Current status and hurdles for CAR-T cell immune therapy
2019-11-02
Key Laboratory of Regenerative Biology,South China Institute for Stem Cell Biology and Regenerative Medicine,Guangzhou Institutes of Biomedicine and Health,Chinese Academy of Sciences,Guangzhou,China
Abstract
Keywords:Cancer,Chimeric antigen receptor T cells,Immune therapy
Studies conducted since the past few decades have revealed the critical roles played by immune cells in cancer surveillance and development.Of the diverse immune cell types,T cells have been the focus of cancer immunology because of their ability to specifically recognize cancer cells via T cell receptors (TCRs)through peptide-major histocompatibility complexes (pMHC)and by triggering strong immune responses.Therefore,the aim of several researchers dedicated to developing cancer immune therapeutic strategies has been to reactivate tumor antigenspecific T cells or redirect bystander T cells to recognize certain antigens expressed by tumor cells with high specificity.The development of checkpoint blockage therapies and cancer vaccines as well as adoptive cell transfer (ACT) technology represents the major translational achievements of the fundamental findings in this field of research.
In recent years,the clinical application of chimeric antigen receptor T (CAR-T) cells has achieved major success in the treatment of several types of hematological malignancies.In 2017,the U.S.Food and Drug Administration approved CAR-T cell therapy for the treatment of relapsed or refractory (R/R)acute lymphoblastic lymphoma (tisagenlecleucel) and diffuse large B-cell lymphoma (axicabtagene ciloleucel).Meanwhile,there is an increase in the number of CAR-T cell clinical trials being registered and ongoing atClinicalTrials.gov.1–4These breakthroughs and progresses emphasize the significant value of ACT therapy and encourage its further development.In this review,our primary aim is to provide a brief introduction on the design of CAR vectors,CARgene delivery,mouse modeling,and current clinical results of CAR-T cell therapies,and to summarize the hurdles faced and potential solutions for these aspects.
1.THE DESIGN OF CAR VECTORS
CARs are recombinant membrane receptor molecules that are generally expressed on the surface of T cells.The extracellular domain of CAR contains monoclonal antibody-derived singlechain variable fragments(ScFv)that mediate binding with tumorassociated antigens (TAAs) and a space/hinge domain typically derived from the FC fragment of IgG to extend ScFv from the T cell membrane.To locate CAR on the cell membrane,a transmembrane fragment follows the extracellular domain.The intracellular domains contain CD3ζ and co-stimulatory domains,which together mimic the canonical first and second signals to induce T cell activation.Upon encountering target tumor cells,CAR binds with TAA and induces the activation of CD3ζ and co-stimulatory signals,followed by triggering of downstream phosphorylation cascades,which then activate T cells to proliferate and elicit effector functions such as cytotoxicity and cytokine secretion.5–7The cytotoxicity of CAR-T cells depends on the secretion of perforin and granzyme and the expression of Fas/Fas ligand,which can directly lyse target cells or mediate apoptosis.IFN-γ,which can upregulate MHC expression by tumor cells,also drives the polarization of M1 macrophages.Secretion of the lymphocyte growth factor IL-2 supports the robust proliferation of CAR-T cells,whereas GMCSF promotes the activation and expansion of myeloid cells to induce systemic immune responses.8The first-generation CAR-T cells utilize only CD3ζ to activate T cells without incorporating a co-stimulatory domain,resulting in poor in vivo antitumor efficacy of these cells.The second-generation CAR-T cells,which generally utilize CD28 or 4-1BB endoplasmic domains as a costimulatory signal,have demonstrated remarkable efficacies in patients with leukemia.9,10Furthermore,the third-generation CAR-T cells incorporated with another co-stimulatory domain together with CD28 or 4-1BB,such as ICOS,CD40,OX40,TLR2,and DAP10,have been reported and functionally evaluated in preclinical models or clinical trials.11–14
2.DELIVERY OF CAR VECTORS INTO T CELLS
A typical technical strategy for manufacturing autologous CART cells with viral vectors primarily includes the following steps:enrichment of PBMCs from peripheral blood by apheresis,magnetic isolation of T cells,activation of T cells,viral transduction,expansion,and harvesting and freezing of cells.15–17Although the reliability of γ-retrovirus and lentivirus has been extensively confirmed,novel gene delivery approaches are still being developed to reduce the possibility of oncogenic mutations caused by stochastic viral integration,enhance the efficiency of large gene transfer,and simplify the manufacturing processes.
Currently,the most commonly used gene delivery approach for CAR-T cell manufacture is the replication of defective γ-retrovirus and lentivirus mediated by gene integration,although some disadvantages are associated with this approach.First,both these viruses are produced by the transient transfection of virusencoding plasmids into packaging cell lines; theoretically,replication-competitive retro/lentivirus (RCR/RCL) could be generated by the recombination of genes encoding the viral genome and structural components.18,19Therefore,the current viral packaging system uses three to four plasmids to separately encode these components.However,this requires the preparation of large amounts of plasmids,which is labor-intensive.Moreover,because of the instability of the virus and its short half-life period,the harvested viral supernatant must be quickly used or cryopreserved.15,20Furthermore,impurities such as plasmid DNA and nucleic acids and proteins released by packaging cells must be detected and removed,which drastically increases process complexity and economic cost.20,21
Another method to deliver CAR into T cells is the transposon/transposase system,which uses electroporation to transfer plasmids into cells and mediates CAR integration with its transposase activities.Both sleeping beauty and PiggyBac (PB)systems have been evaluated forCARgene transfer.22–26Compared with retroviruses and lentiviruses,this approach can significantly reduce the complexity of the manufacturing process and avoid the potential risks caused by RCR/RCL.However,cell viability is more seriously affected by this approach as both electroporation and exogenous plasmids could act as inducers of cell death.Thus,electroporation of mRNA encoding the CAR into the cytoplasm of T cells has been demonstrated to increase cell viability and reduce the genotoxicity and side effects caused by constitutive CAR expression.27,28Clinical trials of mesothelin-specific CAR-T cells based on this strategy are ongoing (NCT01355965).More recently,theCRISPR-CAS9gene editing system has been employed forCARgene transfer.This approach allows the integration of theCARgene into specific sites,such as the TCR α constant (TRAC) locus29; thus avoiding the generation of oncogenic mutations potentially caused by stochastic integration.Meanwhile,the CRISPR-CAS9 system has also been used to generate universal CAR-T cells.The TCR and β-2-microgloblin coding genes are disrupted in these cells to compromise graft-versus-host responses and prevent cells from being attacked by host T cells in allogenic situations.30–32
3.MOUSE MODELS FOR EVALUATING CAR-T CELLS
In general,studies evaluating the antitumor activities of CAR-T cells use both in vitro cell culture-based assays and in vivo tumorbearing mouse models.In vitro assays are used to assess certain aspects or functions,such as cytokine secretion,deregulation,and cytotoxicity,as well as proliferation upon target cell stimulation.These assays can indicate the recognition capability of CAR but are not as helpful for evaluating efficacy and predicting the patient’s response.The tumor-bearing mouse models are at least able to provide a physiological or pathological environment that is similar to the human body to a certain degree.To date,mouse models used for evaluating the efficacy of CAR-T cells can be classified into four types: syngeneic,transgenic,xenograft,and humanized models.
3.1.Syngeneic Models
Syngeneic mouse models typically use immunocompetent mice and murine tumors and T cells.The advantage of using these models is that the composition and the functions of immune system in these models are normal.Because CAR-T cells can trigger systemic immune responses that involve multiple types of immune cells to mediate antitumor activities and adverse effects,such as CRS and neurotoxicity,syngeneic mouse models are extremely useful to study the interaction between CAR-T cells and other host immune cells,such as macrophages and myeloidderived suppressor cells (MDSC),and analyze the synergy between CAR-T cells and other immunostimulating drugs.33–35However,considering the subjective differences in mouse and human biology and the differences in target antigens and CAR sequences,these models cannot model human CAR-T cells targeting human cancer.
3.2.Transgenic Models
Given that syngeneic mouse models do not express human TAAs,transgenic human TAA-expressing immunocompetent mice have been developed.In this model,CAR-T cells are produced by modifying murine T cells with human extracellular domains and murine intracellular domains.This model can reflect the off-target effect of CAR-T cells that may recognize TAA expression in normal tissues.Furthermore,transplantation of murine cancer cell lines that stably express human TAAs in this model enables the recognition of human TAA by human ScFv and induces systemic immune responses elicited by mouse immune cells.36–38
3.3.Xenograft Models
The successful construction of severe combined immunodeficient mice(SCID mice)that possess defects in the development of T and B cells allows xenotransplantation of human tissues and cells,including human cancer cell lines,tumor tissues of patientderived xenografts,and immune cells.In recent decades,SCID mice have been continuously upgraded to generate NOD-SCID and NOD-SCID IL2Rγ-/-mice.The former carries a defect in the innate immune system,and the latter was introduced with an IL-2 receptor γ mutation to completely block its lymphocyte development,including NK cells.This genetic modification drastically enhances the efficiency for the transplantation of human xenografts.39,40Application of these mouse models enables the functional evaluation of human CAR-T cells against human cancer,and they have also been recognized as a common platform for the development of CAR-T cells targeting novel target antigens or disease types.
However,despite the advantages of xenograft mouse models,there are certain drawbacks.First,these models have a compromised immune system,owing to which they cannot model the interplays and crosstalk of CAR-T cells between other host immune cells.41In addition,the native TCR expressed by human T cells can recognize murine xeno-antigen-MHC complexes,thereby leading to hyperactivation of these infused T cells and finally the induction of xenogeneic graft-versus-host disease (xeno GVHD) in these mice.42,43Because of this xenoactivation,the growth kinetics and tissue distribution of infused CAR-T cells in these mice may not be able to simulate that of human conditions,and the lethality of xeno GVHD can interfere in cancer,resulting in death,thereby leading to an inaccurate evaluation of efficacy.
3.4.Humanized Models
To construct human immune system in the immunodeficient mice,the NOD-SCID IL2Rγ-/-mice can be transplanted with human CD34-positive hematopoietic stem and progenitor cells(HSPCs) to reconstitute human lymphoid and myeloid cells in these mice.43Human cancer cells can be subsequently transplanted into these mice after successful reconstitution.CAR-T cells produced from the same donor of HSPCs(e.g.,isolating both T cells and HSPCs from a cord blood sample)can be administered to these mice.41,44Moreover,the construction of a BLT mouse model(co-transplantation with human bone marrow,fetal liver,and thymus in immunodeficient mice) can better mimic normal human T cell development and selection processes in the thymus41and avoid the development of xeno GVHD.It has been reported that the reconstitution rate of human myeloid cells in CD34+ HSPC-transplanted mice is much lower than that under normal conditions,and the maturation of these cells is hindered because of the lack of several types of human cytokines,including stem cell factor,GM-CSF,and M-CSF.Certain types of myeloid cells,such as macrophages,can be activated by CAR-T cells and play important roles in systemic immune responses.Therefore,human cytokine transgenic immunodeficient mice were developed to facilitate the reconstitution and differentiation of myeloid cells from HSPCs.This model has been used to mimic CRS caused by CD19-specific CAR-T cell therapy.45
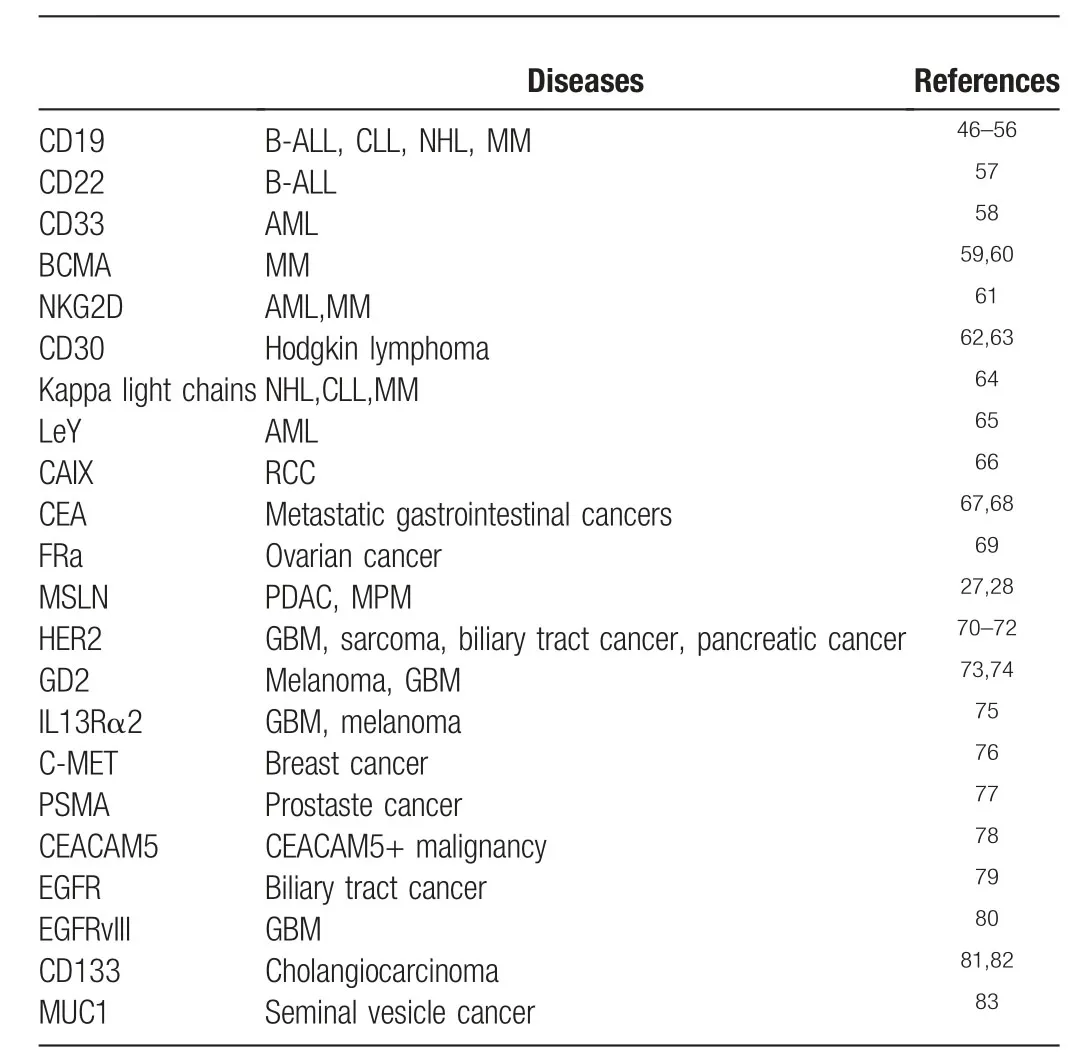
Table1 Target antigens of CAR T-cells in the published clinical trials
4.CLINICAL APPLICATIONS AND HURDLES OF CAR-T CELL THERAPY
Table1 summarized the information regarding the target antigens and disease types from the published results of clinical trials of CAR-T cell therapies.More importantly,we will discuss the current status and hurdles of CAR-T cell therapies for both hematological and solid malignancies reflected by the current clinical results,and summarize those findings from preclinical studies which aim to improve the performance of CAR-T cells.
4.1.Acute Lymphoblastic Leukemia
CD19-specific CAR-T cells containing either CD28 or 4-1BB co-stimulatory domain have shown interesting clinical outcomes in R/R B-cell acute lymphoblastic leukemia (B-ALL),generally with complete remission rates of >70%.Patients typically received a preconditioning regimen comprising cyclophosphamide and fludarabine to deplete lymphocytes and reduce the leukemia burden before CAR-T infusion to induce better expansion and persistence of CAR-T cells.CRS and neurotoxicity were the major life-threatening adverse effects caused by CART cell-triggered immune responses.53,84,85Administration of tocilizumab,an IL-6R antagonistic mAb,has been widely used to alleviate CRS.Moreover,a safe and potent anti-CD19 CAR-T cell therapy was reported recently,as indicated by the fact that no CRS or neurological events were observed and the CR rate remained at >50%.86
However,despite the remarkable CR rate,up to 50% of CAR19 T cell-treated patients suffered from leukemia relapse within 1 year after therapy and were generally resistant to the secondary infusion of the same CAR-T cell product.In some patients,relapse occurred because of the antigen loss of CD19,and the subsequent infusion of CD22 CAR-T cells was able to elicit immune responses in these cases.57Dual-antigen targeting CAR-T cells may also be helpful in the prevention of antigen loss caused by leukemia relapse.87
Nevertheless,the relapsed tumor cells retain CD19 expression in the majority of patients with relapse,and the mechanisms underlying these relapses remain elusive.It is speculated that CAR molecules are immunogenic and can induce host immune responses,which can mediate elimination of infused CAR-T cells.In fact,Turtle et al found that host anti-CAR immune responses are mediated by CD8 T cells in some patients.88The application of humanized ScFv may enhance CAR-T cell persistency and reduce the relapse rate.Vaccination has been reported to improve the persistence of CD19 CAR-T cells in patients; however,the relapse rate remains high.89Furthermore,clinical trials using 4-1BB co-stimulated CAR-T cells have reported better duration of CAR-T cells compared with that of CD28.It has also been suggested that 4-1BB induces T cell exhaustion to a lower extent compared with induced by CD28,and this effect contributes to superior T cell persistency.90However,the choice between CD28 and 4-1BB remains controversial,and a recent study demonstrated better efficacy of CD28-calibrated CAR-T cells.91Moreover,Ruella et al reported that the transduction of single leukemia cells induced resistance to CAR-T cell therapy.92More recently,Hamieh et al found that trogocytosis,the process of antigen transfer from target cells to the surface of CAR-T cells,can induce fratricide and exhaustion of CAR-T cells and mediate tumor escape93;their findings further indicate the complexity and the unknown mechanisms underlying leukemia relapses.
4.2.Acute Myeloid Leukemia
Numerous clinical studies aimed at reproducing the success of CD19-specific CAR-T cells in BALL,CD33,CD123,NKG2D,and Lewis Y-specific CAR-T cells have been reported thus far.Although responses were observed for CD33 and CD123 CAR-T cell therapies,the myeloablative effect caused by these CAR-T cells has hindered their application and the impact prognosis in patients.94–96In contrast,NKG2D chimeric receptor T cells and Lewis Y-specific CAR-T cells exhibited no significant toxicity but limited responses.61In a recent preclinical study,Kim et al developed a novel technology with genetic inactivation of CD33 in hematopoietic stem cells to enable the application of CAR-T cell therapy for the treatment of AML.97Targets that are more specific are urgently required to be confirmed to elicit substantial responses with acceptable adverse effects for patients with AML.CLL1,FLT3-ITD,CD44v6,CD7,and folate receptor β are targets that have already been evaluated in preclinical stages,and their clinical outcomes are worth looking into.96,98
4.3.Lymphoma
The response rate of CD19-specific CAR-T cells in patients with lymphoma (primarily DLBCL and transformed follicular lymphoma,t-FL)is generally in the range of 50%to 80%,and the CR rate is 40%to 60%.A high relapse rate(>60%)has also been observed with long-term follow-up.52,99–101Except for the abovementioned mechanisms responsible for leukemia relapse,lymphoma tissue microenvironment can suppress the activity of CAR-T cells through the expression of PD-L1 or through the recruitment of tumor-associated macrophages to mediate relapse.Co-targeting these targets with CAR-T cells may be effective to overcome this immune suppression.102,103
4.4.Multiple Myeloma
Multiple myelomais plasma cell-derived cancer and has the ability to produce immunoglobulin,which results in tissue damage.Despite significant improvement in the prognosis of patients through targeted therapies,including proteasome inhibitors,epigenetic modulators,immune-stimulating drugs,as well as monoclonal antibodies,MM remains largely incurable.B-cell maturation antigen (BCMA),a membrane receptor expressed during B-cell differentiation into plasma cells,is highly specific for MM cancer cells.48,60,104,105Furthermore,bb2121,a BCMA CAR-T-cell therapy that targets R/R MM,was reported to achieve an 85% objective response rate and a 45% CR rate according to a recent publication,59thereby demonstrated the feasibility of its wide clinical application.
4.5.Solid Tumors
In contrast to the surprising performance of CAR-T cell therapy in hematological malignancies,its clinical application for the treatment of solid cancers remains challenging.Recently published phase I trials have demonstrated that rare CR can be induced in patients with solid cancers.The tested disease types,efficacies,toxicity,and the available targets have been intensively reviewed.106–114It was suggested that factors such as the immunosuppressive tumor microenvironment,poor T cell infiltration and persistency,tumor antigen heterogeneity,and off-target toxicities are major obstructions that limit the efficacy of CAR-T cells against solid cancers.To overcome these impediments,several researchers have attempted to enhance the effector functions,persistency,and alleviation of T cell exhaustion through further modification or optimization of CAR with other signaling domains,such as 4-1BB ligand,115CD27,116CD40 and its ligand,117–120OX-40,11ICOS,12,121,122TLR2,13DAP10,14JAK-STAT,123and cytokines,including IL-7,124,125IL-12,126,127IL-15,128–130and IL-18.131,132Notably,Adachi et al reported a significant synergy between the cytokine IL-7 and the chemokine CCL19 in enhancing CAR-T cell infiltration into the tumor tissue.133
The tumor microenvironment generally comprises immunosuppressive cells such as MDSC and immunosuppressive molecules such as PD-L1 and TGF-β,which dampen T cell responses.Targeting MDSC by administering all-trans-retinoic acid (ATRA),134poly I:C,135or infusing chimeric NKG2D receptor NK cells136has demonstrated synergistic activities with CAR-T cell therapies.Meanwhile,targeting immunosuppressive molecules such as PD-1/PD-L1 and TGF-βreportedly improved the potency of CAR-T cells against certain solid cancers.31,137–141A recent study also revealed that the transcription factor NR4A is a protein that limits CAR-T cell functions,and its inhibition improves the potency of CAR-T cells.142Furthermore,oncolytic viruses(OV) have been suggested to stimulate systemic immune responses and destroy solid tumor microenvironments;therefore,combining the therapeutic strategy of OVs with CAR-T cells could have significant potential in treating solid cancers.143–145
It has been demonstrated that primary cancer cells possess considerable genetic and phenotypic heterogeneities146and can thus escape from the attack of CAR-T cells against single targets.87Induction of host immune responses by CAR-T cells may help prevent this.34,147–149In addition,dual or multispecific CAR-T cells may be used as a strategy to overcome antigen escape.However,the off-target effect may also be amplified because of the potential expression of individual antigens on normal tissues.81A well-designed study conducted by Roybal et al described a precision novel design of tumor recognition by T cells with combinatorial antigen-sensing circuits,150which may prevent the induction of off-target responses.
5.CONCLUSIONS AND PERSPECTIVES
The success of CAR-T cell therapy has been inspiring in the field of cancer immunology,especially in treating B-cell-derived hematological malignancies.However,the manufacturing processes,including the CAR delivery method,need to be further improved.Animal models are being developed and upgraded to better mimic the human body and human cancers.Further research must focus on challenges such as the relapse of B-cell leukemia/lymphoma/MM,lack of available targets for AML,and the low efficacies in treating solid cancers.Adverse effects,including CRS and neurotoxicity,should be considered,better modeled,and rationally intervened,while preserving normal antitumor activities.Participation and cooperation of both researchers and physicians could contribute to the step-by-step resolution of these challenges to improve prognosis for a wide range of patients with cancer.
Acknowledgments
We thank Zhiwu Jiang and Le Qin for their insightful comments on this manuscript.
杂志排行
血液科学的其它文章
- The regulators of BCR signaling during B cell activation
- Novel chimeric antigen receptor T cells based on T-cell receptor-like antibodies
- CAR-NK cell therapeutics for hematologic malignancies: hope is on the horizon
- Long noncoding RNA PCED1B-AS1 promotes erythroid differentiation coordinating with GATA1 and chromatin remodeling
- Advances of adeno-associated virus applied in gene therapy to hemophilia from bench work to the clinical use
- Long non-coding RNAs during normal erythropoiesis