Optimizing the defensive characteristics of mild steel via the electrodeposition of Zn—Si3N4 reinforcing particles
2019-10-31AkndeOluwoleFyomi
I.G.Aknde ,O.O.Oluwole ,O.S.I.Fyomi
a Department of Mechanical Engineering,University of Ibadan,Ibadan,Oyo state,Nigeria
b Department of Mechanical Engineering,Covenant University,ota,ogun state,Nigeria
c Department of Chemical,Metallurgical and Materials Engineering,Tshwane University of Technology,Pretoria,South Africa
Keywords:Polarization Corrosion Microhardness Coating Composite Matrix
A B S T R A C T The effect of Zn—Si3N4 deposition prepared via direct electrolytic co-deposition on mild steel was studied as a result its inherent vulnerability to corrosion in an aggressive environment and failure on the application of load.The experiment was conducted varying the mass concentration of silicon nitride(Si3N4)between 7 and 13 g at cell voltage of 0.3 and 0.5 V,at constant temperature of 45°C.The morphologies of the coated surfaces were characterized using high resolution Nikon Optical Microscope and Scanning Electron Microscope(SEM)revealing that the particles of the Zn—Si3N4 were homogeneously dispersed.The corrosion behaviour was studied using potentiodynamic polarization technique in 3.65%NaCl solution and the microhardness was examined using Brinell hardness testing technique.The result of the corrosion experiment confirmed an improved corrosion resistance with a reduction in corrosion rate from 9.7425 mm/year to 0.10847 mm/year,maximum coating efficiency of 98.9%,maximum polarization resistance of 1555.3 Ω and a very low current density of 9.33×10-6 A/cm2.The negative shift in the Ecorr revealed the cathodic protective nature of the coating.The microhardness was also found to have increased from 137.9 HBN for the unmodified steel to a maximum value of 263.3 HBN for the 0.5Zn—13Si3N4 coated steel representing 90.9%increment in hardness as a result of the matrix grain refining and dispersion-strengthening ability of the incorporated Si3N4 particles.
1. Introduction
In recent time,incessant failure of engineering materials have orchestrated the application of coating technology as a reliable defence mechanism in automotive,manufacturing,aerospace and chemical processing industries[1].Composite coating is of a rare quality, enabling the deposition of insoluble nano-particles of ceramic,metallic and non metallic alloys into electrolytic bath for specified chemical,mechanical and functional properties[2—8].Metal matrix reinforced with nano particles generally exhibit wide range of engineering applications due to improved hardness and ability to withstand corrosion and wear[9—14].Zn incorporated nano composite coatings have been confirmed to posses’good surface properties which is not unconnected to the strengthening effect of the embedded particles[15].However,these properties can be made better through the incorporation of Si3N4nano particles [16]. By virtue of the strength, hardness, good fracture toughness and thermal resistance,silicon nitride based ceramic have been a good choice for structural component application such as ball bearings and cutting tool applications[17—19].Although attempted codeposition of hard particles such as Al2O3,TiO2,SiC,WC,Cr3C2,TiC and diamond on steel had been done focusing more on the improvement of wear resistance[20].In view of this,Zn nanocomposite coating reinforced with Si3N4particles was developed with some incorporated desired properties.Si3N4,being a versatile ceramic was chosen because of its impeccable performance when high strength,high hardness as well as good resistance to thermal shock is required [21—23]. The excellent mechanical properties[24,25]of Silicon nitride ceramic are the reasons for the attraction in numbers of application such as engine components, spacecrafts and high-temperature electronics[26—28].
2. Experimental procedure
2.1. Preparation of substrates
Mild steel of dimension (50 mm×30 mm x 2 mm) whose chemical composition is shown in Table 1 was used as the cathode substrate and 99.9%zinc plate of(60 mm×40 mm x 10 mm)were prepared as anodes.The surface of the steel was well polished with emery papers of different grades as recommend in the previously by authors Ref.[29,30].The samples were surface cleaned by immersion in 0.01 M of sodium carbonate solution at ambient temperature of 25°C for 10 s.They were pickled and activated with 10%hydrochloric acid at ambient temperature for 10 s,followed by quick rinsing in deionized water.
2.2. Deposition of Zn—Si3N4
The bath prepared for the coating process using the parameters in Table 2 was subjected to continuous stirring at 300 rpm and 45°C constant heating throughout the coating process in order to obtain stability in suspension,avoiding particles'agglomeration.Prevention of agglomeration of particles enhances the mobility electrophoresis of the solution[31].The agitations of bath do not only keep the Zn—Si3N4particles suspended in the electrolyte but also assist their mass transportation to the cathode surface.Investigations of various researchers have shown that increased agitation generally enhances the amount of particles in the metal deposit.However,too much or immoderate agitation may influence the movement of the electrodes,alter the charge transfer region and more so,result to a lower quantity of particles in the metal deposit[32].In the plating process,the prepared mild steel being the cathode was positioned in between two zinc plates and connected to the negative terminal of the rectifier in the electrodeposition bath.The zinc(anode)were also immersed and connected to the positive terminal of the rectifier[33,34].The gap between the cathode and anodes was 3 cm.The cathode was positioned equidistance from the anodes.The pH,deposition time,current density and temperature were kept constant as shown in Table 2 varying the mass concentration of Si3N4and cell voltage.The following reactions occurred between zinc and the base metal during the electrodeposition process
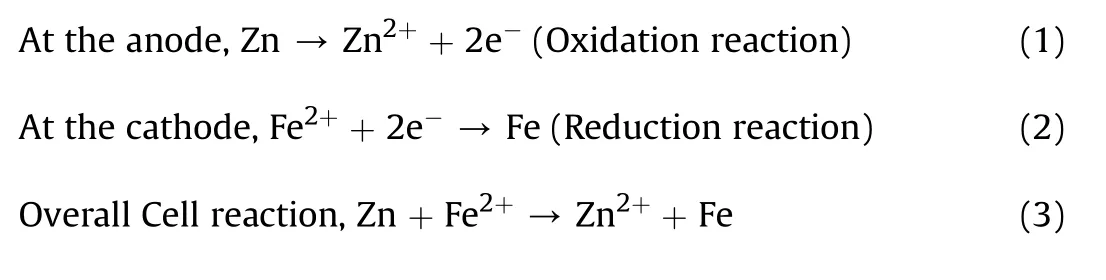
2.3. Characterization of samples and structural test
The surface adhesion and homogeneous dispersion of Zn—Si3N4on the mild steel resulted in the development of remarkable structures.This was examined using high resolution Nikon Optical Microscope and Scanning Electron Microscope(SEM).The images were taken at 100×and 500×magnifications respectively.Potentiodynamic polarization assessment and Brinell technique at a loadof 30 g for a period of 20 s were used to characterize corrosion and hardness of the coated and uncoated steels.Measurements of the coating thickness were carried out using Elcometer microthickness meter guage(Elcometer 456 Model)with the accuracy of±1%.The thickness of all the samples were measured at different points and the thickness average were obtained for each of the samples and recorded as shown in Table 3.
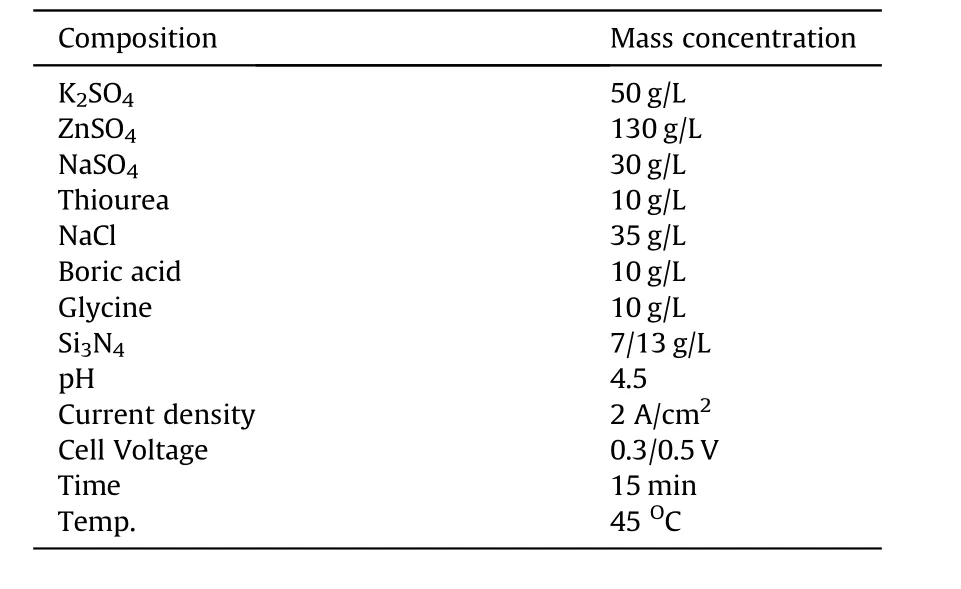
Table 2 Process parameter for Zn—Si3N4 sulphate bath formulation.
2.4. Electrochemical test
A three-electrode cell assembled in a 3.65%NaCl static solution with Autolab PGSTAT 101 Metrohm Potentiostat connected to NOVA 2.1.2 soft ware via computer system was used to examine the anti-corrosion behaviour of the composite coatings at ambient temperature of 25°C.The steel acted as the working electrode,platinum electrode as the counter electrode and Ag/AgCl was made the reference electrode.Potentiodynamic polarization curves were obtained from cathodic potential of-1.5 V to anodic potential of 1.5 V versus open circuit potential at a sweep rate(scan rate)of 0.005 m/s.This was carried out in similitude to our recent research work[35].
2.5. Mechanism of zinc corrosion in 3.65%NaCl solution
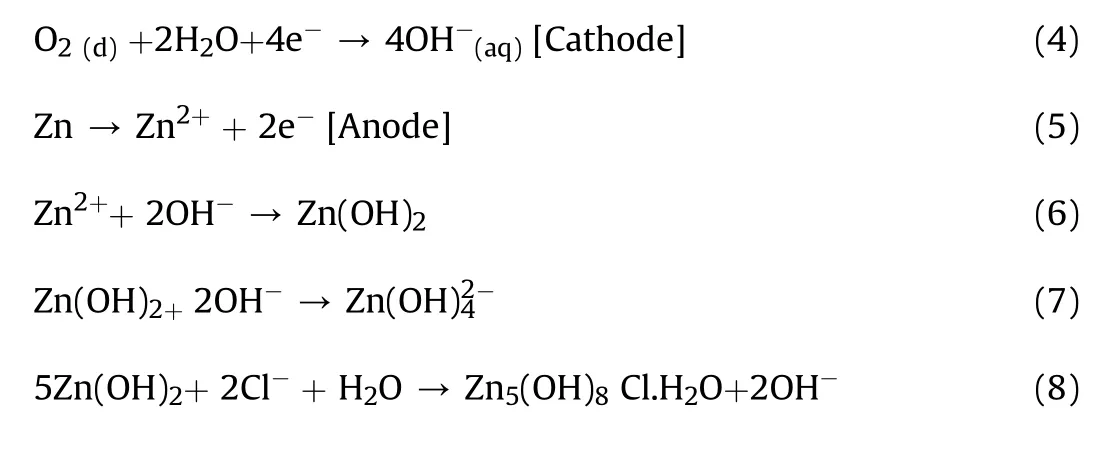
Many researchers have reported zinc hydroxide chloride as corrosion products of zinc in NaCl solution[36—38].Obviously,zinc hydroxide chloride is one of the corrosion layer compounds formed after immersion in 3.65%NaCl solution.A lot of studies on corrosion behaviour of zinc in NaCl solutions have shown that corrosion of zinc precedes two partial reactions[39,40].The cathodic reaction in Eq.(4)corresponds to the reduction of oxygen which could leads to a local increase in pH value in the depth of corrosion damages and consequently resulting to the formation of zinc hydroxide chloride in the pits and their neighbourhood areas[41].The anodic reaction involves the dissolution of zinc as shown by Eq.(5).Expectedly,zinc cation and the hydroxide anion react to form zinc hydroxide presented by Eq.(6).At the active cathodic site,zincate ions is produced as indicated by Eq.(7)provided the pH is large enough.In the presence of Sodium Chloride,Chloride ions(Cl-)migrate to the anodic site where Zinc hydroxide chloride is formed as shown in Eq.(8)

Table 1 Chemical composition of the mild steel used.

Table 3 Itinerary parameters of Zn—Si3N4 alloy co-deposition.
3. Results and discussion
3.1. Structural analysis of uncoated and Zn—Si3N4 deposited mild steel
The Optical micrographs of the uncoated mild steel,Zn—Si3N4coated at 7%w and 13%w are shown in Figs.1—3 respectively.From the structural characterization,as expected,the uncoated mild steel surface as shown in Fig.1 looked rough exhibits the inherent pitting corrosion initiation tendency[42,43].On the other hand,several crystal growths was seen to have developed on the interface of the coated mild steels in Figs.2 and 3 exhibiting a defect free surface.With the 7%w in Fig.2(a)and 2(b),though Fig.2(a)appears smoother but visible flake-like crystalline pattern were seen at the general interface,which are more obvious in Fig.2(b).The unique microstructure displayed by the composite can be linked to the inclusion and homogeneous dispersion of Si3N4nanoparticles in the Zn matrix,promoting the increase in number of nucleation site and impeding crystal growth,resulting in the generation of small nano-sized metal grains[15,44].

Fig.1.Optical micrograph of uncoated mild steel
In the same vein,the 13%w coated mild steel shown in Fig.3(c)and d exhibit similar structures as the 7%w coated but appeared relatively smoother.It is worthy of note that the 0.5Zn—13Si3N4coated steel has the smoothest structure compare to all other coated samples as revealed by the optical microscope.This might be attributed to the increase in mass concentration of Si3N4nanoparticle, minimising the formation of flake-like crystalline pattern.It has been reported that,increasing mass concentration of particles increase the coating thickness leading to less coarse surfaces[45].However,it is worth mentioning that the process parameters can influence the surface texture which may alter the performance and life span of the coating[44].
SEM micrographs Zn—Si3N4coated mild steel at 7%w and 13%w are shown in Figs.4 and 5 SEM images of the samples show that the pitting evolution at the interface was apparently invisible.Good surface topography,morphological and coverage were observed.The well dispersed quality of the coated surface hindered the ingression of the corrosive ion into the metallic interface.The surface of the sample shown in Figs.4(b)and 5(c) appeared rougher,which is in unison with the images of Optical micrograph.
The SEM images,in accordance with the work of author Ref.[46]reveal that the zinc interface clearly displayed nodular structures on the coating network with redefined morphology making it look alluring.Expectedly,the path of nucleation started from the zinc metal as load carrier;the dissemination of the particulates involves the nucleation domains and therefore improving the formed nanocomposites.It is also worthy of note that the SEM micrographs reveal images with low porosity.
3.2. Electrochemical test result of Zn—Si3N4 coated and uncoated mild steel
Potentiodynamic polarization test performed on Zn—Si3N4coated steel confirmed its ability to resist corrosion in 3.65%NaCl solution.The result of the corrosion experiment obtained from the extrapolation of Tafel curve shown in Fig.6 confirmed an improved corrosion resistance with a reduction in corrosion rate from 9.7425 mm/year to 0.10847 mm/year as indicated in Table 4,maximum polarization resistance of 1555.3 Ω and a very low current density of 9.33×10-6A/cm2.This shows that the coating blocked the active sites of the modified steel impeding the exchange of current.The coatings were able to act as barrier,therefore minimising the cathodic evolution and anodic metal dissolution reactions of the mild steel[47,48].The presence of Si3N4nanoparticles decreases the concentration of chloride ion,resulting in a lower current density in the charge transfer controlled and mixed potential region. At the mixed potential region, the value of corrosion potential depends on the rate of both the cathodic as well as anodic reaction.The rate of the charge transfer reaction at the interface depends not only on the applied potential but also on the concentration of reacting species prevailing at the electrode surface[49].
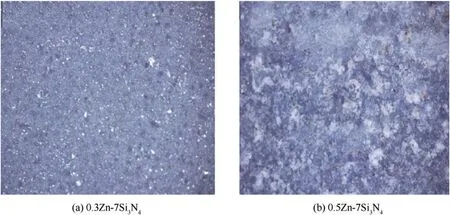
Fig.2.Optical micrographs of(a)0.3 Zn—7Si3N4 coated mild steel and(b)0.5 Zn—7Si3N4 coated mild steel.
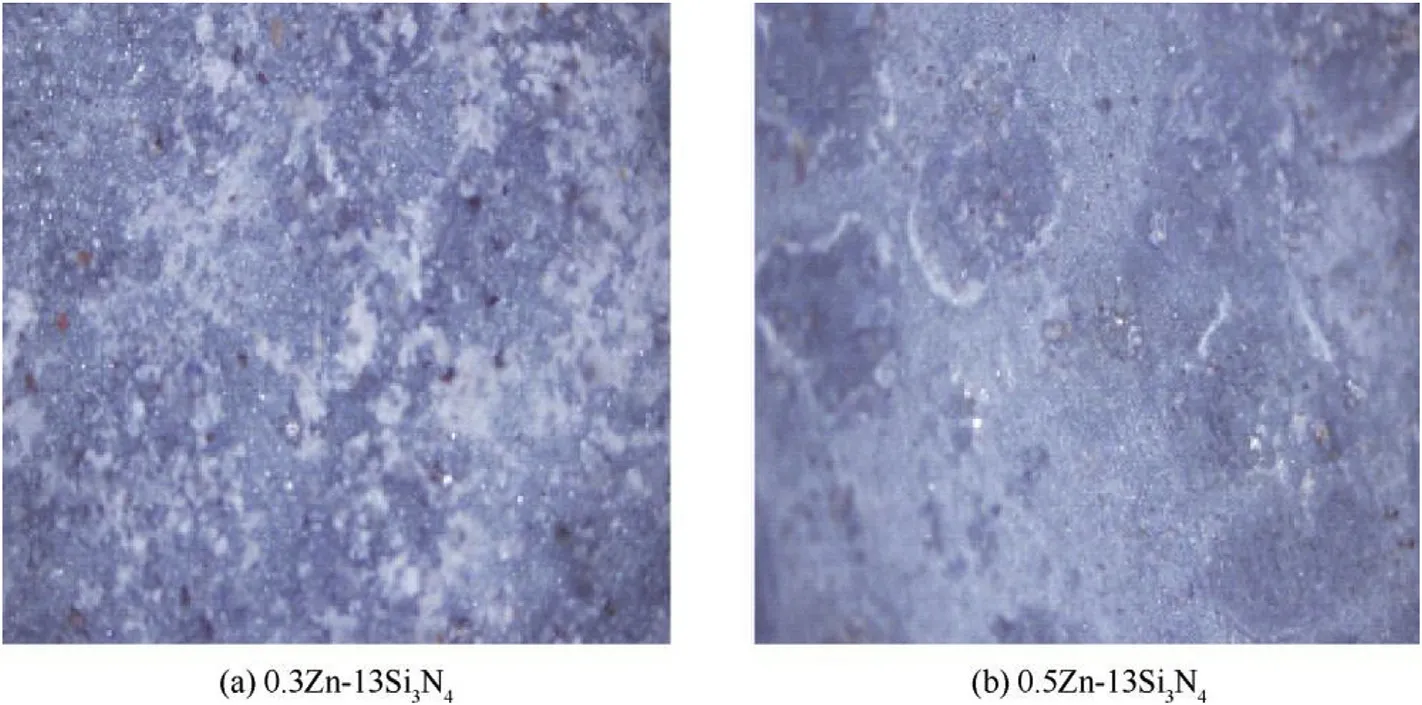
Fig.3.Optical micrographs of(c)0.3 Zn—13Si3N4 coated mild steel and(d)0.5Zn—13Si3N4 coated mild steel.
Fig.4.SEM micrographs of(a)0.3 Zn—7Si3N4 coated mild steel and(b)0.5Zn—7Si3N4 coated mild steel.
From the results obtained,all the corrosion parameters favoured 0.5Zn—13Si3N4coated sample.This may be as a result of the increased concentration of Si3N4leading to the reduction of the adsorption of chloride ion in aggressive environment[50,51].It can also be traced to the nature and adhesiveness of the passive film produced by 0.5Zn—13Si3N4on the surface of the coated steel or chemical stability of the samples[52].The negative shift in the Ecorrconfirms the cathodic protective nature of the coating[53,54].Generally,Zn—Si3N4was found to have reduced the current density of all the coated samples.The reduction in current densities could be attributed to blockage of the active sites of the steel by the Zn—Si3N4particles.
Fig.7 shows the OCP versus time curves for the uncoated and Zn—Si3N4coated steel in 3.65% NaCl static solution. Carefully examining the OCP vs.time curves,it can be seen that the presences Zn—Si3N4shifted the potential of the steady-state to more negative values.The notable shift is attributed to the predominant cathodic effect of Si3N4on the mild steel indicating the cathodic reaction is relatively more affected than the anodic reaction [1,51]. It is important to note that the OCP vs.time curve for the coated and uncoated samples were near straight line indicating that steady state potential was attained[54].
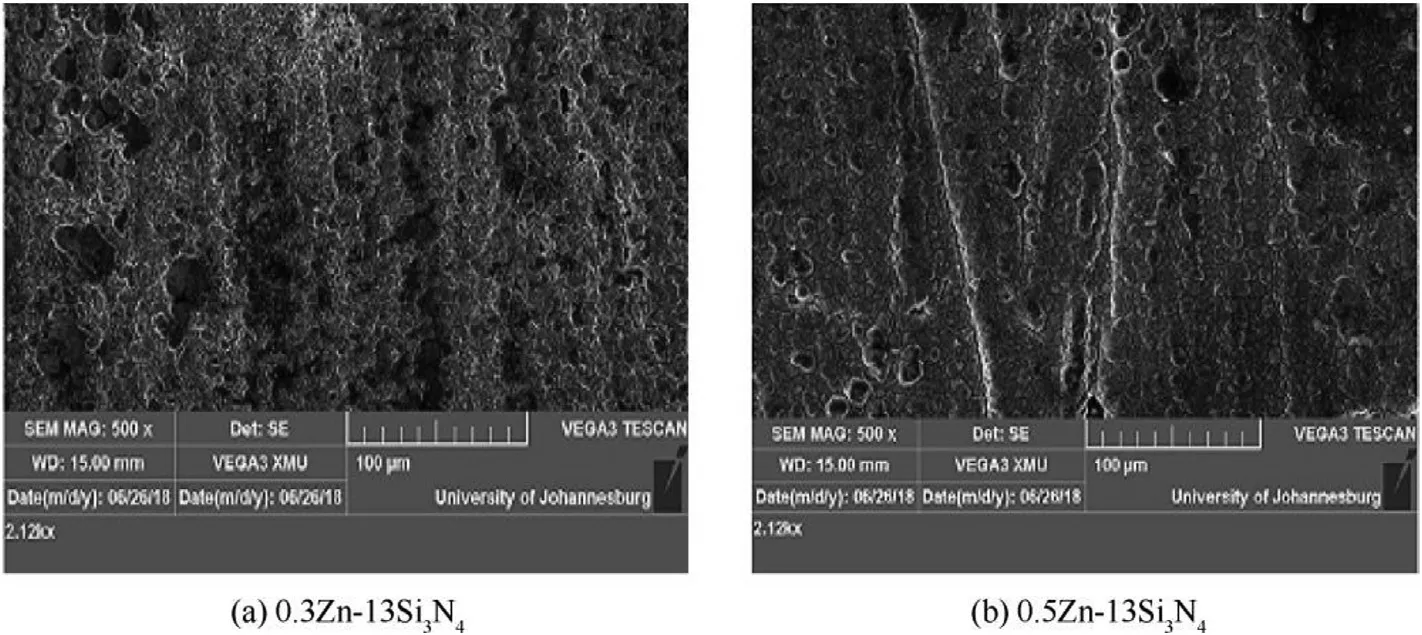
Fig.5.SEM micrographs(c)0.3 Zn—13Si3N4 coated mild steel and(d)0.5Zn—13Si3N4 coated mild steel.
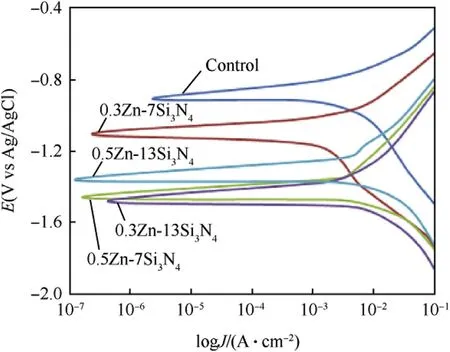
Fig.6.Potentiodynamic Polarization curves for uncoated and Zn—Si3N4 Composite coated mild steel in 3.65%NaCl medium.
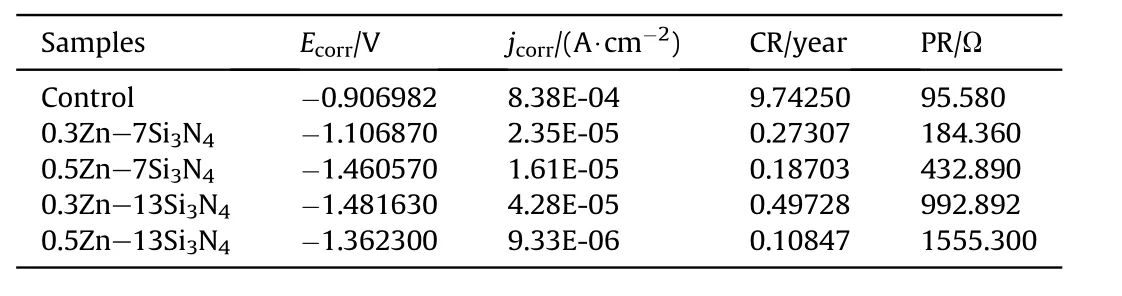
Table 4 Potentiodynamic Polarization parameters for uncoated and Zn—Si3N4 Composite coated mild steel in 3.65%NaCl medium.
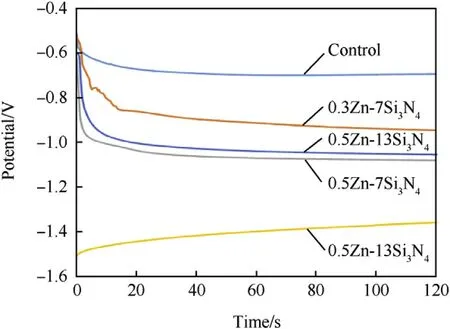
Fig.7.Evolution of open circuit potential(OCP)vs.exposure time for the uncoated and Zn—Si3N4 Composite coated mild steel in 3.65%NaCl medium.
3.3. Coating efficiency
The percentage coating efficiency(%CE)was calculated using equation(9)[55—59].

jcorr,the corrosion current densities for the coated samples and jocorr,corrosion current density for the uncoated sample.It can be seen in Fig.8 that 0.5Zn—13Si3N4coated steel exhibits the highest coating efficiency of 98.9%.This might be due to the higher concentration of Si3N4and the value of cell voltage,thereby influencing the increase in deposition of the particles leading to higher value of thickness,175.7 μm and effective covering of the steel,preventing the ingression of chloride ion[45,51].Generally the coating efficiency of Zn—Si3N4was found to be on a high side with the minimum value of 94.9%.
3.4. Microhardness analysis of Zn—Si3N4 coated and uncoated samples
The microhardness results obtained for the Zn—Si3N4coated and uncoated steel were displayed in the chart in Fig.9.These values were gotten using the Brinell hardness test technique.This is in accordance to ASTM A-370[60].Upon comparison,0.5Zn—15-Si3N4has the highest hardness value of 263.3 BHN.This represents 90.9%increment in the hardness compared to the unmodified mild steel.
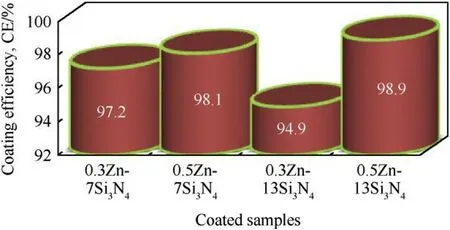
Fig.8.Coating efficiency of Zn—Si3N4.
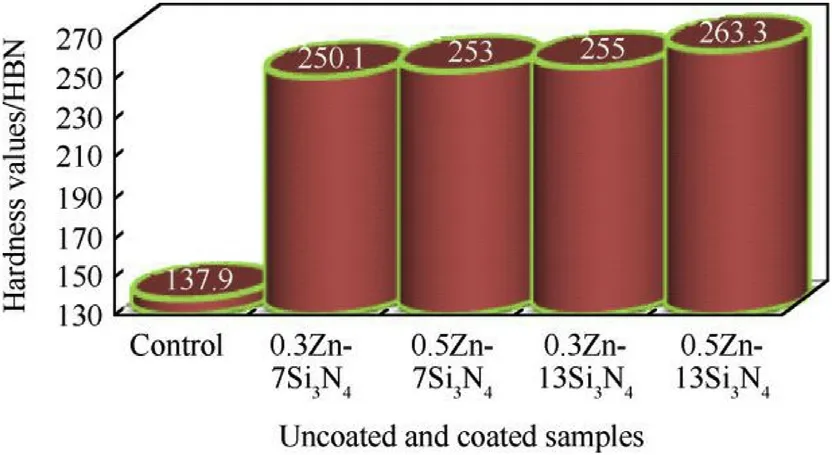
Fig.9.Brinell hardness value for Zn—Si3N4 coated and uncoated samples.
In general,the hardness value for all the coated samples with varying additives showed an increase. The improvement in microhardness could be attributed to the formation of adhesive mechanism of the composite coating on the substrate sample,strain energy imposed by the particles in the matrix on the periphery of the composite coated steel and operating factors such as bath constituents and other processing parameters[61—63].
4. Conclusions
(1)Zn—Si3N4nanocomposite coatings have been successfully produced.
(2)The modified mild steel displayed strong strengthening behaviour with maximum hardness value of 263.3 HBN which amounts to 90.9%increment in hardness compared to the unmodified steel
(3)0.5Zn—13Si3N4proved to be the optimum bath loading for improved corrosion resistance,hardness and better surface structure.
(4)The result of the corrosion experiment on 0.5Zn—13Si3N4coated mild steel confirmed an improved corrosion resistance with a reduction in corrosion rate from 9.7425 mm/year to 0.10847 mm/year,coating efficiency of 98.9%,polarization resistance of 1555.3 Ω and a very low current density of 9.33×106A/cm2.
(5)The negative shift in the Ecorrreveals the predominant cathodic protective nature of the coating.
Acknowledgments
This is to acknowledge Department of Mechanical Engineering,University of Ibadan for the P.hD opportunity.Prof.Fayomi Ojo Isaac Sunday of Surface Engineering Research Centre,Covenant University Ota,Nigeria is deeply appreciated for the provision of laboratory facilities and technical advice.
杂志排行
Defence Technology的其它文章
- Modeling on the shock wave in spheres hypervelocity impact on flat plates
- Insensitive high explosives:IV.Nitroguanidine—Initiation&detonation
- Effect of energy content of the nitraminic plastic bonded explosives on their performance and sensitivity characteristics
- Effect of wave shaper on reactive materials jet formation and its penetration performance
- A comparative study for the impact performance of shaped charge JET on UHPC targets
- The role of crystal lattice free volume in nitramine detonation
