Multifaced regulator: RNA binding proteins and their roles in hematopoiesis
2019-11-02YueRenYueHuoWeiqianLiFangWangJiaYu
Yue Ren, Yue Huo, Weiqian Li, Fang Wang,*, Jia Yu,*
aKey Laboratory of RNA and Hematopoietic Regulation, Chinese Academy of Medical Sciences, Beijing, PR China; bState Key Laboratory of Medical Molecular Biology, Department of Biochemistry and Molecular Biology, Chinese Academy of Medical Sciences, School of Basic Medicine Peking Union Medical College, Beijing, PR China.
Abstract
Keywords:Hematopoiesis, RNA binding protein
1.RBPS ARE DIVERSIFIED REGULATORS INVOLVED IN DIFFERENT REGULATION PATHWAYS
So far,there are approximately 20,000 protein-coding genes in human genome,of which over 1500 have been annotated as RNA binding proteins (RBPs) that regulate the biogenesis, fate, and function of RNAs.1
Conventional RBPs take part in the formation of ribonucleoprotein complex and govern RNA processing to maintain fundamental gene expression.2RBPs recognize and bind to specific sequences or structures in RNA molecules via a set of defined RNA binding domains (RBDs),3although studies on decoding ribonucleoprotein structures revealed the existence of protein-RNA interactions that do not require classical RBDs,4-9and this unconventional type of protein-RNA interaction is more common than anticipated.
The canonical regulation mode of an RBP is to take “good care”of its target RNAs on different aspects;however,emerging evidences have indicated that the RNA-modulating pathway is not universally applicable, as some RBPs can be recruited by RNA, while the others can work independently, for instance,PKR (interferon-induced, double-stranded RNA-activated protein kinase)and RIGI(retinoic acid-inducible gene I protein)can be activated by abnormal RNA molecules derived from virus replication10-12and TRIM25 can activate the production of interferon upon the stimulation of viral RNA molecules.13Furthermore, several RBPs have been reported to act on chromatin, thus regulating gene transcription.For example,Lin28A binds to genomic regions near the transcription start sites and activates gene transcription by interplaying with DNA methylcytosine oxidase (Tet1).14Another study in Arabidopsis thidopsis revealed that AGO1,a classical RBP involved in RNA interference, could also bind to specific chromatin regions with SWI /SNF complex to activate gene expression.15Moreover, it was reported that hnRNP U(heterogeneous nuclear ribonucleoprotein U)bound to the active chromatin regions and facilitated the maintenance of the 3D structure of chromatin together with CTCF, RAD21 and other chromatin structural proteins.16
Currently, the traditional conception of RBPs is being overturned gradually, and increasing evidences prompt us to redefine the role of RBP in multiple biological processes not only as a key regulator of RNA metabolism.
2.RBPS PLAY CRITICAL ROLE IN HEMATOPOIESIS AT THE POSTTRANSCRIPTIONAL LEVEL
Hematopoiesis is a stepwise process that requires multilayered regulation to keep a subtle balance between hematopoietic stem cell(HSC)stemness maintenance anddownstreamlineagecommitment.Any perturbation in the balance would lead to severe pathological phenotypes.On the other hand, efforts to discover novel key regulators or pathways that regulate the hematopoietic homeostasis may offer great opportunities to manipulate the composition of hematopoieticcellpool,thussheddinglightsonclinicalmanagement of malignant hematological diseases.
Although numerous studies focused on transcriptional regulation have proved the crucial role in hematopoiesis,17,18the pluripotency of HSC and its capacity to differentiate into all blood cell subtypes by cell fate decision make it an extraordinary complex system that must be controlled precisely at multiple levels.Besides the classical “checkpoint” function on the way from DNA to RNA,the regulatory events occurring on the RNA molecules have won their reputation as a detrimental process in hematopoiesis in recent years.This posttranscriptional regulation mechanism forms an additional level for the rapid and precise orchestration of protein expression during hematopoiesis.19Importantly, it is well-recognized that RBPs are the key players involved in this regulation network.
So far, several RBPs have been identified to regulate hematopoiesis via regulating RNA splicing, RNA modification,RNA translation,or RNA decay.The RNA splicing is the major component of RBP-regulated pathways.For example, the RBP Rbm15 regulates the splicing of c-Mpl receptor and affects the thrombopoietin signaling pathway, thus contributing to the quiescence and proliferation of HSC.20Another splicing factor Srsf2 has a role in HSC production and hematopoietic reconstitution in mouse model.21,22Previously, we identified QKI5 could activate the processing of primary miR-124-1 and decreased QKI5 during erythropoiesis led to concomitant reduction of mature miR-124, which facilitated erythrocyte maturation.23Furthermore, we also confirmed that RBP KSRP promoted the processing of primary miR-129, thus inducing granulocyte differentiation at the level of monocyte-macrophage differentiation.24The most well-characterized modification on RNA is N6-methyladenosine(m6A),25which is dominated by the“writer” protein METTL3 and METTL14.26,27METTL3 depletion induces loss of m6A modification, which then triggers the expression of hematopoietic differentiation-associated genes;therefore, Mettl3-deficient mice displayed T cell activation deficiency.28RBP Musashi-2 (MSI2), a repressor of mRNA translation, has a key role in HSC self-renewal and lineage determination by modulating TGF-β signaling pathway.29RBP ZFP36 has been reported to control erythroid differentiation by regulating RNA decay.30,31However, the current knowledge of RBP's function in hematopoietic system is still very limited and mainly focus on the RNA-binding-associated activities; thus,further investigation of multifunctional potency of the hematologic RBPs is highly demanded.
3.INSIGHTS INTO THE MULTILAYERED REGULATORY POTENCY OF NOVEL RBPS IN HEMATOPOIETIC SYSTEM
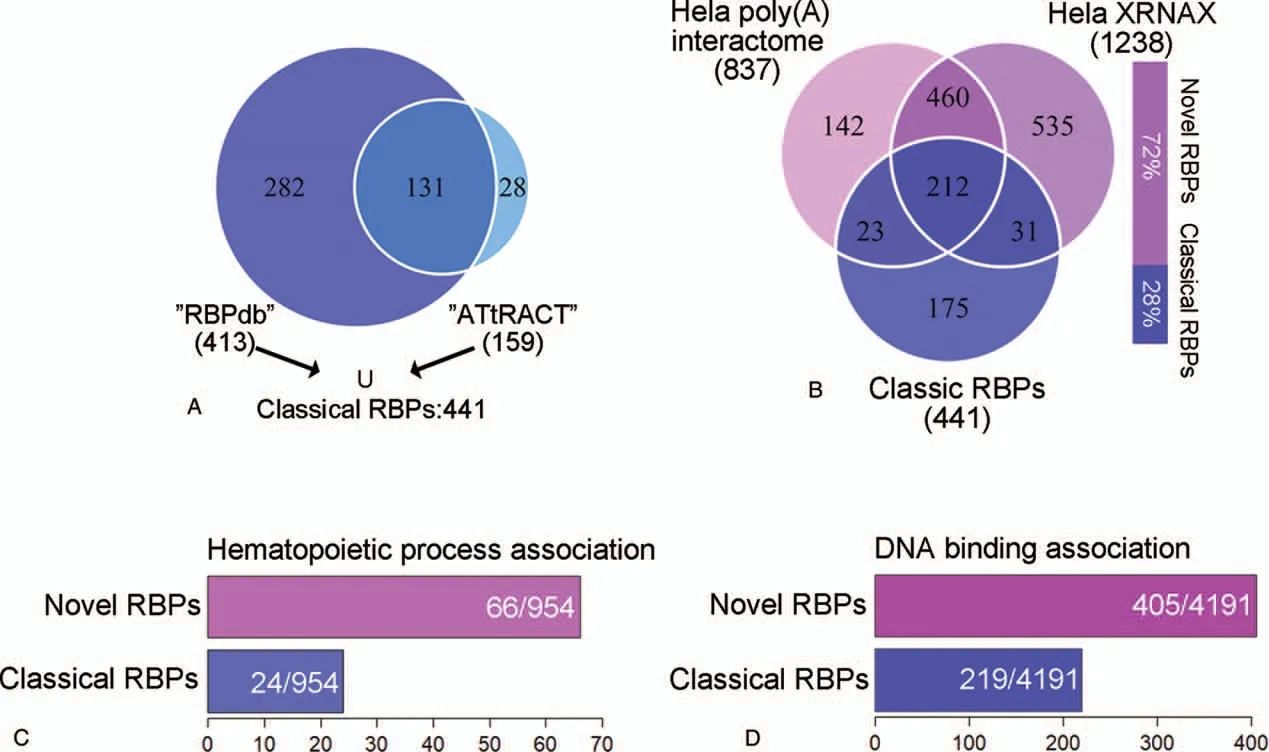
Figure 1.Outline of newly identified RBPs and their potential function in hematopoietic system.A: Venn diagram of RNA binding proteins in the RBPdb and ATtRACT database.The union of these two sets represents the classical human RNA binding proteins,consisting of 441 proteins.B:Venn diagrams comparing the 441 classical RBPs to the 1137 novel RBPs.The novel RBPs are identified by XRNAX-derived RNA binding proteomes or poly(A) interactomes.The histogram described the proportion of novel and classical RBPs.C:The histogram describes the 66 novel and 24 classical RBPs associated with the hematopoietic process.The hematopoiesis-related terms are collected in gene ontology database(http://geneontology.org/),with the following keywords:hemopoiesis;hematopoietic;erythrocyte; megakaryocyte; monocyte; neutrophils; lymphocyte; myeloid.D:There are 405 novel and 219 classical RBPs identified in high-throughput studies defining the human dsDNA interactomes.
Currently benefited from the development of large-scale screening approaches for novel RBPs, we can have a glimpse of the newly identified multipotent RBPs in hematopoiesis.According to two most commonly used databases, RBPdb and ATtRACT,there are only 441 classical RBPs annotated(Fig.1A).However,Castello et al identified 837 proteins that could bind to RNA directly using a poly(A)-baiting protein capture screening system.32Among these recognized RBPs, 602 were found to interact with RNA for the first time (Fig.1B).Another outstanding work carried out by Trendel et al described a method named“XRNAX”,which extracted protein-cross-linked RNAs based on organic phase separation.33“XRNAX” could purify broad-spectrum RNA and also provided a new protocol for RNA binding protein screening,which picked up 1238 RBPs,among them 995 were newly identified (Fig.1B).These two studies revealed in total 1137 novel RBPs that accounted for up to 70% of all RNA-protein interaction events as compared to classical ones that only hit less than 30%.
As for hematopoietic system, we realized that the importance of RBP in hematopoiesis should be recharacterized because of the ever-expanding list of RBPs.Therefore, we obtained 954 hematopoiesis-associated genes from QuickGO database.34,35When we investigated the gene sets,only 24 classical RBPs were involved in hematopoietic system.However,66 RBPs from those newly discovered RBP set were found to be associated with different pathways in hematopoiesis (Fig.1C).The increasing number of RBPs demonstrated its crucial role of RNA-mediated functions in hematopoietic system.
Indeed, the field of RBPs is far beyond our current understanding, because not only a good deal of potential RBPs are needed to be uncovered,but also the inadequate knowledge of the multilayered regulation capacity of RBPs.A research profiling the human protein-DNA interactome revealed 4191 DNA binding proteins (DBPs).36It is notable that when we looked into this large set of DBPs, we observed 405 newly identified RBPs and 219 classical RBPs (Fig.1D).These discoveries prompted us to reconsider the definition between DBP and RBP,and the multiple regulatory potentials of RBP on both posttranscriptional and transcriptional levels.For the hematopoietic system,we found 37 RBPs that were predicted to have the ability to bind both RNA and DNA.They accounted for about 40% in all hematopoietic RBPs, suggesting a rather common phenomenon for RBPs to have multifunction in one system.Moreover, from the Encode project,37we found 92 RBPs with their defined ChIP-seq data, further proving that RBPs indeed have multiple regulatory capacities, while 7 of them were functionally proved in hematopoietic system.
Taken together, in complex systems such as hematopoiesis,regulators tend to function precisely at multiple levels.Case studies and large-scale screening have demonstrated that RBPs could regulate gene expression at different levels.Development of new techniques will definitely provide new insights of RBPs and their novel regulatory pathways.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (81530007 and 31725013), CAMS Innovation Fund for Medical Sciences (2017-I2M-3-009 and 2017-I2M-1-015), CAMS Young Talents Award Program(2018RC310013),the National Key Research and Development Program of China (2016YFA0100601), the National Key Basic Research Program of China(2015CB943001),the CAMS(2017-I2M-B&R-04), and Grant from Medical Epigenetics Research Center, CAMS (2017PT31035).Author contributions: J.Yu and F.Wang supervised the article.Y.Ren wrote the first draft.Y.Huo performed bioinformatics analysis and made the figure.W.Li revised the article.
杂志排行
血液科学的其它文章
- Successful ex vivo expansion of mouse hematopoietic stem cells
- A chemotaxis model to explain WHIM neutrophil accumulation in the bone marrow of WHIM mouse model
- Interleukin-12 supports in vitro self-renewal of long-term hematopoietic stem cells
- The disruption of hematopoiesis in tumor progression
- Progress of cGVHD pathogenesis from the perspective of B cells
- Molecular mechanisms for stemness maintenance of acute myeloid leukemia stem cells