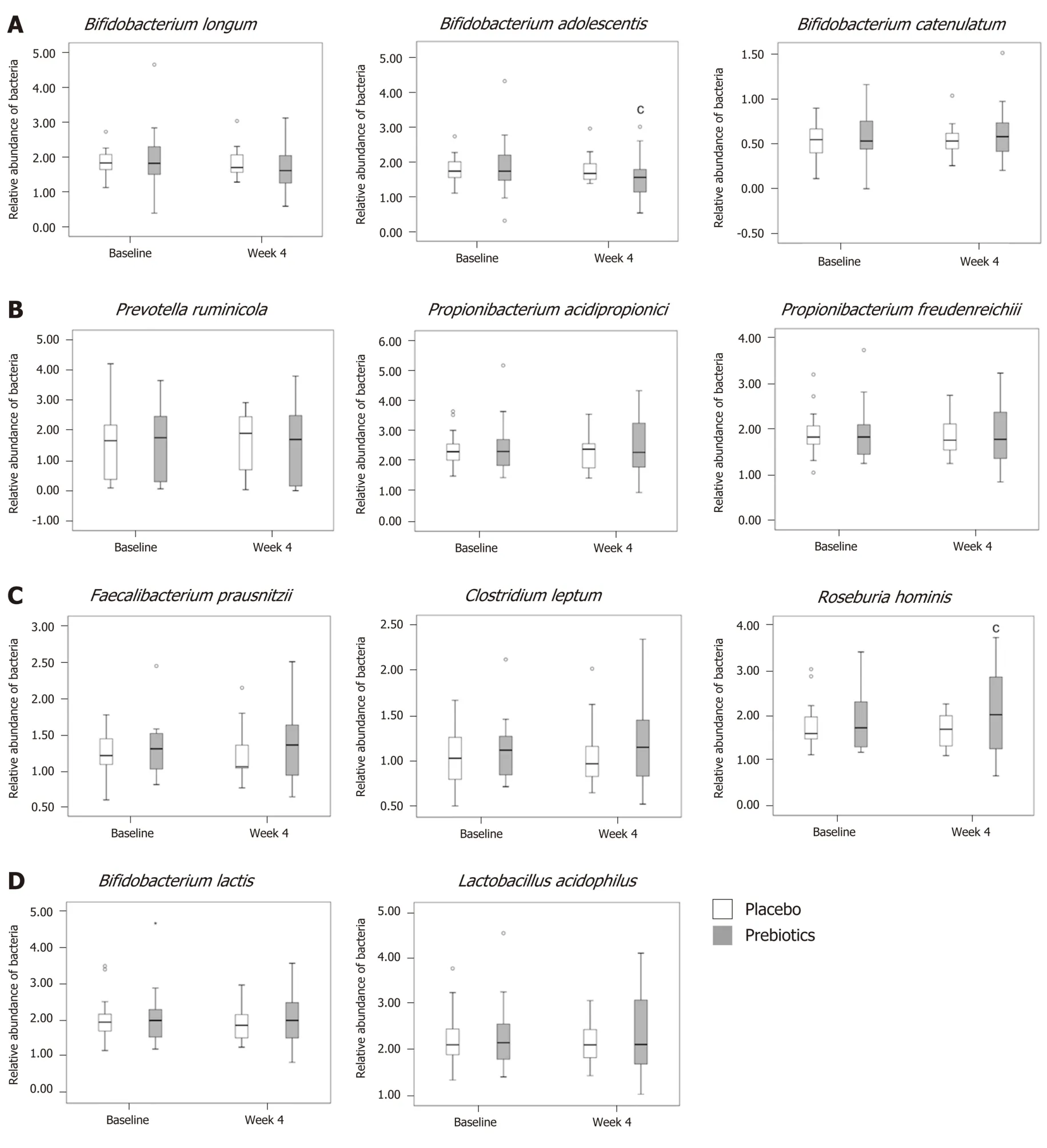
Figure 3 Analysis of Relative abundance. A: Relative abundance of acetate-producing bacteria; B: Propionate-producing bacteria; C: Butyrate-producing bacteria;D: Prebiotic-sensitive bacteria. In the prebiotics group, the relative abundance of Bifidobacterium adolescentis was decreased and that of Roseburia hominis was increased after 4 wk of intervention. Box plots show the 25th and 75th percentiles, median, and range. Outliers are expressed as a small circle. cP < 0.05. Significantly different from the values at baseline, using the Student’s t-test.
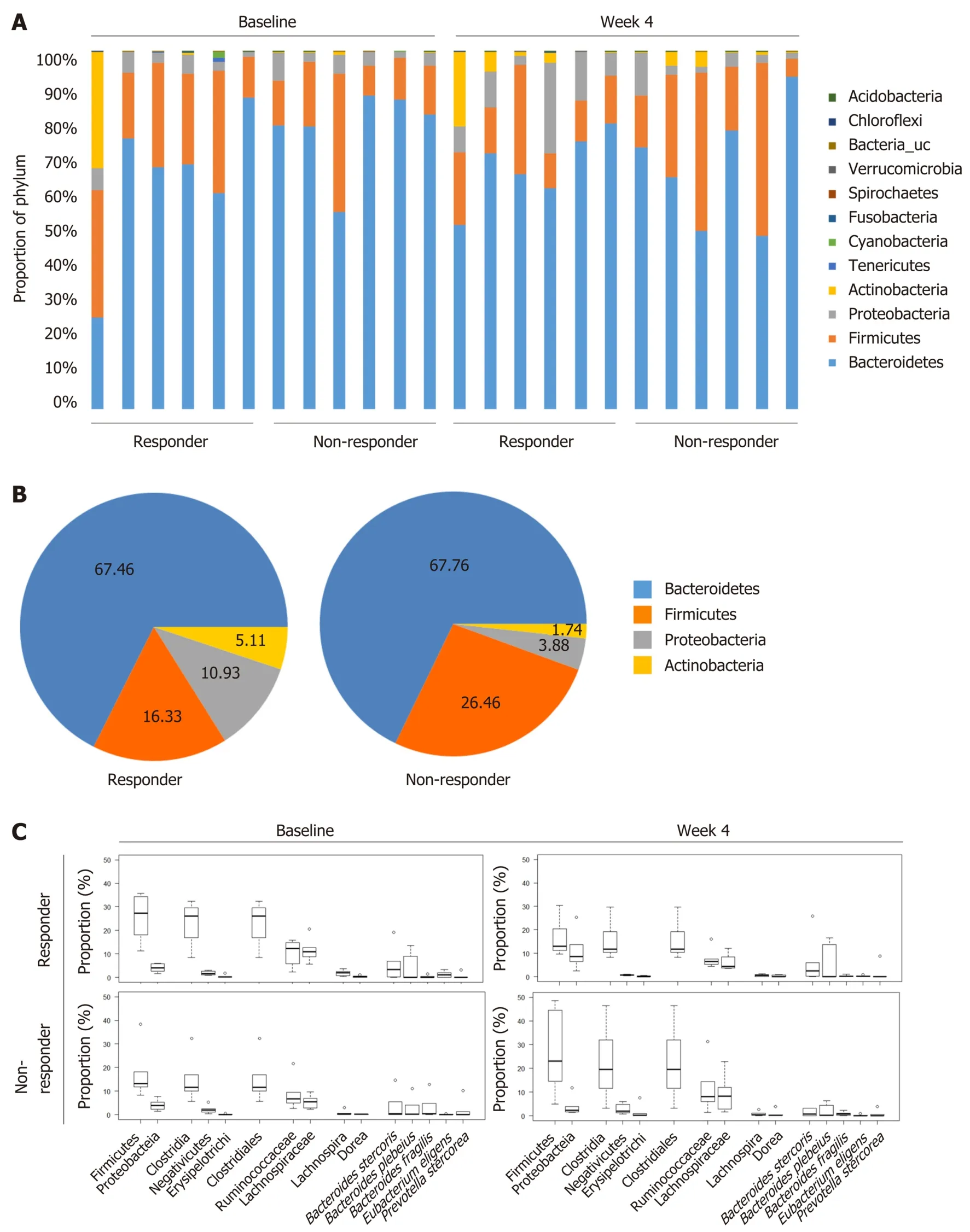
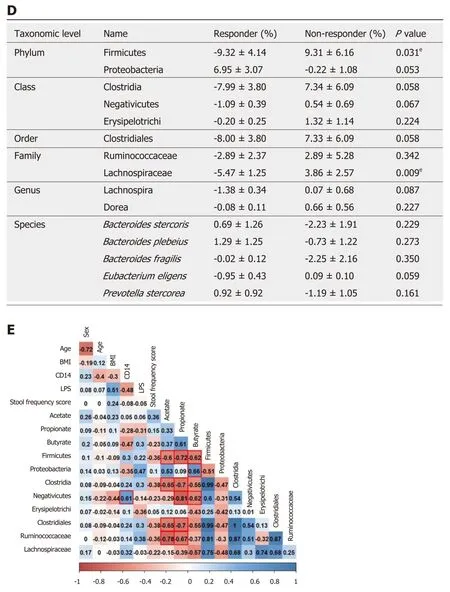
Figure 4 Microbial community analysis between responders and non-responders treated with prebiotics. A: Gut microbiome phylum profile of the responder and non-responder groups at baseline and week 4; B: Major microbiome phylum profile at week 4; C and D: Changes in the relative abundance of subordinate taxa(from baseline to week 4); E: Correlations of gut microbiota with serum endotoxemia markers and fecal short chain fatty acids. The abundances of the phylum Firmicutes, the class Clostridia, and the order Clostridiales were reduced in the responders after 4 wk of intervention representing the inverse associations with several fecal short chain fatty acids. Box plots show the 25th and 75th percentiles, median, and range. Outliers are expressed as a small circle (C). eP < 0.05. Significantly different from the values at responders, using the Student’s t-test (D). The number in each box indicates coefficient of correlation. The blue color implies a positive correlation, while the red color indicates a negative correlation. Statistically significant correlation is highlighted with a red line (E). CD: Cluster of differentiation; LPS:Lipopolysaccharide; BMI: Body mass index.
ARTICLE HIGHLIGHTS
Research background
Constipation is a common functional gastrointestinal disorder. However, its etiology is multifactorial and there is no medicine for remedy. Constipation is not only related to other gastrointestinal disease including irritable bowel syndrome or colorectal cancer but also lowers the quality of life. Therefore, it is essential to find proper supplement that controls the symptoms.
Research motivation
Recently, several evidences suggest that the gut dysbiosis is associated with the occurrence of constipation. However, most of studies have revealed superficial relationship between gut microbiota and constipation for some of western population.
Research objectives
In this study, we focused on prebiotics that might regulate gut dysbiosis and constipation. We assessed the efficacy of the prebiotic UG1601 in suppressing constipation-related adverse events in subjects with mild constipation. Furthermore, we investigated the relationship between gut dysbiosis and constipation.
Research methods
Adults with a mild constipation were randomized to receive either prebiotics or placebo supplements for 4 wk. Gastrointestinal symptoms and stool frequency were evaluated. Serum endotoxemia markers, fecal short-chain fatty acids (SCFAs), relative abundance of SCFAproducing bacteria and the gut microbial community in the responders and non-responders in the prebiotics supplementation group were determined.
Research results
After prebiotic usage, serum cluster of differentiation (CD) 14 and lipopolysaccharide (LPS)concentrations were significantly decreased. Fecal SCFAs concentrations did not differ between groups, while the relative abundance ofRoseburia hominis, a major butyrate producer was significantly increased in the prebiotic group. The abundances of the phylum Firmicutes and the family Lachnospiraceae that were correlated with SCFAs were deceased in the responders within the prebiotic group.
Research conclusions
Changes in gut microbiota comp osition including a decrease in the phylum Firmicutes and an increase in butyrate-producing bacteria following prebiotic UG1601 supplementation might contribute to improvement of symptom and endotoxemia.
Research perspectives
This study suggests endotoxemia markers including CD14 and LPS are correlated with constipation through alteration of gut microbial composition. To elucidate causality,investigation of other clinical factors that are related to constipation and gut dysbiosis was needed. Also, clinical study involved various age and population will be needed.
ACKNOWLEDGEMENTS
We would like to thank the participants involved in this study.
杂志排行
World Journal of Gastroenterology的其它文章
- Role of liver biopsy in hepatocellular carcinoma
- Ultrasound-based techniques for the diagnosis of liver steatosis
- lnsulin-like growth factor 2 mRNA-binding protein 1 promotes cell proliferation via activation of AKT and is directly targeted by microRNA-494 in pancreatic cancer
- Nucleus tractus solitarius mediates hyperalgesia induced by chronic pancreatitis in rats
- Sustained virologic response to direct-acting antiviral agents predicts better outcomes in hepatitis C virus-infected patients: A retrospective study
- Endoscopic retrograde cholangiopancreatography in children with symptomatic pancreaticobiliary maljunction: A retrospective multicenter study
