Interleukin-12 supports in vitro self-renewal of long-term hematopoietic stem cells
2019-11-02ShnshnZhngMikoMoritZhoWngJunOoehrSenZhngMinerXieHitoBiWenyingYuXiofngWngFngDongJinhongWngShihuiStoshiYmzkiHideoEm
Shnshn Zhng, Miko Morit, Zho Wng, Jun Ooehr, Sen Zhng, Miner Xie,Hito Bi, Wenying Yu, Xiofng Wng, Fng Dong, Jinhong Wng, Shihui M,Stoshi Ymzki,,*, Hideo Em,*
aInstitute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College,Tianjin, China; bProject Division of Advanced Regenerative Medicine, Institute of Medical Science, University of Tokyo, Tokyo,Japan; cLaboratory of Stem Cell Therapy, Center for Experimental Medicine, Institute of Medical Science, University of Tokyo,Tokyo, Japan
Abstract
Keywords:Ex vivo expansion, Hematopoietic stem cells, Interleukin-12, Self-renewal
1.INTRODUCTION
Most adult bone marrow HSCs reside in a quiescent state at any given time and infrequently cycle for self-renewal or differentiation into blood lineages.1-3Cytokines play essential roles regarding when hematopoietic stem cells (HSCs) begin cycling.4Previous studies have revealed that interleukin (IL)-3, IL-6,IL-11,5-7thrombopoietin (TPO),8and granulocyte-colony stimulating factor,9in combination with stem cell factor(SCF), can drive HSCs into the cell cycle.These cytokines generally share intracellular signal transduction pathways.However, whether these cytokines have different effects on HSC self-renewal and differentiation is currently unknown.
There is a functional heterogeneity among HSC populations.10-17In the mouse, various classifications of HSC subsets with different names have been proposed.13,14,16,18In this study,short-term (ST) and long-term (LT) HSCs were defined at the clonal level and used in subsequent experiments.ST-HSCs reconstituted myeloid,B-lymphoid,and T-lymphoid lineages at a specific time,and their myeloid reconstitution levels decreased by 6 months after transplantation.The myeloid reconstitution levels of LT-HSCs were maintained or increased for 6 months or longer after transplantation.Moreover,myeloid reconstitution alone is sufficient to detect LT-HSCs, and lymphoid reconstitution may not be essential for LT-HSCs.19
The IL-12 family comprises IL-12, IL-23, IL-27, and IL-35.20Their subunits are shared to form heterodimeric ligands and their receptors.IL-27 directly supports myeloid differentiation but not self-renewal in HSCs.21Neither IL-23 nor IL-35 seems to have any effect on HSCs (HE, unpublished data, 2017).Two decades ago,IL-12 in combination with SCF,Flt3 ligand,or IL-11 was shown to stimulate hematopoietic progenitors.22-25However,whether IL-12 acts directly on transplantable HSCs and supports their self-renewal and/or differentiation has never been definitively examined.
We have previously shown that TPO and SCF together support in vitro self-renewal division in serum-free cultures to some extent.8,26,27In this study,we compared the direct effects of IL-12 and TPO in combination with SCF on ST-and LT-HSC fates at the single-cell level.We found that both TPO and IL-12 support the self-renewal of ST-and LT-HSCs in culture,but that IL-12 is superior at supporting LT-HSC self-renewal.
2.METHODS
2.1.Mice
C57BL/6(B6)mice were purchased from Beijing HFK Bioscience,Co.(Beijing, China) or Japan SLC (Shizuoka, Japan).Ly5 congenic B6 mice (B6-Ly5.1) were bred and maintained at the State Key Laboratory of Experimental Hematology or purchased from Sankyo Lab (Tsukuba, Japan).Eight- to 10-week-old female mice were used for all experiments.All experimental protocols using mice were approved by the institutional animal care and use committees at the Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, and at the Institute of Medical Science, University of Tokyo.
2.2.Isolation of HSC1 cells
Bone marrow cells were obtained from the femora, tibiae, and iliac crests of 8- to 10-week-old female B6 or B6-Ly5.1 mice.c-Kit-positive cells were enriched by anti-c-Kit antibody-conjugated MACS beads(Miltenyi Biotechnology,Germany)and stained with a lineage cocktail consisting of allophycocyanin (APC)-eFluor 780-conjugated anti-B220 (RA3-6B2, eBioscience,Waltham, MA), Gr-1 (RB6-8C5, eBioscience), and Ter-119(TER-119, eBioscience) antibodies.Cells were also stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD34(RAM34, eBioscience), APC-conjugated anti-c-Kit (2B8, eBioscience),phycoerythrin-cyanine 7(PE-Cy7)-conjugated anti-Sca-1 (D7, eBioscience), brilliant violet 421-conjugated anti-CD48(HM48-1, eBioscience), PE-conjugated anti-CD150 (TC15-12F12.2, Biolegend), and PerCP-eFluor 710-conjugated anti-CD41(eBioMWReg30,eBioscience)antibodies.CD150+CD41-CD48-CD34-c-Kit+Sca1+Lineage-(CD150+41-34-KSL) cells,which were designated HSC1, were sorted with a FACSAria III(BD Biosciences).
2.3.Serum-free culture components
S-Clone(Sanko Junyaku,Japan)had previously been provided in a package of SF-O3 medium; the supplement contained insulin,transferrin, bovine serum albumin (BSA), and other factors.Recently, BSA was excluded from the manufacturer's package.Thus, we used rHSA purchased from Albumin Bioscience(Huntsville, AL) or Sigma-Aldrich.Dulbecco Modified Eagle Medium (DMEM), Roswell Park Memorial Institute (RPMI)1640 medium, Ham F-12 medium, insulin (I), transferrin (T),selenium (S), ITS, and ITS with ethanolamine (ITS-X, 51500-056) were purchased from Thermo Fisher Scientific (Waltham,MA).
2.4.New standard serum-free medium
We optimized all of our serum-free culture conditions.Our new standard serum-free medium consisted of 0.5mg/mL rHSA(Albumin Bioscience,1001),1×ITS-X(Thermo Fisher Scientific,51500-056), 10mM HEPES (Sigma Aldrich, H0887), 0.1mM MEM nonessential amino acids (Thermo Fisher Scientific,11140050), 2mM L-glutamine (Thermo Fisher Scientific,25030081), 0.055mM 2-mercaptoethanol (Thermo Fisher Scientific, 21985023), and 0.5mg/mL streptomycin/penicillin(Thermo Fisher Scientific, 10378016) in Ham F-12 medium(Thermo Fisher Scientific, 21700026).
2.5.Single-cell culture
Briefly, 60 HSC1 cells were individually sorted into a 96-well U-bottom plates, with each well receiving 200μL of the new standard serum-free medium supplemented with 50ng/mL recombinant murine SCF(Peprotech,250-03),50ng/mL recombinant murine TPO (Peprotech, 315-14), and 50ng/mL recombinant murine IL-12(Peprotech,Rocky Hill,NJ,210-12).Single cells were cultured at 37°C in a humidified atmosphere with 5%CO2for 7 days.The cell numbers in each well was visually counted every day using an inverted microscope.The first division kinetics was obtained based on the cumulative number of wells where ≥2 cells were found during 7 days of culture.The second-division kinetics were obtained based on the cumulative number of wells where ≥3 cells were found.The third-division kinetics were obtained based on the cumulative number of wells where ≥5 cells were found.
2.6.Serial competitive repopulation
Competitive repopulation was performed on 10 to 40 test donor cells(test cells)purified from the bone marrow of B6-Ly5.1 mice together with 5×105or 10×105bone marrow cells (competitor cells)from B6 mice.When the cultured cells were tested,10 to 40 cells were cultured in serum-free medium for 7 days.SCF,TPO,and IL-12 were used at 50ng/mL each.Cultured cells were mixed with competitor cells and injected into B6 mice irradiated twice at a dose of 4.75 Gy after a 3-hour interval(total 9.5 Gy).Peripheral blood cells from the recipient mice were analyzed at the indicated time points after transplantation.
For the peripheral blood analysis, red blood cells were lysed with buffer containing 0.15M NH4Cl,10mM KHCO3,and 0.1 mM EDTA in water (pH 7.2).Cells were stained with FITC-conjugated anti-CD45.1(A20,eBioscience),PE-conjugated anti-CD45.2 (104, eBioscience), PE-Cy7-conjugated anti-CD4(GK1.5, eBioscience), APC-conjugated anti-CD8a (53-6.7,eBioscience), PerCP-Cy5.5-conjugated anti-B220 (RA3-6B2,eBioscience), and APC-eFluor780-conjugated anti-Mac-1/Gr-1(M1/70 and RB6-8C5, eBioscience) antibodies.Cells were analyzed on a Canto II (Becton Dickinson, Franklin Lakes,NJ).The percentage of test donor-derived cells(%test cells)was calculated with the following formula:(%Ly5.1+cells)×100/(%Ly5.1+test cells + % Ly5.2+cells).When the percentage of test cells was 0.5 or greater,the recipient mouse hematopoietic system was considered to be reconstituted with test cell-derived cells(positive mice).Mac-1/Gr-1+cells were considered to be of a myeloid lineage.B220+cells were considered to be of a B-lymphoid lineage.CD4+and CD8+cells were considered to be of a T-lymphoid lineage.
For secondary transplantation, bone marrow cells were harvested from the femora, tibiae, and iliac crests from each recipient mouse.Bone marrow cells from all recipient mice were pooled,and a half femur-equivalent or 2×107bone marrow cells were transplanted into lethally irradiated mice.Peripheral blood from the recipient mice was analyzed at the indicated time points after transplantation.For further serial transplantation,the same protocol was used.
2.7.Single-cell transplantation
Single HSC1 cells were sorted from B6-Ly5.1 murine bone marrow and transplanted into lethally irradiated B6 mice,along with 5×105bone marrow cells from a B6 mouse.Single HSC1 cells were cultured in the new standard serum-free medium with the indicated cytokines for 7 days and transplanted into lethally irradiated B6 mice along with 5×105bone marrow cells from a B6 mouse.For secondary transplantation, 2×107bone marrow cells from each recipient mouse were transplanted into a lethally irradiated mouse.
2.8.Single-cell RT-PCR
Single cells were sorted into 48 wells of a 96-well plate where each well contained 10μL of reverse transcription and specific target amplification mixture consisting of 2.5μL 0.2×primers containing all 48 sets of primers,5.0μL 2×reaction mix,0.5μL Superscript III,and 2.0μL Tris-EDTA buffer.Reverse transcription was performed at 50°C for 15minutes.The samples were incubated at 95°C for 2 minutes,followedby22 cyclesof 95°Cfor 15secondsand60°Cfor4 minutes.Five microliters from each of the samples was mixed with 20μL of Tris-EDTA.Then,2.7μL of the diluted samples was mixed with3.0μLTaqmanuniversalPCRmastermix(AppliedBiosystems)and 0.3μL sample loading buffer (for a total of 6.0μL sample loading mix).After 3.0μL of 20×of each set of primers was mixed with 3.0μL of assay loading reagent(a total of 6.0μL assay loading mix),5μL of the sample loading mix and 5μL of the assay loading mix were applied to a 48-chip array, and 48×48 reactions were prepared by an integrated fluidics circuit controller.The chip was set on a Fluidigm BioMark and incubated at 95°C for 10minutes,followed by 40 cycles of 95°C for 15seconds and 60°C for 60 seconds.Data were analyzed with BioMark Real-time PCR analysis software(Fluidigm,SanFrancisco,CA).The PCRprimers purchased from Thermo Fisher are listed in Supplemental Table 1(http://links.lww.com/BS/A1).When threshold cycle values (Ct) were <27.65,cells were considered to express the gene(positive cells).
2.9.Statistical analysis
To test whether the mean of a variable dif fered between 2 groups,unpaired t test or Mann-Whitney test was performed.To compare 3 or more groups,nonparametric ANOVA testing was performed.
3.RESULTS
3.1.Ex vivo expansion of ST-HSCs and LT-HSCs
We prepared test donor cells(test cells)from B6-Ly5.1 mice and competitor cells from B6-Ly5.2 mice in this study.Initially, we used CD34-/low, c-Kit+, Sca-1+, and lineage-(CD34-KSL)cells28,29as our HSC-enriched population.Forty freshly isolated CD34-KSL cells were transplanted into lethally irradiated mice along with 1×106competitor cells,and bone marrow cells from the recipient mice were serially transplanted 3 more times.Figure 1 shows all results obtained from serial competitive repopulation.The percentage of total test cell-derived cells in the peripheral blood (% test cells) showed that varying degrees of reconstitution occurred among the primary recipients, but these gradually decreased over the serial transplantation.
To compare the effects of IL-12 with TPO in combination with SCF on HSCs in culture, 40 CD34-KSL cells were cultured in serum-free SF-O3 medium with its supplement and BSA for 7 days and subsequently transplanted into lethally irradiated mice,along with 1×106competitor cells.The percentage of test cells 1 month after primary transplantation in SCF+TPO culture was significantly greater than that in freshly isolated cells and SCF+IL-12 culture.The levels of reconstitution given by SCF+TPO cultures but not SCF+IL-12 cultures remained significantly greater than those given by freshly isolated cells after secondary transplantation.Overall, however, the reconstitution levels of SCF+TPO cultures ultimately decreased.As a result, a monophasic wave of reconstitution was observed.In contrast, the reconstitution levels given by SCF+IL-12 cultures decreased after primary transplantation.But, low levels of reconstitution were maintained after secondary transplantation.Surprisingly, the reconstitution levels increased 5 months after tertiary transplantation.As a result, a biphasic wave of reconstitution was observed.It would be interesting to determine whether different types of HSCs contributed to the formation of these monophasic and biphasic waves.Nevertheless,both ST(<6 months)and LT(>6 months)reconstitution activities were increased in SCF+TPO and SCF+IL-12 cultures, but their reconstitution kinetics markedly differed.
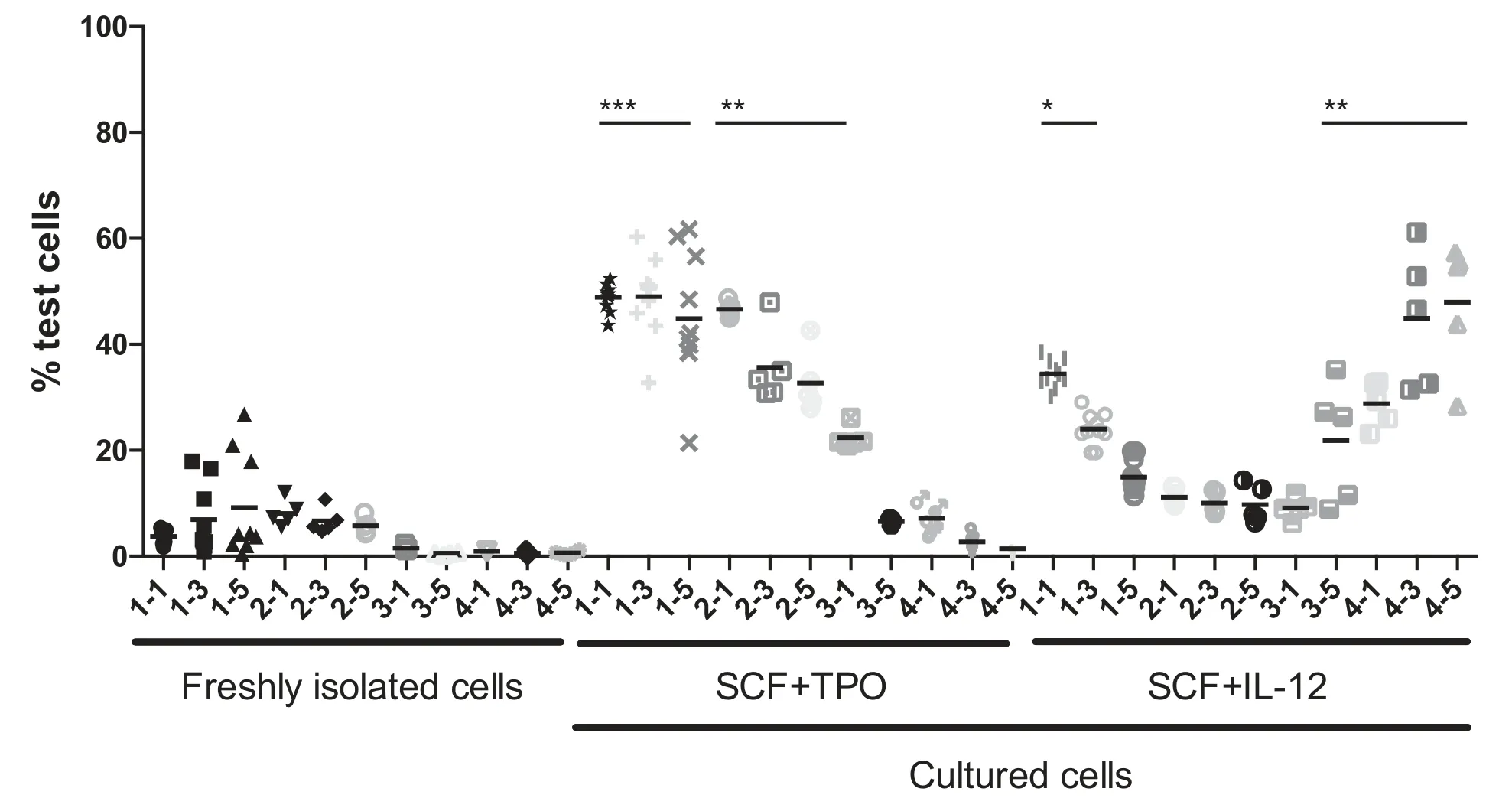
Figure 1.Effect of IL-12 treatment on HSCs revealed by serial competitive repopulation assays.Forty CD34-KSL cells were transplanted into 10 lethally irradiated mice along with 1×106 bone marrow competitor cells(freshly isolated cells).Forty CD34-KSL cells were cultured with SCF+TPO or SCF+IL-12 in SF-O3 medium,its supplement,and BSA for 7 days and then transplanted into 10 lethally irradiated mice along with 1×106 bone marrow competitor cells.Peripheral blood samples from the recipients were analyzed at 1,3,and 5 months(indicated as 1-1,1-3,and 1-5,respectively)after transplantation.The percentage of test cells is equal to the percentage of Ly5.1+cells in the peripheral blood.For serial transplantation,bone marrow cells were collected from one femur from all the surviving recipients and pooled for each group.Bone marrow cells equivalent to those in a half femur were transplanted into 5 lethally irradiated mice.Peripheral blood from the recipients was analyzed 1,3,and 5 months(indicated as 2-1,2-3,and 2-5,respectively);1 and 5 months(indicated as 3-1 and 3-5,respectively);and 1,3,and 5 months(indicated as 4-1,4-3,and 4-5,respectively)after secondary,tertiary,and quaternary transplantation,respectively.*P<.05;**P<.01;***P<.001(nonparametric ANOVA).BSA = bovine serum albumin; HSC = hematopoietic stem cells; IL = interleukin; SCF = stem cell factor; TPO = thrombopoietin.
3.2.Further enrichment of LT-HSCs
We attempted to reproduce our important transplantation results described; however, it was difficult to do so.There have been many such cases in previous studies as well.5Thus,we sought to address two fundamental problems in HSC culture: the heterogeneity of HSCs among purified cells, and batch-to-batch differences of serum-free media.When these problems are solved,it should be possible to clonally analyze cultured cells, enabling the dissection of the reconstitution waves.
To reduce heterogeneity in the HSCs, CD150+CD41-CD34-KSL (CD150+41-34-KSL) cells16were used as a more purified source of HSCs.CD150+41-34-KSL and CD150-41-34-KSL cells are subpopulations within CD34-KSL cells and were named HSC1 and HSC2, respectively, provided in the Supplemental Figure 1 (http://links.lww.com/BS/A2).We first compared the HSC1 and HSC2 cells with CD34-KSL cells by transplantation assay.Forty CD34-KSL cells, 20 HSC1 cells, or 20 HSC2 cells were transplanted into lethally irradiated mice, along with 1×106competitor cells.These cells were cultured with SCF+TPO in SF-O3 medium containing its supplement for 7 days and then similarly transplanted.Figure 2 shows 12-month follow-up monitoring for the recipient mice after transplantation.LT(>6 months) reconstitution was observed after transplantation with CD34-KSL cells.A comparable level of LT reconstitution was observed after transplantation with HSC1 cells.The reconstitution levels of freshly isolated HSC1 cells were significantly greater than those of freshly isolated HSC2 cells,supporting our previous observations.16Interestingly, the ST repopulation activities at 1 month after transplantation were significantly increased in CD34-KSL cells and HSC1 cells but not HSC2 cells after culture.Varying levels of LT reconstitution activities were detected in the cultured cells derived from CD34-KSL cells and HSC1 cells.The reconstitution levels of cultured HSC1 cells were significantly greater than those of cultured HSC2 cells.Both ST- and LT-HSC activities in HSC1 cells were enhanced by in vitro culture.Based on these data, we concluded that the HSC activities of CD34-KSL cells both before and after culture are well-represented by HSC1 cells.If HSC1 cells were used,more LT-HSCs would generate ST-HSCs and LT-HSCs in culture.
3.3.New serum-free culture conditions
This study next sought to address the problems associated with serum-free media conditions.To culture HSCs,many researchers routinely use commercially available serum-free media.Most of these media contain BSA,resulting in batch-to-batch variation.27In this study, we attempted to establish our own serum-derived protein-free medium in which all components were chemically defined.
A serum-free medium called S-Clone, consisting of SF-O3 medium, its supplement, BSA, and other components, has regularly been used as one of the best formulations of serum-free media.16,26,30,31The BSA previously included in the S-Clone package has recently been removed and is now provided separately by the company.We attempted to replace BSA with recombinant human serum albumin (rHSA).27In this study,3 different batches of rHSA were tested with competitive repopulation assays.Ten HSC1 cells were transplanted into lethally irradiated mice,along with 5×105competitor cells.Ten HSC1 cells were also cultured with SCF+TPO in SF-O3 medium,its supplements, and 0.1 or 0.5mg/mL rHSA.As shown in Supplemental Figure 2 (http://links.lww.com/BS/A3), regardless of the different batches of rHSA used,similar degrees of ST and LT reconstitution activities were observed in the groups of cultured cells with 0.5mg/mL rHSA; however, reconstitution activities in cultured cells with 0.1mg/mL rHSA-1 and -2 were significantly lower than those with 0.5mg/mL rHSA-1 and -2,respectively.
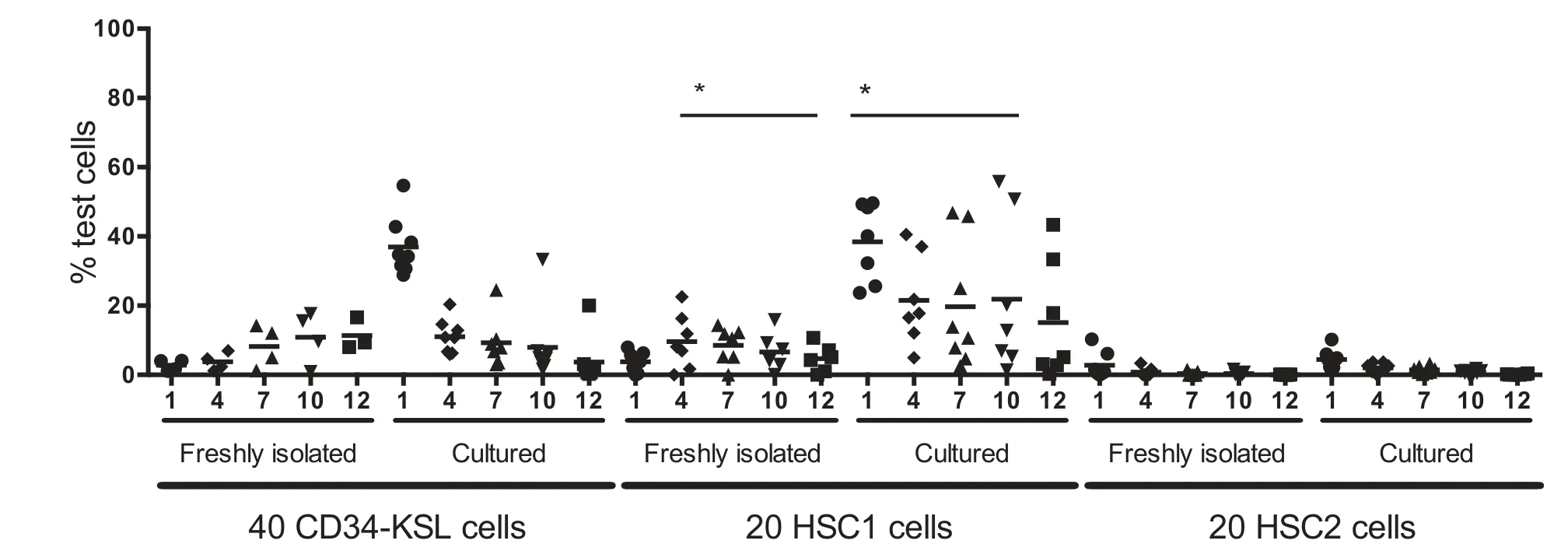
Figure 2.HSC1 cells represent CD34-KSL cells in culture.Forty CD34-KSL cells,20 CD150+41-34-KSL cells(HSC1),or 20 CD150-41-34-KSL cells(HSC2)were transplanted into lethally irradiated mice along with 1×106 competitor cells.The cells were cultured with SCF and TPO in SF-O3 medium,its supplement,and rHSA for 7 days and then transplanted into lethally irradiated mice along with 1×106 competitor cells.Recipient mice were followed for 12 months after transplantation.*P<.05 (unpaired t test).HSC = hematopoietic stem cells; rHSA = recombinant human serum albumin; SCF = stem cell factor; TPO =thrombopoietin.
We next compared the following basic media formulations and their mixtures:SF-O3,DMEM,RPMI 1640,and Ham F12(F-12) media.As shown in Figure 3A, F-12 medium supported HSC activity to a significantly greater extent than DMEM or RPMI 1640 medium(P<.05),supporting previous observations that the balance in amino acid components plays an important role in HSC maintenance.33However, significant differences were not observed among SF-O3,F-12,DF,DR,and DFR media.Next, we compared the SF-O3 supplement, which reportedly contains insulin (I), transferrin (T), and other factors, with I, T,selenium(S),the combination of these components(ITS),and ITS plus ethanolamine (ITS-X).As shown in Figure 3B, individual supplementation with I,T,or S did not support the maintenance of HSC activities in vitro.Interestingly,the further supplementation of the combination of the 3 additional components (ITS)supported HSC activities.ITS-X, but not ITS, supported the HSCs activities to a greater extent than the SF-O3 supplement.To confirm the effect of ITS-X,LT-HSC activity with F-12 and ITS-X was compared with that of F-12.As shown in Figure 3C,ITS-X supported both ST- and LT-HSC activities.We next compared SF-O3 medium with F-12 medium supplemented with ITS-X.As shown in Figure 3D, F-12 medium supported both ST- and LT-HSC activities to a significantly greater extent than SF-O3 medium.We used these established culture conditions as the new standard serum-free culture conditions in the following experiments.

Figure 3.Establishing new serum-free conditions.Various formulations of media and supplements were tested in the presence of rHSA,SCF,and TPO.(A)SF-O3 medium was compared with DMEM,RPMI 1640,Ham F-12(F-12),a 1:1 mixture of DMEM and F-12(DF),a 1:1 mixture of DMEM and RPMI 1640(DR),and a 1:1:1 mixture of DMEM,F-12,and RPMI 1640(DFR)media.HSC-supporting activity was evaluated 3 months after transplantation by competitive repopulation assay.The percentage of test cells in F-12 was significantly greater than those in DMEM and RPMI(*P<.05,nonparametric ANOVA).(B)The HSC-supporting activity in the SF-O3 supplement was compared with that in human insulin, transferrin, selenium, the combination of the 3 factors (ITS), and ITS + ethanolamine (ITS-X)4 months after transplantation by competitive repopulation assay.Forty CD34-KSL cells and 1×106 bone marrow cells were used as the test and competitor cells,respectively.(C)HSC-supporting activity of ITS-X was examined in the presence of F-12,SCF+TPO,and rHSA by competitive repopulation assay.Ten HSC1 cells and 5×105 bone marrow cells were used as the test and competitor cells,respectively.Recipient mice were followed for 12 months after transplantation.(D)HSC-supporting activity of F-12 medium was compared with that of SF-O3 medium in the presence of SCF+TPO,ITS-X,and rHSA by competitive repopulation assay.Ten HSC1 cells and 5×105 bone marrow cells were used as the test and competitor cells, respectively.Recipient mice were followed for 12 months after transplantation.*P <.05(Mann-Whitney test).DMEM=Dulbecco Modified Eagle Medium;HSC=hematopoietic stem cells;rHSA=recombinant human serum albumin; RPMI = Roswell Park Memorial Institute medium; SCF = stem cell factor; TPO = thrombopoietin.
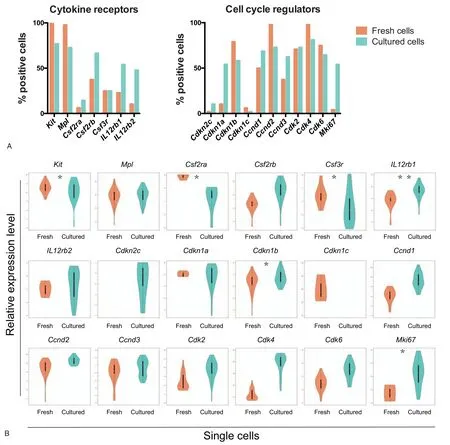
Figure 4.Single-cell RT-PCR.(A)The percentages of gene expressing cells in 48 cells(%positive cells)are shown for cytokine receptors and cell-cycle regulators.Single HSC1 cells were analyzed by RT-PCR.Single HSC1 cells were incubated with SCF for 24hours and analyzed by RT-PCR.Cells were considered as positive cells when threshold cycle values (Ct) were <27.65.(B) The relative expression levels of cytokine receptors and cell-cycle regulators are shown.The relative expression levels were defined as (27.65-Ct).*P<.05 and**P<.01 (unpaired t test).HSC = hematopoietic stem cells; SCF = stem cell factor.
3.4.IL-12 receptor expression in HSCs
Reverse transcription-polymerase chain reaction (RT-PCR) was performed on freshly isolated single HSC1 cells.This analysis was also performed on single cells 24hours after culture in the new standard serum-free medium with SCF because our previous results suggested that few HSC1 cells expressed IL-12 receptor,but its expression was upregulated during in vitro culture.33Figure 4 shows the expression of genes selected from a total of 48 genes examined(Supplemental Table 1 and Supplemental Fig.3,http://links.lww.com/BS/A4).Most single HSC1 cells expressed c-Kit and c-Mpl.Single cells still expressed these cytokine receptors 24hours after culture.The IL-12 receptors consist of β1 and β2 subunits (IL-12rb1 and IL-12rb2, respectively).The expression of IL-12rb1 and IL-12rb2 was detected in 11 and 5 of the 48 HSC1 cells,respectively,though only 1 cell expressed both IL-12rb1 and IL-12rb2.After culture with SCF for 24hours,21 cells(44%of the cells)expressed both receptors,suggesting that IL-12 receptor expression was induced by SCF.The upregulation of Tgfrb1 and Tgfrb2, and the common β chain (Cdf2rb) were also observed.The upregulation of the IL-12 and TGF-β receptors might be tightly associated with cell-cycle progression,because Mki67 and members of the cyclin and cyclin-dependent kinase families were expressed after culture with SCF(Fig.4 and Supplemental Fig.3, http://links.lww.com/BS/A4).
3.5.Early division kinetics of HSCs in the presence of IL-12
We noted that a portion of HSC1 cells expressed CD48.Since it has been reported that HSCs do not express CD48,34,35we added the marker CD48 for the isolation of HSC1 cells from this experiment (Supplemental Fig.4, http://links.lww.com/BS/A5).We then used serum-free single-cell cultures to examine the first,second,and third division kinetics of HSC1 cells in the presence of either SCF+TPO or SCF+IL-12.As shown in Figure 5A,in the presence of SCF+TPO,82.7±7.9%(mean±SD,n=5)of HSC1 cells underwent the first division within 7 days of culture, and most of the cells subsequently proceeded to the second and third divisions.In the presence of SCF+IL-12, 75.0±14.5% (mean±SD,n=5)of HSC1 cells underwent the first division in 7 days of culture.The first division in the SCF+IL-12-treated cells was delayed approximately 2 days compared with the SCF+TPO-treated cells.HSC1 cells never divided in the absence of cytokines.Similarly, HSC1 cells did not divide in the presence of IL-12 alone.Therefore, it was assumed that in the presence of SCF, 1 day or more was required to respond to IL-12; such time was likely required for upregulation of the IL-12 receptor by SCF.The cells slowly and continuously divided with SCF+IL-12(Supplemental Table 2, http://links.lww.com/BS/A6).The addition of IL-12 to the SCF+TPO culture increased the number of cells per well only on later days of culture.Taken together,these data clearly demonstrated that HSCs slowly divide in SCF+IL-12,whereas HSCs rapidly divide in SCF+TPO.
3.6.Effect of IL-12 on ST and LT reconstitution potential in HSCs
Using HSC1 cells as test donor cells and our new standard serumfree culture,we again compared the reconstitution activity of the cultured cells by serial competitive repopulation assay,as before.Ten HSC1 cells were directly transplanted into lethally irradiated mice with 5×105competitor cells.Ten HSC1 cells were cultured in the new standard serum-free medium with SCF+TPO,SCF+IL-12,or SCF+TPO+IL-12 for 7 days and transplanted into lethally irradiated mice with competitor cells.After 9 months, bone marrow cells from the recipient mice were pooled and transplanted into lethally irradiated mice.
As shown in Figure 5B, cells cultured with SCF+TPO or SCF+TPO+IL-12 showed significantly increased ST reconstitution activities,such as at 1 month after transplantation compared with the freshly isolated cells.Varying degrees of LT reconstitution were observed in mice transplanted with the SCF+TPO,SCF+IL-12, and SCF+TPO+IL-12 cultured cells.However, after secondary transplantation, the reconstitution activity in SCF+IL-12 cultures was significantly greater than in freshly isolated cells and SCF+TPO or SCF+TPO+IL-12 cultures (P<.001).All myeloid,B-cell and T-cell lineages were well-reconstituted 6 months after secondary transplantation(Supplemental Fig.5,http://links.lww.com/BS/A7).These data, which are consistent with the data in Figure 1,showed that IL-12 supported LT-HSC self-renewal to a greater extent than TPO.LT-HSCs that responded to TPO and those that responded to IL-12 likely overlapped, because the additional effect of IL-12 to SCF+TPO culture was relatively small.
3.7.Transplantation of cells derived from single HSCs in culture
Single HSC1 cells were transplanted into 30 lethally irradiated mice with 5×105competitor cells.Single HSC1 cells were cultured in the new standard serum-free medium with SCF+TPO,SCF+IL-12, or SCF+TPO+IL-12 for 7 days.All the cells in an individual well were mixed with competitor cells and individually transplanted into 30 lethally irradiated mice.This was a clonal analysis because single-cell-derived cells were transplanted.Figure 6A shows the reconstitution data for each recipient mouse 1,4,7,and 10 months after transplantation.In total,10,10,6,and 7 mice appeared to be positive(>0.5%test cells)after transplantation with freshly isolated cells,cells cultured with SCF+TPO,cells cultured with SCF+IL-12,and cells cultured with SCF+TPO+IL-12, respectively.LT reconstitution was detected in 3 mice transplanted with freshly isolated single cells and one mouse each transplanted with cells cultured with SCF+TPO,SCF+IL-12,and SCF+TPO+IL-12, though the myeloid reconstitution levels were very low in all of the positive mice.In these experiments,STHSCs were present in isolated HSCs more than LT-HSCs.These data thus indicated that single ST-HSCs can generate one or more ST-HSCs in the presence of SCF+TPO or SCF+IL-12.In another word,SCF+TPO or SCF+IL-12 can support in vitro self-renewal of ST-HSCs.
The same transplantation experiment was repeated.Unfortunately, some of the recipient mice died during the experiment.Ultimately,7,3,2,and 3 recipients appeared to be positive after transplantation with freshly isolated cells,cells cultured with SCF+TPO,cells cultured with SCF+IL-12,and cells cultured with SCF+TPO+IL-12, respectively.Figure 6B depicts the reconstitution data for the freshly isolated cells and cells cultured with SCF+IL-12 obtained from the analysis of each recipient mouse 1,2,4,and 6 months after primary transplantation,as well as 2,4,6,and 9 months after secondary transplantation.LT-HSC activities from the secondary recipient mice were detected in 4 and 1 mice transplanted with freshly isolated cells and cells from SCF+IL-12 cultures,respectively,compared to none of the mice transplanted with cells from the SCF+TPO and SCF+TPO+IL-12 cultures(data not shown).Myeloid reconstitution was detected until the end of the analysis in 3 mice transplanted with freshly isolated HSC1 cells and 1 mouse transplanted with cells from the SCF+IL-12 culture.These data supported that single LT-HSCs can generate 1 or more LT-HSCs in the presence of SCF+IL-12.In another words, SCF+IL-12 can support in vitro self-renewal of LT-HSCs.
4.DISCUSSION
Serum-free culture conditions are essential for controlling the in vitro proliferation and differentiation of HSCs.In this study,we established a simple serum-free culture medium completely composed of defined substances to serve as a standard serum-free culture medium.Commonly used tissue culture protocols often require fetal bovine serum as a source of nutrients.However,serum contains a variety of proteins that have positive and negative effects on cells in culture.This problem persists in serumfree culture because serum albumin fractionated from animal blood, such as BSA, is used in most serum-free media.Lipids,growth factors, hormones, and many other unidentified factors are contaminants in BSA.27As a result, this type of serum-free media frequently exhibits batch-to-batch variability; moreover,several formulations are unstable when stored at 4°C for unknown reasons,which likely causes difficulties in reproducing data between different laboratories.Despite their frequent use,the constituent components of commercially available serum-free media are usually not available to the public.In this study, we found that 2 basic components, rHSA and ITS-X, in F-12 medium can sufficiently support in vitro HSCs self-renewal.Hopefully, our new standard serum-free culture conditions will allow us to reproduce and compare ex vivo expansion data not only from our own laboratory but also from other laboratories.
Serial transplantation of cultured cells with SCF+TPO exhibited an initial reconstitution wave (monophasic wave),but serial transplantation of cultured cells with SCF+IL-12 exhibited an initial and second reconstitution waves (biphasic wave).It was very likely that the first reconstitution wave was formed by ST-HSCs derived from ST-HSCs and LT-HSCs, and the second reconstitution wave was formed by LT-HSCs derived from LT-HSCs(Figs.1,2,and 5).Basically,similar reconstitution patterns were also observed at the clonal level (Fig.6).
HSC1 cells were separated from HSC2 cells(Supplemental Fig.1,http://links.lww.com/BS/A2)based on their CD150 expression levels.34HSC1 cells were enriched in LT-HSCs,whereas HSC2s were enriched in ST-HSCs (Fig.2), which is consistent with previous studies.14,16,35Notably,HSC1 cells could self-renew in vitro significantly more than HSC2 cells(Fig.2).However,both ST-HSCs and LT-HSCs continued to coexist among the HSC1 cells (Fig.6).Because of the subtle changes associated with the sorting gates in flow cytometry, particularly the CD150 gate,more ST-HSCs were sometimes isolated than LT-HSCs, or vice versa.In the single-cell transplantation experiment shown in Figure 6A, where more ST-HSCs were isolated, in vitro selfrenewal of ST-HSCs was supported by both SCF+TPO and SCF+IL-12.In the single-cell transplantation experiment shown in Figure 6B, where more LT-HSCs were isolated, in vitro selfrenewal of LT-HSCs was supported to a greater extent by SCF+IL-12 than SCF+TPO.These data suggested that only ST-HSCs can generate ST-HSCs,whereas LT-HSCs can generate both STHSCs and LT-HSCs.Whereby a monophasic reconstitution wave was created by ST-HSCs and a biphasic reconstitution wave was created by both ST- and TL-HSCs.
Latent HSCs have been defined as HSCs that are able to show reconstitution 4 months or later after transplantation.14Since CD150highcells are more enriched in latent HSCs than CD150medcells,it was relevant to test whether latent HSCs were responsible for the ex vivo expansion of LT-HSCs with SCF+IL-12.LT-HSCs or latent HSCs generated in SCF+IL-12 culture, but not in SCF+TPO culture, might have been responsible for the unique kinetics observed for LT reconstitution by serial transplantation(Figs.1 and 5).Theoretically, these LT-HSCs may slowly but extensively self-renew after transplantation,but serial transplantation is required to drive them into the cell cycle again.36
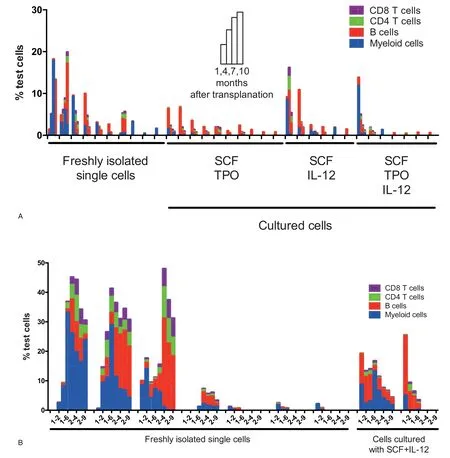
Figure 6.Transplantation with cells derived in vitro from single cells.(A)Thirty lethally irradiated mice received freshly isolated single HSC1 cells along with 5×105 competitor cells(freshly isolated single cells).Single HSC1 cells were cultured in the new standard serum-free conditions with SCF+TPO,SCF+IL-12,or SCF+TPO+IL-12 for 7 days,and transplanted along with 5×105 competitor cells(cultured cells).Peripheral blood from the recipients was analyzed 1,4,7,and 10 months after transplantation.(B)Thirty lethally irradiated mice received freshly isolated single HSC1 cells or cells derived from single HSC1 cells in the new standard serumfree culture with SCF+IL-12 for 7 days along with 5×105 competitor cells.Peripheral blood was analyzed 1 and 2(indicated as 1-2)and 4 and 6(indicated as 1-6)months after transplantation.Bone marrow cells were individually collected from 25 and 24 surviving recipients of freshly isolated cells and cultured cells,respectively,and 2×107 bone marrow cells from each donor were transplanted into a lethally irradiated mouse.Mice were analyzed 2 and 4(indicated as 2-4)and 6 and 9 months(indicated as 2-9)after secondary transplantation.(A,B)Data only depict the reconstituted mice.Blue columns show the percentage of test cellderived myeloid cells.Red columns show the percentage of test cell-derived B-lymphoid cells.Green columns show the percentage of test cell-derived CD4 T cells,and purple columns show the percentage of test cell-derived CD8 T cells.HSC = hematopoietic stem cells; IL = interleukin; SCF = stem cell factor; TPO =thrombopoietin.
HSCs have a limited potential to self-renew under physiological conditions, as well as after transplantation.12,37This should also theoretically be the case for in vitro HSC self-renewal.Conceptually,as HSCs divide more,the probability of the loss of self-renewal potential increases.HSCs divided more slowly in the presence of SCF+IL-12 compared with SCF+TPO (Fig.5A;Supplemental Table 2, http://links.lww.com/BS/A6).TPO activates the Jak2/Stat3 and Stat5 signal transduction pathways in HSCs.26Consistent with observations in T cells,38IL-12 presumably activates Jak2/Stat4 signal transduction pathways in HSCs.The differences in the signal transduction efficiency should lead to different division rates.The fate decision itself may not necessarily differ between the SCF+TPO and SCF+IL-12 cultures.Slow and continuous division should be an advantage for HSCs to maintain the self-renewal potential because the number of division that they undergo becomes smaller, and sufficient time is given them to repair DNA damage and maintain the telomere length.This hypothesis should be formally verified by further study.Nevertheless, this study demonstrated that IL-12,but not TPO,can be used for the ex vivo expansion of a subset of LT-HSCs.
ACKNOWLEDGMENTS
This work was supported by grants from the National Key Research and Development Program of China Stem Cell and Translational Research(2017YFA0104903,2016YFA0100600,and 2017YFA0103400),the Ministry of Science and Technology of China (2015CB964403 and, 2011CB964801), the CAMS Initiative for Innovative Medicine (2016-I2M-1-017 and 2017-I2M-1-015),and the National Natural Science Foundation of China(81470279,81670105,81421002,81400077,and 81500085).
杂志排行
血液科学的其它文章
- Successful ex vivo expansion of mouse hematopoietic stem cells
- Cell cycle regulation and hematologic malignancies
- Will immune therapy cure acute myeloid leukemia?
- Engineered human pluripotent stem cell-derived natural killer cells: the next frontier for cancer immunotherapy
- Hematopoietic stem cell metabolism and stemness
- Epigenetic regulation of hematopoietic stem cell homeostasis