CAR-NK cell therapeutics for hematologic malignancies: hope is on the horizon
2019-11-02KailinXuHaiCheng
Kai-lin Xu*,Hai Cheng
aBlood Diseases Institute,Xuzhou Medical University,Xuzhou,China; bDepartment of Hematology,The Affiliated Hospital of Xuzhou Medical University,Xuzhou,China
Abstract
Keywords:Hematology,Immunotherapy,NK cells
1.INTRODUCTION
In the field of cancer immunotherapy,chimeric antigen receptor(CAR)-T-cell therapy has immense significance.In fact,in vivo,the topmost player against cancer is not T cells,but natural killer(NK)cells.1Compared with CAR-T cells,CAR-NK cells have the following advantages for application in the immunotherapy of blood cancers:
1.allogeneic NK cells are associated with less severe graft-versushost disease (GVHD)2,3;
2.mature NK cells have a shorter life span in vivo4; and
3.in addition to the ability of directly identifying multiple stimulatory molecules on the surface of tumor cells,NK cells can enhance the specificity of killing tumor cells through CAR modification.5,6
The purpose of constructing CAR-NK cells is to create a novel strategy for activating NK cells and enhance their antitumor effects by genetic modification.CAR-NK cells inherit the basic structural framework and the transfection method of CAR-T cells.The CAR construct consists of the following three components: an extracellular antigen-recognition domain,a transmembrane domain,and an intracellular signaling domain.CAR molecules can be roughly divided into the following three generations:first-generation CARs fused into target-specific scFv to specifically activate NK cells7; second-generation CARs incorporated into cytoplasmic signaling domains of T-cell costimulatory receptors; and third-generation CARs with multiple costimulatory domains8–10(Fig.1).In this review,we emphasize the effectiveness of CAR-NK cell therapy in preclinical and early clinical studies using CAR-NK cells.
2.ROLE OF CAR-NK CELLS IN B-ALL
Research on the application of CAR-NK cells in the treatment of relapsed and refractory acute B-cell lymphoblastic leukemia(R/R B-ALL)has just begun.Preclinical research can be roughly divided into the following two categories:CAR-NK cell therapy designed to alleviate the serious side effects caused by traditional CAR-T-cell treatment,and the other includes CAR-NK cell therapy developed for preventing relapse after CAR-T-cell treatment.Oelsner et al11transduced cytokine-induced killer(CIK)cells with a lentiviral vector encoding a CAR that carried a CD28-CD3ζ-CD19 structure.These targeted CIK cells exhibited high cytotoxicity in vitro and in vivo.This was the first evidence showing that CAR-modified CIK cells may be used as a promising immunotherapeutic strategy for treating R/R B-ALL.Subsequently,different research groups confirmed that CAR-modified NK92 cells could exert antileukemic effects.12,13Moreover,CAR-NK cells were found to be not only as effective as CAR-T cells but also less toxic.14
Being the most promising targeted therapy,CAR-T-cell therapy has attracted widespread research attention in recent years.CAR-T cells that specifically recognize the B-cell surface antigen CD19 have demonstrated significant success in clinical trials against R/R B-ALL,5,15–23and two CAR-T cell products have been approved by the FDA.5,24However,treatmentinduced selection indicates that leukemic cells with altered or lost CD19 expression could be considered as the cause of treatment failure and relapse.6,25Thus,CAR-NK92 cells were designed to express an fms-like tyrosine kinase 3 (flt3)-specific CAR(CD28-CD3ζ) and an inducible caspase-9 (icasp9) suicide gene to improve safety.26Another strategy that can be used to overcome antigen escape is to design CAR-NK cells that express a CAR-targeting CD22,which simultaneously secretes a CD19-specific T-cell engager to induce bystander T cells to kill leukemia cells.27Several clinical trials on CAR-NK cells are also being conducted(Table1).Hence,the optimization of the intracellular signal domain of CAR and the antitumor effect of CAR-NK cells are aspects that have yet to be confirmed.
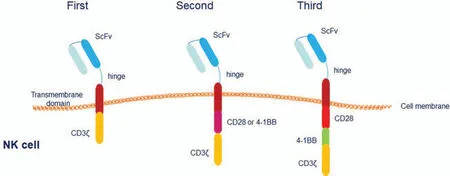
Figure1.Structure of different generations of CARs.
3.ROLE OF CAR-NK CELLS IN T-ALL
Results of basic research and clinical trials investigating the role of CAR-T cells in B-ALL are highly promising; however,there are still tremendous challenges in designing CARs for relapsed and refractory T-cell lymphocytic leukemia.As several antigens are shared by leukemia cells and normal cells,CAR T cells clear not only leukemia cells but also normal cells,even including CAR-T cells themselves.
You et al28constructed two second-generation CARs that can recognize CD7,one carrying a monovalent antibody(CD7-CARNK-92MI) and the other carrying a bivalent antibody (dCD7-CAR-NK-92MI).They compared the killing effects of the two types of CAR-NK cells on leukemia cells.Both the CAR-NK cell types exhibited specific antileukemic activity.Xu et al29compared the function of two intracellular domains,4-1BB and 2B4.Both CARs exhibited specific antitumor activity against T-cell lymphoma cell lines.Analysis of the killing effects using a mouse model revealed that CAR-2B4-NK cells were more potent than CAR-4-1BB-NK cells.
4.ROLE OF CAR-NK CELLS IN B-NHL
A multicenter phase 2 clinical trial on 101 patients with relapsed and refractory non-Hodgkin’s lymphoma (R/R NHL)demonstrated that the overall response rate and the complete remission rate after CAR-T-cell treatment reached 82% and 54%,respectively.The overall survival rate at 18 months was 52%.30It remains unclear why responses to CD19 CAR-T-cell therapy are more frequent and deeper in ALL than those in NHL.Purvey et al31described preliminary data on CAR-NK cells at the 2017 ASH Meeting.They examined various lymphoma cell lines and primary lymphoma cells,and their experiments demonstrated that CD19 CAR-NK cells can effectively kill tumor cells.To increase the targeting specificity of amplified NK cells,Chu et al32constructed an anti-CD20 CAR-NK cell line using peripheral blood NK cells.The anti-CD20 CAR-NK cells exhibited significantly enhanced cytotoxicity in vitro and prolongedsurvival in xenotransplanted mice.However,the transient persistence of CAR-NK cells limits their therapeutic efficacy.To prolong the persistence time of CAR-NK cells in vivo and reduce treatment-related side effects,researchers have optimized the structure of CAR and introduced the induced suicide gene andIL-15gene.Such a novel design demonstrated remarkable survival in a lymphoma model.33An ongoing phase I/II trial is investigating the side effects and the best dose of CD19-CD28-zeta-2A-iCasp9-IL15-transduced cord blood NK cells(NCT03579927).
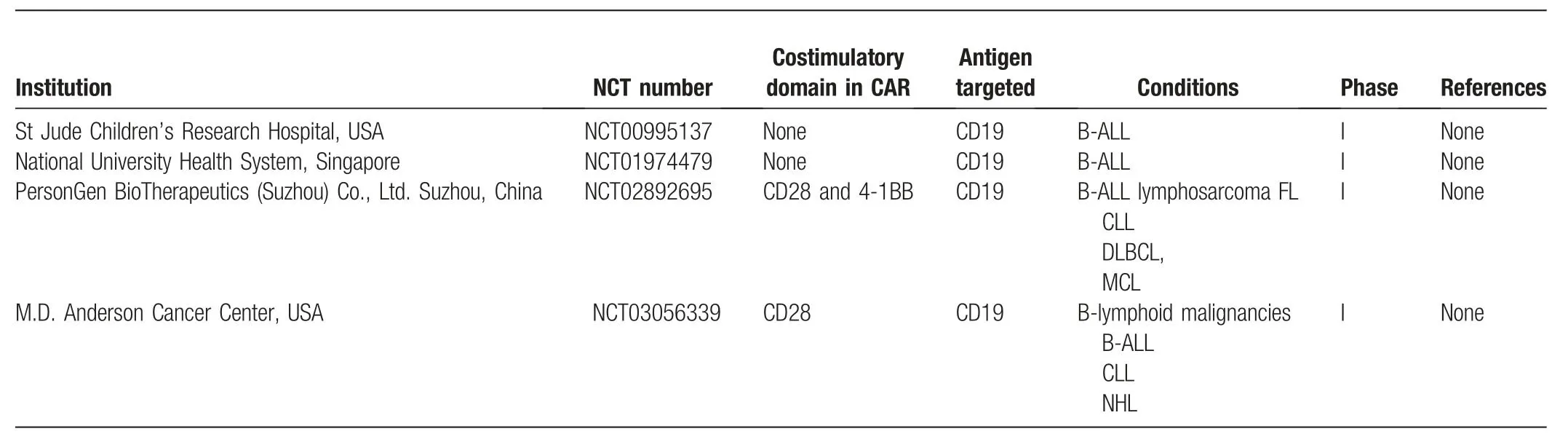
Table1 Summary of CAR-NK cell clinical trials for ALL
5.ROLE OF CAR-NK CELLS IN T-NHL
Peripheral T-cell lymphoma(PTCL)accounts for 10%to 15%of NHL.Compared with B-NHL,PTCL has limited treatment measures and poor prognosis.34–37Because PTCL develops from mature T cells,these cells highly and commonly express CD4 antigen.Therefore,CD4 comprises an ideal target for CAR therapy.Pinz et al38constructed an anti-CD4 CAR,in which the sequence of the single variable Fc chain of the extracellular domain for CD4 antigen recognition was derived from a commercialized anti-CD4 antibody,which ensured the specificity and the high affinity of CAR for recognition of the target antigen.The application of third-generation CAR structure has been reported to prolong the life span of CAR-NK cells in vivo.The results revealed that CD4 CAR-NK cells could effectively inhibit the growth of lymphoma cells and prolong the survival time of mice.A more significant finding is that CD4 CAR-NK cells had no significant effect on the function of hematopoietic progenitor cells while killing tumor cells in vivo.Another potential target for T-cell malignancy is CD5.39,40The majority of T-cell malignancies express CD5,whereas only a few lymphocyte subsets and thymocytes express CD5.41–43Although the application of anti-CD5 CAR-T cells yielded good results,there is evidence of crossreaction due to the intrinsic CD5 expression of CAR-T cells.44NK cells,which are CD5-negative,may be more feasible for the immunotherapy of T-cell malignancy.45Moot et al46made an interesting attempt to investigate the CD5 CAR.The CD5 on T cells was knocked out by the CRISP-Cas9 technique,and then CD5-scFv-CAR-T was constructed.To increase the affinity of CAR-NK cells to CD5,the variable lymphocyte receptor (VLR)structure was introduced.The affinity of VLR to specific antigens is known to be much higher than that of IgG antibodies.The functions of CD5-scFv-CAR-T,CD5-VLR-CAR NK92,and CD5-scFv-CAR NK92 cells were compared,which revealed that all the three types of CAR cells can recognize and kill tumor cells in vitro.However,the function and activity of CAR-T cells were decreased after knocking out CD5 compared with those of CARNK cells,suggesting that CD5 is a necessary element for maintaining the normal function of CAR-T cells.CD5-VLR-CAR NK92 and CD5-scFv-CAR NK92 cells demonstrated comparable tumor-killing activity in vitro; however,in vivo,only CD5-scFv-CAR NK92 cells prolonged the survival of mice.Although this preclinical study is preliminary,it confirmed for the first time that CAR-NK cells may be more advantageous than CAR-T cells in the treatment of T-cell malignancies.Furthermore,the antigenrecognition domain of different species may not increase the binding affinity of CARs.47
6.ROLE OF CAR-NK CELLS IN MM
Multiple myeloma (MM) occurs in middle-aged and older people aged >45 years and poses a serious challenge to human health.Although the treatment and prognosis of MM have been improved significantly over the past 20 years,MM still remains an incurable hematologic malignancy with a poorer overall prognosis,indicating the urgent need for new treatments.CART-cell therapy for relapsed/refractory MM (R/R MM) has demonstrated remarkable success.48–50MM encounters a similar dilemma as acute myeloid leukemia (AML) in CAR-NK cell treatment,the most important of which is the choice of the target antigen.Because MM cells are highly heterogeneous and the current surface markers for MM stem cells are still unclear,achieving a complete cure of MM is still difficult in clinical practice,and there is also remission after CAR-T-cell therapy.The choice of therapeutic targets is one of the key factors in the application of CAR-NK cells for treatment.The cell surface membrane protein CS1 may become a possible target for CARNK cell treatment.Since CS1 is ubiquitously expressed on the surface of MM cells,it is only expressed at low levels on NK cells and some T-cell subsets.51–53Although Chu et al54demonstrated that CS1 CAR-NK cells can clear MM cells,there was also evidence of cross-reaction.Jiang et al55generated two NK cells carrying an anti-CD138 scFv or anti-CD19 scFv structure and demonstrated the in vitro antitumor activity of both these CARNK cells.However,they did not report the results of in vivo experiments,due to which it is unclear whether these CAR-NK cells are effective in mouse model.Traditional strategies used in the construction of CARs include the introduction of specific antibody sequences to mediate the recognition of cancer cells by CAR-modified cells.However,this strategy has the disadvantage of the loss of tumor cell antigen.To overcome these limitations,Leivas et al56constructed CAR-NK cells carrying the NKGD2 receptor.The in vitro experiments confirmed that this novel CAR-NK cell is effective against the majority of MM cell lines.A multicenter phase I dose-escalation trial using a secondgeneration BCMA-targeted CAR-NK cell is ongoing,and we expect that we would obtain more information about the safety and feasibility of this strategy (NCT03940833).
7.ROLE OF CAR-NK CELLS IN AML
In 2015,AML affected approximately one million people and resulted in 147,000 deaths worldwide.57,58In patients aged >65 years,therapy for AML has been unsatisfactory.Despite extensive research efforts over the years,the prognosis of AML in elderly subjects has not yet been significantly improved.59In preclinical models,cord blood-derived CD123 CAR NK cells exhibited antileukemic activity in vitro.60Like the preparation of CAR-T cells,the transfected CAR-NK cells are highly heterogeneous as untransfected NK cells.The presence of these cells may have an effect on the antitumor effect of CAR-NK cells.After cell sorting,CAR-NK cells were found to exhibit enhanced activity against the CD123+AML cell line and primary human leukemia cells.61The first-in-human clinical trial(NCT02944162) of CD33 CAR-NK92 cells in patients with AML revealed that CAR-NK92 cells can be safely used,although their effect was not obvious.62In addition to the abovementioned clinical trials,there are currently two ongoing clinical trials of CAR-NK cell treatment with CD33-targeted AML(NCT02892695,NCT02944162).
8.PERSPECTIVE
Clinical research pertaining to the application of CAR-NK cells is still in the preliminary stage.The focus is still on how to reduce the cost,improve the efficacy,and reduce the occurrence of adverse reactions.Questions that are yet to be answered include how to select the ideal target,how to define the optimal CAR structure,and how to optimize the preparation procedure of CAR-NK cells.Advancements in basic research and additional clinical trials would be helpful to address these issues.
杂志排行
血液科学的其它文章
- The regulators of BCR signaling during B cell activation
- Novel chimeric antigen receptor T cells based on T-cell receptor-like antibodies
- Long noncoding RNA PCED1B-AS1 promotes erythroid differentiation coordinating with GATA1 and chromatin remodeling
- Advances of adeno-associated virus applied in gene therapy to hemophilia from bench work to the clinical use
- Long non-coding RNAs during normal erythropoiesis
- Current status and hurdles for CAR-T cell immune therapy