Advances of adeno-associated virus applied in gene therapy to hemophilia from bench work to the clinical use
2019-11-02XiaoleiPeiMingzheHanLeiZhang
Xiaolei Pei,Mingzhe Han,Lei Zhang
aState Key Laboratory of Experimental Hematology,Institute of Hematology and Blood Diseases Hospital,Chinese Academy of Medical Sciences and Peking Union Medical College,Tianjin,China; bTianjin Key Laboratory of Gene Therapy for Blood Diseases,Institute of Hematology and Blood Diseases Hospital,Chinese Academy of Medical Sciences and Peking Union Medical College,Tianjin,China; cCAMS Key Laboratory of Gene Therapy for Blood Diseases,Institute of Hematology and Blood Diseases Hospital,Chinese Academy of Medical Sciences and Peking Union Medical College,Tianjin,China; dHematopoietic Stem Cell Transplantation Center,Institute of Hematology and Blood Diseases Hospital,Chinese Academy of Medical Sciences and Peking Union Medical College,Tianjin,China
Abstract
Keywords:Adeno-associated virus,Hemophilia,FVIII,FIX,Clinical trial
1.INTRODUCTION
Hemophilia is a coagulation factor deficiency disorder caused by a single gene mutation in one of the X-linked coagulation factor genes.1,2Hemophilia A and B are the most common forms of hemophilia.Hemophilia A,which accounts for the highest prevalence,is caused by a coagulation factor VIII deficiency.Hemophilia B,meanwhile,indicates a coagulation factor IX deficiency.The occurrence of hemophilia A is approximately four times that of hemophilia B.Due to the irreplaceable role of factor VIII and factor IX in the coagulation cascade,loss of these genes would lead to a series of dysfunctions in the coagulation system,including spontaneous bleeding or abnormal bleeding after surgery and trauma,the bleeding level depending on the functional coagulation factor level in the circulation.Fifty percent to 150% of coagulation factor VIII is recognized as the normal range,while 6% to 49% of normal coagulation factor VIII or IX is treated as mild hemophilia,and people usually experience bleeding after injury,trauma,or surgery.Moderate hemophilia is the term used for the presence of 1% to 5% of normal coagulation factor VIII or IX; sufferers experience spontaneous bleeding with only slight provocation.Less than 1%of coagulation factor VIII or IX is recognized as severe hemophilia,which presents as spontaneous bleeding in the joints,brain,and muscles.
A brief history of hemophilia treatment covers this disease from unsolved to completely cure and from treatment using serum precipitation to gene therapy.In the 1960s and 1970s,FVIII in human serum was found and collected for infusion into hemophilia A patients.In 1965,Dr.Judith Graham Pool3found a simple way to cryoprecipitate a high concentration of FVIII from normal human serum and then infuse it to control heavy bleeding,allowing blood banks to produce and store large amounts of FVIII for use in surgical procedures for hemophiliacs.In 1970,frozen-dried plasma-derived factor concentrates were used.Desmopressin was identified and developed to treat mild hemophilia and von Willebrand disease.4,5The first recombinant factor VIII was approved by the FDA in 1992,and the first recombinant factor IX product was approved 5 years later.The first generation of coagulation factors precipitated from human blood was easy to obtain,and their supplement was abundant,but immune responses,especially the antigen–antibody reaction between different people,and high risks of viral infection were unavoidable.The second generation of coagulation factors,including human recombinant FVIII and FIX,avoided the disadvantages of the risk of virus infection and immune response from the plasma products and were independent of the blood bank.Furthermore,the safety and activity of the recombinant coagulation factors were optimized gradually through elimination of albumin protein and animal source contaminations and nano-classical purification.6–9The optimization of coagulation factor activity utilizedbioengineering tofind higher-activity variants or longer halflife mutants/fragments,such as the FVIII Padua mutant;10FIXPadua was initially identified as the etiology of a rare X-linked thrombophilia with a missense mutation,which presents with a spontaneous venous thrombosis at a young age.10Extended half-life(EHL) FVIII,which provides for prophylactic regimens with a decreased infusion frequency,was another advance in recombinant coagulation factor protein therapy.11–14Despite the advantages of recombinant wild type (WT) and variant coagulation factors,the price of these products is still high due to their frequent infusion rates.
In the last decade,viruses such as lentivirus,adenovirus,and adeno-associated virus(AAV)have attracted increasing attention as gene therapy carriers.Among these carrier viruses,AAV has been proven to be an ideal tool in gene therapy for hemophilia.
2.THE ADVANTAGE OF GENE THERAPY,ESPECIALLY AAV,TO CURE HEMOPHILIA
Adeno-associated virus is a small virus that can infect humans and other kinds of mammals.AAV is constructed from 60 highly homogenous proteins and one strain of genome DNA.The capsid of AAV is an icosahedron assembled from 20 VP1 proteins,20 VP1 proteins,and20VP3proteins;the three protein groups share the same N-terminal sequence.The genome of AAV contains two inverted terminal repeat (ITR) sequences,which consist of an enhancer,a promoter,a gene sequence,and a polyA sequence.The two ITR sequences are located at the 5’and3’termini of the genome and help to form the proper genome structure by forming concatemers.To date,AAV has been recognized as not being pathogenic,inducing only a mild immune response and rarely integrating its DNA into the host genome.Therefore,AAV has increasingly been considered an ideal carrier for gene therapy in recent decades.
AAV carrying theFVIIIorFIXgene has already been successfully used in preclinical research and clinical trials for treating FVIII-or FIX-deficient hemophilia,respectively.To get rid of the helper adenovirus,highly purified particles and decreased cytotoxic contamination were obtained,and plasmids containing the essentialadenovirus helpergenes,includingE1A,E1B,E2,andE3,were employed to package AAV particles withrepandcapcarrying plasmids and target genes carrying ITR plasmids.
In previous research and clinical trials,AAV-FVIII and AAVFIX were successfully used in the human body.The amount of AAV administered ranged from 2e11 to 6e13/kg,and the amount of AAV particles applied in the clinical trials was relatively higher than that of other viruses,such as lentivirus and CMV; a high packaging efficiency is an important characteristic of AAV.AAV particles were originally produced by gene-carrying ITR plasmids,rep–cap plasmids and adenovirus helper systems.Although the packaging efficiency of this system was high,the pathogenic elements from adenovirus could not be avoided,which was a main safety concern in clinical trials.Xiao15constructed the four adenovirus helper genes into one plasmid,successfully avoiding adenovirus element contamination.Researchers could then switch focus to managing capsid immunogenicity and the integration of the AAV genome into the host genome.
The size of the genome of single-stranded AAV (scAAV)genome capability is 4.7 kbp,which means that the length of the enhancer,promoter,target gene,and polyA together should exceed 4.7 kbp.The size of the genome of self-complementary AAV(scAAV)is 2.5 kbp,which is much less than that of scAAV but displays a higher transfection efficiency and gene expression level.16For coagulation factor IX,the original human gene length is 1.4 kbp,and it is easy to package the entire ORF region into ssAAV and scAAV.Furthermore,the FIXR338L(Padua)variant carried by AAV5 or AAV8 displayed ~8-fold activity compared to that of WT FIX10and has recently been employed in several clinical trials (Table1).The discovery of the FIXR338L varianthelped to reduce the administration of AAV particles to maintain the same coagulation factor activity as the WT while reducing immunogenicity and liver damage.
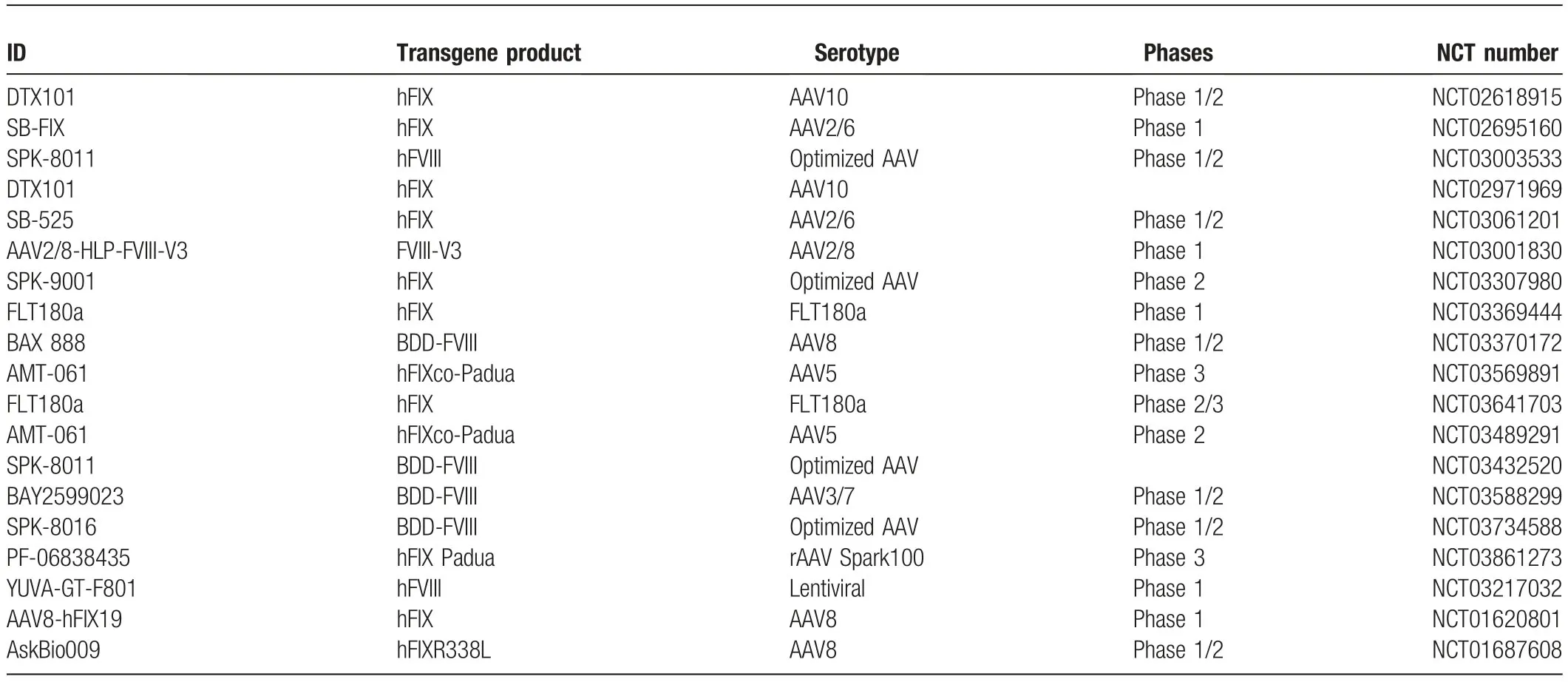
Table1 The AAV related detail in the clinical trials registered at NCT.
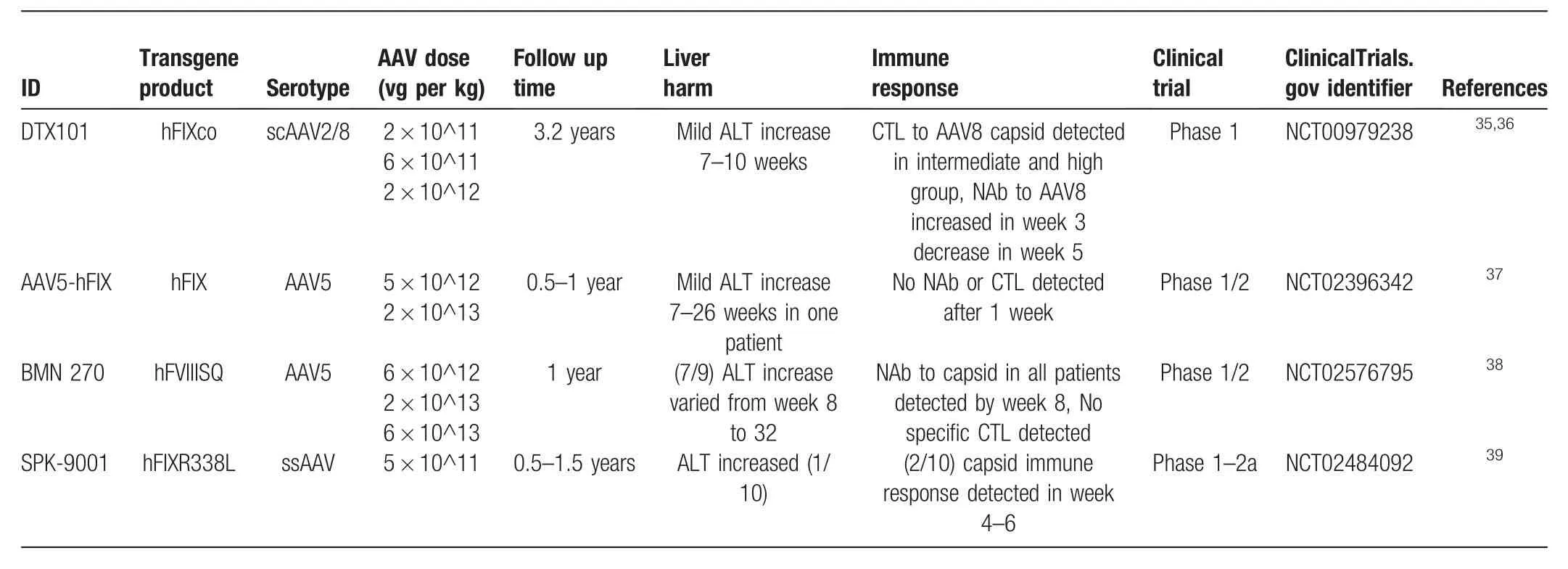
Table2 The AAV employed clinical trials,which have been completed,were summarized according the technical aspects.
The endurance of AAV-transfected FIX in clinical trials is on a“year’s scale.”The half-lives of coagulation factors FIX and FVIII are very short,between 18 to 24h and 12h,respectively.17According to the observations from clinical trials,one-time administration of AAV particles resulted in the maintenance of coagulation factor levels >1%for 12 to 24 months(Table2),and the duration of coagulation factors showed no correlation with the number of administered particles.Because the transgene could stably exist in the host cell,the gene expression time was longer than 1 year in all completed clinical trials.Another advantage of AAV applied in hemophilia therapy is the rare insertion occurrence of its genome.Unlike genome-integrating viruses,which raise high safety concerns in clinical use for their high possibility of genome integration and random insertions,WT AAV has one inserting site,human chromosome 19(AAVS1),as a result of the elements of AAV,including the ITR sequences and rep protein,binding to AAVS1 with very low efficiency.18,19According to Weitzman’s study,the specific AAVS1 integration frequency was 0.1%,random integration frequencies were difficult to detect.19Therefore,several clinical trials concerning the long-term observation of transgene expression delivered by AAV are being carried out.
Liver injury after AAV injection is a very common phenomenon in clinical trials but is very mild compared with other carriers.The immune response induced by liver injury could reduce transgene expression levels.After AAV particles enter hepatocytes,they travel to the prenuclear region20,21and uncoat their capsid,which is then degraded by proteasomes.The remaining capsid fragments are presented on the surface and initiate the immune response.22,23On the other hand,AAV-carrying hepatocytes can also sense virus invasion by cytoplasmic sensors such as MDA5,RIG-I,and MAVS to initiate internal responses and apoptosis.24,25According to the results of clinical trials,AAV particles could induce a very mild liver injury 1 week after administration,as indicated by an elevation of the ALT level in the peripheral blood serum.These ALT elevations were seemingly transient and not related to transgene level decrease.Most of the patients who displayed liver injury received antiimmune treatment.
3.A BRIEF SUMMARY OF HEMOPHILIA CLINICAL TRIALS WORLDWIDE FROM DRUG TO GENETHERAPY
The first case of AAV as a gene therapy tool in a clinical trial started in 2004 and was sponsored by Avigen(NCT00076557).The number of clinical trials concerning hemophilia A/B treated by AAV-transgenes has been increasing over the last 10 years.As shown in Table3,approximately 368 clinical trials about hemophilia have been initiated.Among these trials,234 attempted to explore and cure hemophilia A,while 108 cases were associated with hemophilia B.Over the last 10 years,56 hemophilia A trials tried using recombinant human coagulationfactor VIII,and 11 cases tried employing AAV to carry theFVIIIgene.Meanwhile,13 hemophilia B trials used recombinant coagulation factor IX as the agent in the replacement strategy,and 11 used AAV carrying theFIXgene as a gene therapy strategy.Despite several unsolvable obstacles in recombinant human coagulation factor VIII or IX,such as inhibitor generation and the short half-life of FVIII and FIX in the human body,AAV gene therapy has begun to gain increased traction as a hemophilia treatment.
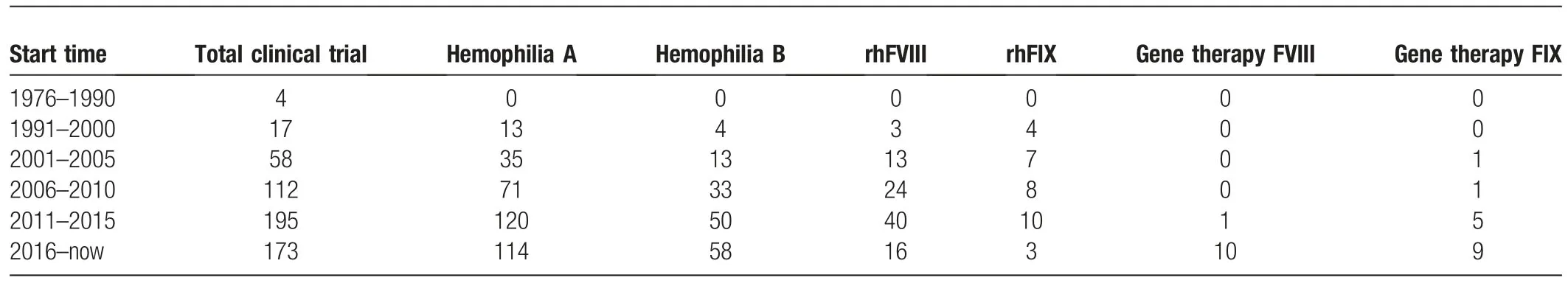
Table3 The distribution of targeting diseases including hemophilia A/B,treatment approaches including protein products and gene therapies in clinical trials were summarized by the time.
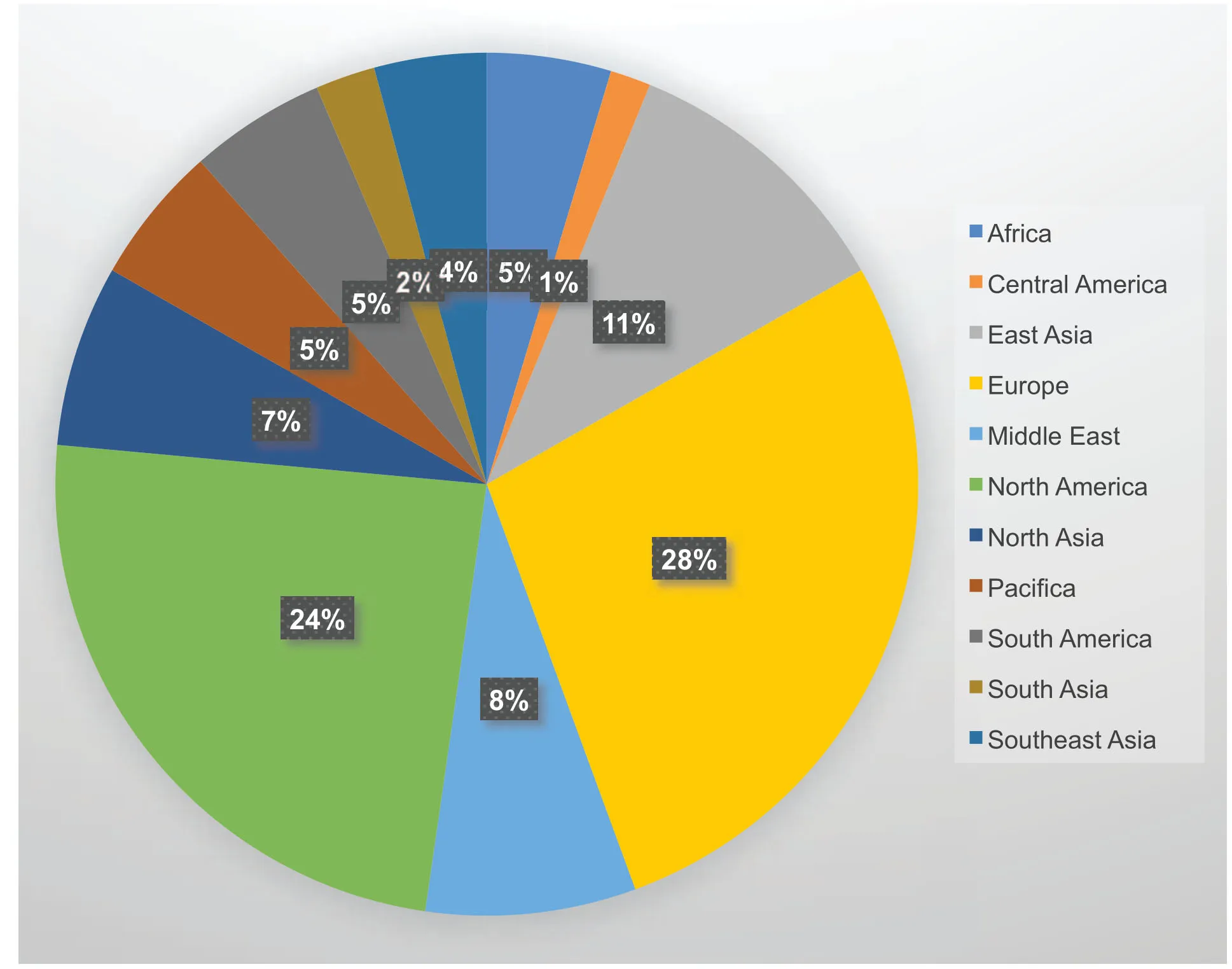
Figure1.The world-wide distribution of clinical trials for hemophilia.In this graph,the clinical trial including on-going and completed were summarized and not limited to gene therapy.And the original data was from clinicaltrials.gov.
Hemophilia has received worldwide attention in recent decades.One hundred clinical trials covering treatment involving drugs,inhibitors,recombinant proteins,and gene therapy have been initiated.As shown in Figure1,clinical trials running in North America and Europe contributed more than 50% of all trials worldwide,which is obviously related not to the population distribution but to the different regions’development levels.Two reports describing hemophilia B clinical trials using retrovirusbased gene therapy treatments were published in 199326and 1996.27In Qiu’s study,the implantation of autologous fibroblasts genetically modified to secrete clotting factor IX by a retrovirus restored the plasma FIX protein in two patients,increasing protein levels over 2-fold after several injections of fibroblast cells and persisting for more than 420 days.27Another clinical trial for hemophilia B was approved in February 2003 using a recombinant adeno-associated viral vector(AAV)-2 encoding human FIX.This trial was performed in collaboration with Beijing Vector Gene Technology Company(VGTC)and Fudan University.
Four clinical trials investigating gene therapy for treating hemophilia have been completed,three for hemophilia B and one for hemophilia A.As summarized in Table2,AAV2,AAV8,AAV5,or an optimized AAV were used,the WTs of which have been proven to display good tropism for the liver.28,29Human coagulation FIX with the R338L mutant is a gain-function variant originally from Chang’s research,which was found to have much higher coagulating activity than WT.10,30Even though FIX-R338L is small enough to be packaged by normal AAV,self-complementary AAV was employed in some preclinical hemophilia B studies and resulted in a higher transduction efficiency.31,32For hemophilia A,the whole length of the WT human coagulation FVIII ORF is approximately 2.3 kbp,while the B-domain deleted recombinant FVIII ORF was approximately 4.3 kbp,which displayed a comparable activity with that of the WT.33,34BDD-FVIII could only be packaged by single-strain AAV(ssAAV)under the condition that the promoter should not exceed 1000bp,which limited the choice of promoter.
In all completed AAV-associated gene therapy hemophilia clinical trials,only mild liver injury,indicated by ALT level,was induced,and administration of an immunosuppressor could rescue the ALT elevation,which means AAV gene therapy is safe enough with regards to the mild liver injury.As summarized in Table2,35–39in 2 of 4 completed clinical trials,neutralizing antibodies to the capsid were reported,which were generated in the high dose group.CTL responses to the capsid were reported in two clinical trials.No neutralizing antibodies against the transgene protein or ITR were found in any of the clinical trials.
To enhance the tropism and transduction efficiency of AAV particles,in the ongoing clinical trials,more capsid types are employed,including AAV2,5,6,7,and 10(Table1).Furthermore,more optimized capsids are reported.Zheng’s study found that the polyploid AAV generated from AAV2 and AAV8 displayed a higher transduction efficiency and capability to escape from neutralizing antibodies in vivo40and also established a novel method to discover AAV capsid mutants that had more potent tissue tropism and neutralizing antibody resistance.41
Altogether,AAV would be an ideal tool to cure hemophilia,a disease caused by the mutation of a single gene whose length is no more than 5 kbp.
4.THE MAIN OBSTACLE IN GENE THERAPY OF HEMOPHILIA,ESPECIALLY AAV-BASED THERAPY
Currently,capsidimmunogenicityisnot clearlyunderstood.The AAV capsid could help AAV particles enter target cells and protect the genome as it passes from the endosome to the lysosome.42,43After the capsid proteins break up and are unpackaged in the perinuclear region,the capsid protein is degraded in the lysosome or by the proteinase degradation pathway,and sequentially,the degraded product is carried by certain proteins to the cell membrane and presented as an antigen by MHC I/II molecules either directly or indirectly.44,45The neutralizing antibodies against the AAV capsid might be originally induced by liver injury.39The function of the capsid is similar to that of a two-sided sword.Researchers worldwide are trying to distinguish the difference between the “good side” and the “bad side” of the AAV.One important finding was the basic region of the VP1 protein(BR1),which might dominate the AAV trafficking to the perinuclear region and might contain the important amino acid lysine as a main modifying target of the proteasome.44Therefore,it may be an ideal strategy to mutate the lysine in theBR1–3 sequence into otheramino acids to avoid proteasome targeting and maintain normal trafficking capability.
According to the results of the completed clinical trials,no neutralizing antibodies against FVIII and FIX have been detected,which is very common after recombinant FVIII or FIX administration in the clinic and is the main reason for the failure of replacement strategies failure.46Therefore,inhibitors of FVIII and FIX are not the main obstacle for AAV gene therapy to hemophilia.However,according to the reports of those four completed clinical trials,two questions have been raised:
1.In the first week post-AAV injection,the FVIII or FIX level would decrease very quickly from high to low,even with respect to the amount of liver injury,the mechanism of which still remains unclear;
2.In almost all patients from the four clinical trials,the stable coagulation factor level was much lower than the physical level;there is an invisible red line existing,so what’s the nature of this red line?
For question 1,when high dose AAV particles were injected into patients,AAV particles would enter the hepatocytes,and after degradation by proteasomes,the capsid would be presented on the cell surface.The host immune system,especially CTLs,can recognize and terminate infected hepatocytes.Based on this hypothesis,immunosuppressors should be added immediately after AAV administration.In one of our previous studies,empty AAV particles could induce a stronger CTL response,and greater numbers of empty capsid particles were presented as antigens than the full particle,45which revealed that the CTL response to the capsid might lead to a sharp decrease in the coagulation factor in the first week post-AAV injection.
For question 2,CTL responses or neutralizing antibodies to the capsid may not be the main reason why transgene expression levels remained low,according to the clinical trials’ reports.In addition to the immunogenicity of the capsids,the genomic DNA or transcript RNA carried by AAV has also been found to initiate an inflammatory response.The most recent research by Wenwei24revealed that dsRNA produced by AAV could trigger the innate immune response after AAV gene therapy at a later time point depending on the double-stranded RNA (dsRNA)sensor MDA5,which may be responsible for the FIX loss 10 weeks after AAV administration in the clinical trials.Unlike immunotolerance,the hepatocytes infected by AAV could still sense the existence of the viral genome,and by some unclear mechanism,they might make the viral gene silent or themselves undergo apoptosis.Under this hypothesis,the long-term observation of AAV gene therapy would demonstrate much lower levels of or eliminated coagulation factors after 2 to 3 years.
5.PERSPECTIVES
To date,the use of AAV in gene therapy for the treatment of hemophilia has been very promising.AAV has irreplaceable advantages compared with other therapeutic methods,including recombinant proteins,neutralizing antibodies,and other viruses,as mentioned above.According to recent reports,capsid optimization would be the main direction of AAV gene therapy development.By optimizing the AAV capsid,AAV particles would achieve higher transduction efficiency and more specific tropism and result in a weaker immune response.Rosario’s study revealed that the triple mutation Y731F/Y705F/T492V on the AAV6 capsid could enhance the transduction efficiency in microglia cells in mouse.47The triple mutation of Y444F,Y500F,and Y730F on the AAV2 capsid was reported to have a much higher transduction efficiency than WT in mouse retinal cells.48For hemophilia,this triple mutant had the highest level of in vivo gene transfer to murine hepatocytes,approximately threefold more efficient than the best single mutants,and ~30- to 80-fold higher compared with WT AAV2 capsids.49Selecting an ideal capsid mutant from the capsid mutant library is a reasonable strategy in preclinical research.As mentioned above,Zheng established a mutant library and found their mutant capsid by using a mouse model,which displayed good tropism to muscle.41In a study regarding AAV targeting HIV-infected T cells,researchers used directed evolution successfully to select for mutants with improved tropism for a T cell line in the presence of HIV-1,and two of the four cap mutants showed a significant increase in the amount of cell-associated genomes compared to WT AAV2.50
In addition to redesigning the capsid directly,there are some other studies trying to find a chaperone for AAV capsids to enhance their transduction efficiency,change their tropism and reduce their pathogenesis.The heparin-binding motif on the AAV capsid was found by Kern,located on arginine-484,-487,-585,and -588 and lysine-532.51In one of our previous studies,we found that several serum proteins could bind to AAV capsids,such as albumin,transferrin,and LDL,which could not only enhance the AAV8 transduction efficiency but also help with escape the immune response.45A similar study revealed that mouse C-reactive protein and platelet factor 4 could enhance the AAV6 and AAV8/9 transduction efficiency in a murine model.52We believe that future efforts will lead to the discovery of a universal binding protein or molecule with a high affinity that could change the tropism of AAV to any needed tissue.
Although the clinical dose of AAV could induce little harm to patients,the mild immune response to the AAV capsid may lead to gene therapy failure.Therefore,the simultaneous use of a lower dose of AAV particles with the maintenance of the transgene expression level could be a strategy to avoid immune clearance.Although human coagulation factor IX with the R338L mutation has high coagulation activity compared to the WT,the FIX activity level restored in the patients is still limited.B-domain deleted recombinant FVIII is small enough for AAV to package its gene; however,its coagulation activity is still low.Therefore,finding a novel mutant of the genes that have a shorter ORF and higher activity should be the primary direction of preclinical research.
杂志排行
血液科学的其它文章
- The regulators of BCR signaling during B cell activation
- Novel chimeric antigen receptor T cells based on T-cell receptor-like antibodies
- CAR-NK cell therapeutics for hematologic malignancies: hope is on the horizon
- Long noncoding RNA PCED1B-AS1 promotes erythroid differentiation coordinating with GATA1 and chromatin remodeling
- Long non-coding RNAs during normal erythropoiesis
- Current status and hurdles for CAR-T cell immune therapy