CARMA3/NF-κB signaling contributes to tumorigenesis of hepatocellular carcinoma and is inhibited by sodium aescinate
2019-10-28HuiHouWeiXiangLiXiaoCuiDaChenZhouBinZhangXiaoPingGeng
Hui Hou, Wei-Xiang Li, Xiao Cui, Da-Chen Zhou, Bin Zhang, Xiao-Ping Geng
Abstract BACKGROUND Primary hepatocellular carcinoma (HCC) is a very malignant tumor in the world.CARMA3 plays an oncogenic role in the pathogenesis of various tumors.However, the function of CARMA3 in HCC has not been fully clarified.AIM To study the biological function of CAEMA3 in HCC.METHODS Tissue microarray slides including tissues form 100 HCC patients were applied to access the expression of CARMA3 in HCC and its clinical relevance. Knockdown and overexpression of CARMA3 were conducted with plasmid transfection.MTT, colony formation, and apoptosis assays were performed to check the biological activity of cells.RESULTS Higher expression of CARMA3 in HCC was relevant to poor prognostic survival(P < 0.05). Down-regulation of CARMA3 inhibited proliferation and colony formation and induced apoptosis in HCC cell lines, while increasing its expression promoted tumorigenesis. We also found that sodium aescinate (SA), a natural herb extract, exerted anti-proliferation effects in HCC cells by suppressing the CARMA3/nuclear factor kappa-B (NF-κB) pathway.CONCLUSION Overexpression of CARMA3 in HCC tissues correlates with a poor prognosis in HCC patients. CARMA3 acts pro-tumorigenic effects partly through activation of CARMA3/NF-κB. SA inhibits HCC growth by targeting CARMA3/NF-κB.
Key words: CARMA3; Nuclear factor kappa-B; Sodium aescinate; Hepatocellular carcinoma; Tumorigenesis; Liver cancer
INTRODUCTION
Hepatocellular carcinoma (HCC) ranked the sixth in the most common malignant tumors and is the most frequent primary liver cancer[1], with 841080 new cases and 781631 death in 2018[2]. Although the molecular mechanisms research and treatment for HCC have progressively advanced in the past, it is still the third leading cause of cancer related death[3]. CARMA3 is a member of the membrane-associated guanylate kinase (MAGUK) superfamily which acted as scaffold proteins. There are other two members in MAGUK family, CARMA1 and CARMA2[4]. All three proteins contained caspase recruitment domain, were known CARD11, CARD14 and CARD10,respectively[5]. CARMA3 is required for GPCR- and PKC-induced nuclear factor kappa-B (NF-κB) activation by activating BCL10 and MALT1, acted as a protumorigenic role in different types of solid malignant tumor[6]. NF-κB signal pathway is commonly activated and exerts critical roles in the progression of HCC[7]. Here, we hypothesized CARMA3 might play an oncogenic role in HCC by activating NF-κB,and explored the relationships between CARMA3 expression and clinicopathological characters. Furthermore, we found Sodium Aescinate (SA), a natural plant extracts derived from the seeds of the horse chestnut tree (also known as aesculus hippocastanum) exerted anti-tumor effects in possibly by inactivating CARMA3/NFκB signaling.
MATERIAL AND METHODS
Patients and specimens
Tumor tissues and paired adjacent liver tissues were obtained from 100 HCC patients who had undergone liver resection at the First Affiliated Hospital of Anhui Medical University and the Second Hospital of Anhui Medical University between 2000 and 2015 (100 pairs of HCC tumor tissues and adjacent liver tissues, randomly selected from an HCC tissue bank of 527 patients). All included patients were confirmed by pathological diagnosis with primary HCC. Those who had received pre-operative anti-tumor therapy or extrahepatic metastasis confirmed by computed tomography,magnetic resonance imaging, or positron emission tomography were excluded.Tumor differentiation and grade were classified by the Edmondson grading system.Follow-ups were performed every three months in first year, then every 6 mo postoperatively. The deadline of this research was June 30, 2017. Serum alphafetoprotein, liver function test, and liver ultrasonography results were recorded routinely. Survival time was measured from the date of first operation to the death date of the patient or the last follow-up. Disease free survival (DFS) time was defined the period between the operation day and the date when recurrence was confirmed.Written consent was obtained from all enrolled patients. This study was approved by the ethics committees of The First Affiliated Hospital and the Second Hospital of Anhui Medical University.
Tissue microarray (TMA) analysis
Surgically excised tumor specimens were fixed with 10% neutral formalin and embedded in paraffin. TMAs were made (Ai-We-Er Company, Wuhan, China). Each TMA block carried 25 pair of tissues, and the sections were 4 μm thick.Immunostaining was performed using the polink-2 plus polymer HRP detection system (PV-9001, ZSBIO Company, Beijing, China). The sections were deparaffinized in xylene, rehydrated with gradient alcohol, and then boiled in 0.01 M citrate buffer(pH 6.0) for 3 min in a pressure cooker. Hydrogen peroxide (0.3%) was applied to block endogenous peroxide activity, and the sections were incubated with normal goat serum solution (10% in TBST) for 1 h at room temperature to reduce nonspecific binding. Primary antibody (CARMA3, 1:50 dilution, Abcam, USA; NF-κB, 1:200 dilution, CST, USA) was incubated at 4 °C overnight. The secondary antibody(SPN9001, ZSBIO Company, Beijing, China) was incubated at room temperature for 1 h. Two independent blinded investigators examined all tissue slides randomly.
Cell lines and cell culture
Human hepatocellular carcinoma cell lines HepG2 and Hep3B and normal liver cell line HL-02 were obtained from The Cell Bank of Chinese Academy of Sciences. All the cells were cultured in DMEM medium containing 10% fetal bovine serum (Gibco,Gaithersburg, MD, USA) in a humidified incubator with 5% CO2at 37 °C, and the medium was renewed every three days.
Construction of CARMA3 overexpression and knockdown cells
pEGFP-P2A-hCARMA3 and pGpU6-GFP plasmids (GenePharma, Shanghai, China)were transfected into HepG2 and Hep3B cells with Lipo6000TM transfection reagent(Beyotime, Shanghai, China) according to the manufacturer's instructions. Empty vectors ware used as negative controls.
MTT assay
Cells in the logarithmic growth phase were seeded into 96-well culture plates at a density of 3 × 103cells/well and then treated with SA at concentrations of 10, 20, 30,and 40 μmol/L for 24, 48, and 72 h separately. The control group was treated with the same amount solvent (DMSO) as the treatment groups. Afterwards, 10 μL of MTT solution (2.5 mg/mL) was added to each well to incubate the cells at 37 °C for 4 h.DMSO (150 μL) was added to dissolve the resultant formazan crystals each well. The OD value was detected at 570 nm using a KHB ST-360 microplate reader (KHB,Shanghai, China). Cell growth inhibition rate was calculated using the following formula: 1 - ODexperiment/ODcontrol.
Cell apoptosis assay
Apoptosis was monitored using the Annexin V/propidium iodide (PI) method, and its rate was detected by flow cytometry with an Annexin V-FITC apoptosis detection kit (BD Biosciences, New York, United States). The cells were seeded in 6-well plates and treated with SA (40 μmol/L) for 48 h, and then harvested by trypsinization. After washing twice with cold phosphate-buffered saline, the cells were re-suspended in binding buffer at a concentration of 1 × 106cells/mL. After transferring 100 μL of the solution (1 × 105cells/mL) into a 5-mL culture tube, 5 μL of FITC Annexin V and 5 μL of PI were added for reaction 15 min in the dark, which was followed by adding 400 μL of binding buffer into the tube and analyzing by flow cytometry.
Western blot analysis
The cells were lysed in RIPA buffer in an ice bath for 30 min, and centrifuged at 15000 rpm for 20 min at 4 °C. The supernatant was stored at -80 °C until analyses. The protein concentration was measured using the BCA kit (Beyotime, China). Equal amounts of proteins were loaded onto a 10% SDS-polyacrylamide gel for electrophoresis and transferred by electroblotting to a polyvinylidene difluoride membrane (Millipore, Boston, MA, United States). The membrane was blocked with 5% BSA for 1 h at room temperature. Primary antibodies against CARMA3 (Abcam,Cambridge, MA, United States) at 1:100 dilution or NF-κB (CST, Danvers, MA, USA)at 1:1000 dilution was incubated at 4 °C overnight. The secondary antibody (ZB-2301,ZSBIO Company, Beijing, China) was added and incubated at room temperature for 1 h. The signal intensity of each band on the membrane was measured with Odyssey(LI-COR) ImageJ software.
Cell immunofluorescence staining
Ten thousand cells were seeded on the cover slides in 12-well plates. Next day, the cells were fixed (10 min) with 4% formaldehyde and then permeabilized and blocked with 0.1% TBS-Triton plus 1% normal goat serum for 1 h at room temperature.Primary antibodies were incubated overnight at 4 °C. The secondary antibody CY3(Beyotime, Shanghai, China) was used at a dilution of 1:200 and incubated for 1 h at room temperature. DAPI was used to stain the cell nuclei for 2 min.
Colony formation assay
HCC cells were seeded into 6-well plates at a density of 600-1000 cells/well and treated with 40 μmol/L SA. Forty-eight hours later, the culture medium was renewed and regularly cultured for 1-2 wk until visible colonies appeared. After being fixed with methanol for 15 min, the cells were stained with crystal violet for 20 min before washing with tap water and air-drying. The colony formation rate was calculated with the following formula: Colony formation efficiency = (number of colonies/number of cells inoculated) × 100%. All the experiments were repeated three times independently.
Statistical analysis
Baseline variables of patients that were considered clinically relevant with the oncological outcomes were carefully chosen for inclusion and were analyzed by univariate analysis (Mann-Whitney U for continuous variable or Chi-square test for categorical variables). The survival and DFS analyses were performed by the Kaplan-Meier method and log-rank Cox test was used for test. Statistical analyses were performed with SPSS software, version 19.0 (IBM, Armonk, NY, United States).P < 0.05 was set as the significant difference.
RESULTS
Increased CARMA3 expression is found in human HCC tissue
The clinical characteristics of patients are shown in Table 1. All patients had undergone R0 resection confirmed by pathological diagnosis. The immunohistochemistry staining of TMA showed that CARMA3 was mainly located in the cytoplasm of tumor cells. CARMA3 expression was positively correlated with NF-κB expression in tissues (P < 0.05, Figure 1D). CARMA3 expression was higher in HCC tissues than in adjacent liver tissues from same patients and associated with tumor differentiation (Figure 2), TNM stage, and the diameter of tumor (> 5 cm). No significant difference was found between CARMA3 expression and gender, age, HBV,AFP level, Child-Pugh class, liver cirrhosis, tumor number, or microvascular invasion.
CARMA3 predicts a poor prognosis in HCC patients
Among 100 enrolled patients, intact follow-up records were kept in 93 patients. Based on the levels of CARMA3 expression, patients were divided into a low CARMA3 expression group and high CARMA3 expression group. Patients with high CARMA3 expression had a significantly shorter overall survival period (P < 0.05, Figure 1A and B) and lower disease-free survival rate (P < 0.05, Figure 1C)
CARMA3 promotes HCC cell growth and colony formation and inhibits apoptosis by targeting NF-κB
Regulation of CARMA3 expression was performed in HepG2 and Hep3B cells (Figure 3A). Reducing CARMA3 expression down-regulated NF-κB expression, inhibited proliferation and colony formation capacity, and induced an increase in early apoptosis of HCC cells. On the contrary, up-regulating CARMA3 expression increased NF-κB expression, promoted HCC cell growth and colony formation, and blocked cells to enter into the early apoptosis process (P < 0.05, Figure 3B-D).
Sodium aescinate counteracts CARMA3 induced pro-tumorigenic effects by targeting CARMA3/NF-κB
Here, we found that SA significantly inhibited cell proliferation and colony formation capacity and enhanced early apoptosis in a dose dependent manner (IC50: 46 μM, P <0.05), but no difference was observed in hepatic cells, HL-02 (Figure 4A). An interesting phenomenon showed that SA reduced CARMA3 and NF-κB expression in HCC cells (Figure 5A and B). For further confirming its anti-tumor effects by targeting CARMA3, SA was employed to treat HCC cells in which CARMA3 expression was up-regulated. It reverted the effects of promoting proliferation and colony formation capacity and inhibition of apoptosis induced by CARMA3 (Figure 4B-D).
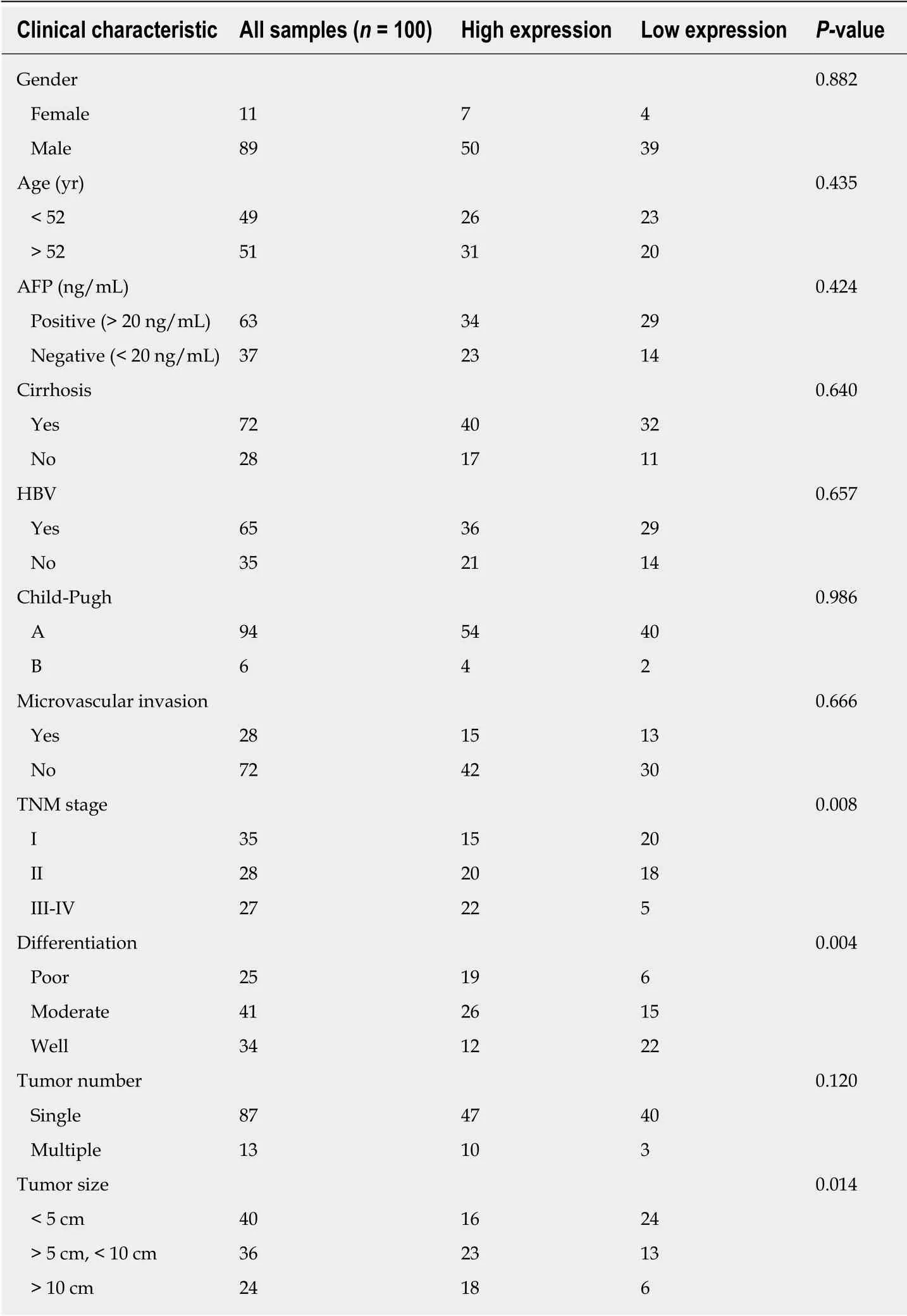
Table 1 Relationship between CARMA3 expression and clinical characteristics of hepatocellular carcinoma patients
DISCUSSION
CARMA3 functions as a scaffold protein that assembles multiple proteins involved in the activation of certain membrane signaling which contributes to carcinogenesis,inflammation, and immunity[8]. Despite its putative roles in oncogenesis in a variety of solid tumors, the effects of CARMA3 in HCC have not been clearly elucidated yet. In this study, we observed that CARMA3 expression was increased in HCC tissues in comparison with non-cancerous liver tissues. High CARMA3 expression in HCC was positively associated with some unfavorable clinicopathological characteristics:advanced TNM stage, poor tumor differentiation, and larger tumor size. This oncogenic function has been observed in certain types of malignant tumors[9].Furthermore, we also found that high CARMA3 expression positively correlated with NF-κB expression in HCC tissues, which predicted a poor prognosis in HCC patients by decreasing both overall survival time and DFS time. Through specific CARD to CARD homophilic interaction, CARMA3 promotes the activation of NF-κB signaling pathway in immunity cells[10]. Here, we hypothesized that CARMA3 might exert protumorigenic effects in HCC partly by increasing NF-κB expression. We increased and decreased endogenous CARMA3 expression in HCC cells using constructed plasmids in vitro. It was observed that increasing CARMA3 enhanced NF-κB expression in HCC cells and promoted cell proliferation and inhibited apoptosis, while decreasing endogenous CARMA3 expression suppressed NF-κB expression and cell proliferation but induced more apoptosis. These results suggest that CARMA3 exerts a protumorigenic role in the progression of HCC partly through the NF-kB signal pathway.
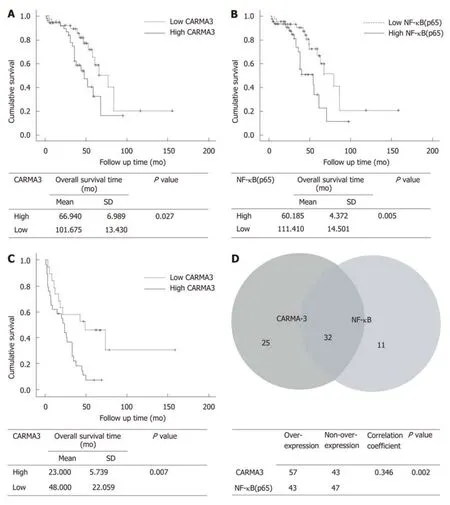
Figure 1 CARMA3 and nuclear factor kappa-B (p65) are correlated with clinical prognosis in hepatocellular carcinoma patients. A: High CARMA3 (n = 54)expression suggested shorter overall survival time (P = 0.027); B: High nuclear factor kappa-B (NF-κB) (p65) (n = 40) expression indicated shorter postoperative overall survival (P = 0.005); C: Hepatocellular carcinoma (HCC) patients with high CARMA3 expression had a shorter disease free survival time than those with low CARMA3 expression (P = 0.007); D: Correlation between CARMA3 and NF-κB expression in HCC tissue (P = 0.002). NF-κB: Nuclear factor kappa-B.
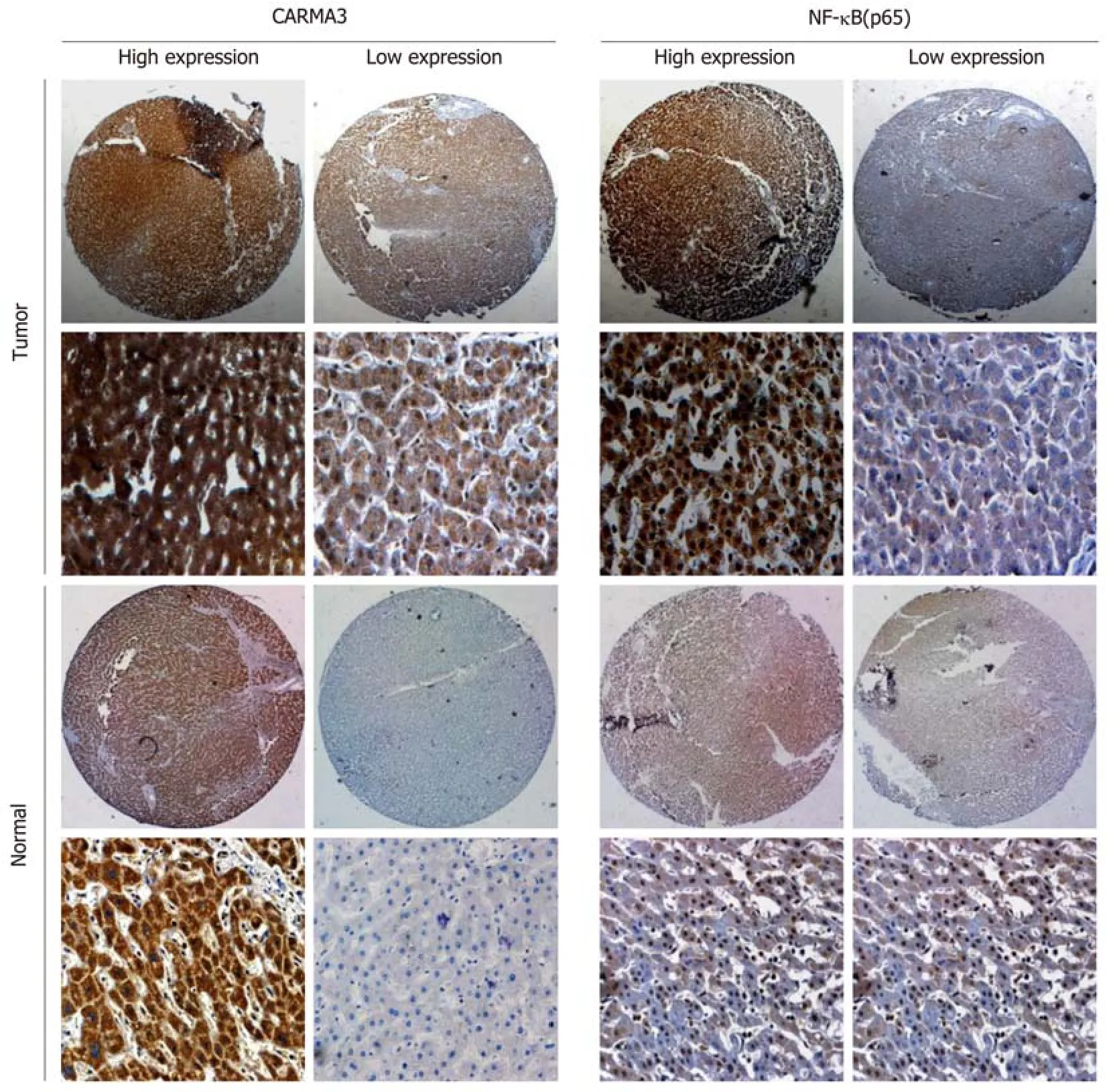
Figure 2 Expression of CARMA3 and nuclear factor kappa-B (p65) in hepatocellular carcinoma tumor tissues and the adjacent liver tissues. Magnification,×100. NF-κB: Nuclear factor kappa-B.
Due to the medical complexity of patients and heterogeneity of HCC, appropriate treatment for HCC remains a challenge. The potential use of CARMA3 as a therapeutic target for HCC has not been explored. In our laboratory, we observed that SA, a natural plant extract which has been widely administrated to alleviate inflammation reactions in trauma patients, inhibited HCC cell proliferation and induced apoptosis. The same SA concentration did not induce the significant growth arrest on hepatocytes. Interestingly, SA reduced CARMA3 and NF-κB expression in HCC and suppressed HCC cell growth. In previous research, SA exerted anti-tumor effects in various tumor cells via the NF-κB, JAK/STAT, and MAPK/iNOS pathways[11]. IKK complex is a core kinase which consists of IKKα, β, and γ subunits and mediates the phosphorylation of p65, which induces the activation of the NF-κB signaling pathway[12]. SA counteracts NF-κB activation by inhibiting activation of IKK through blocking ubiquitination of IKK-γ[13]. However, the mechanism by which SA blocks the ubiquitination of IKK-γ has not yet been fully clarified. In this research, we found that SA might inhibit NF-κB activation partly by mediating CARMA3.CARMA3 physically interacts with IKK-γ and causes IKK-γ polyubiquitination but has no effects on the other two subunits[14]. Deletion of CARMA3 impaired the activation of IKK even in the presence of an activator of NF-κB[15-17]. For further confirmation that SA exerts anti-tumor effects dependent on CARMA3/ NF-κB, SA was employed to treat HCC with high CARMA3 expression. SA inhibited HCC growth and reduced the expression of CARMA3 and NF-κB. These findings might introduce a new insight that SA inhibits HCC by decreasing CARMA3 expression,thus hindering the IKK-γ ubiquitination process, suppressing activity of the IKK complex, and inducing inactivation of NF-κB.
In conclusion, we found that CARMA3 plays an oncogenic role in the progression of HCC partly by activation of NF-κB. CARMA3 expression levels in HCC tissue positively correlate with a poor prognosis of HCC patients. Sodium aescinate effectively inhibits the growth of HCC cells partly through the CARMA3/NF-κB signaling pathway. These findings might provide a new potential therapeutic tactic for HCC.
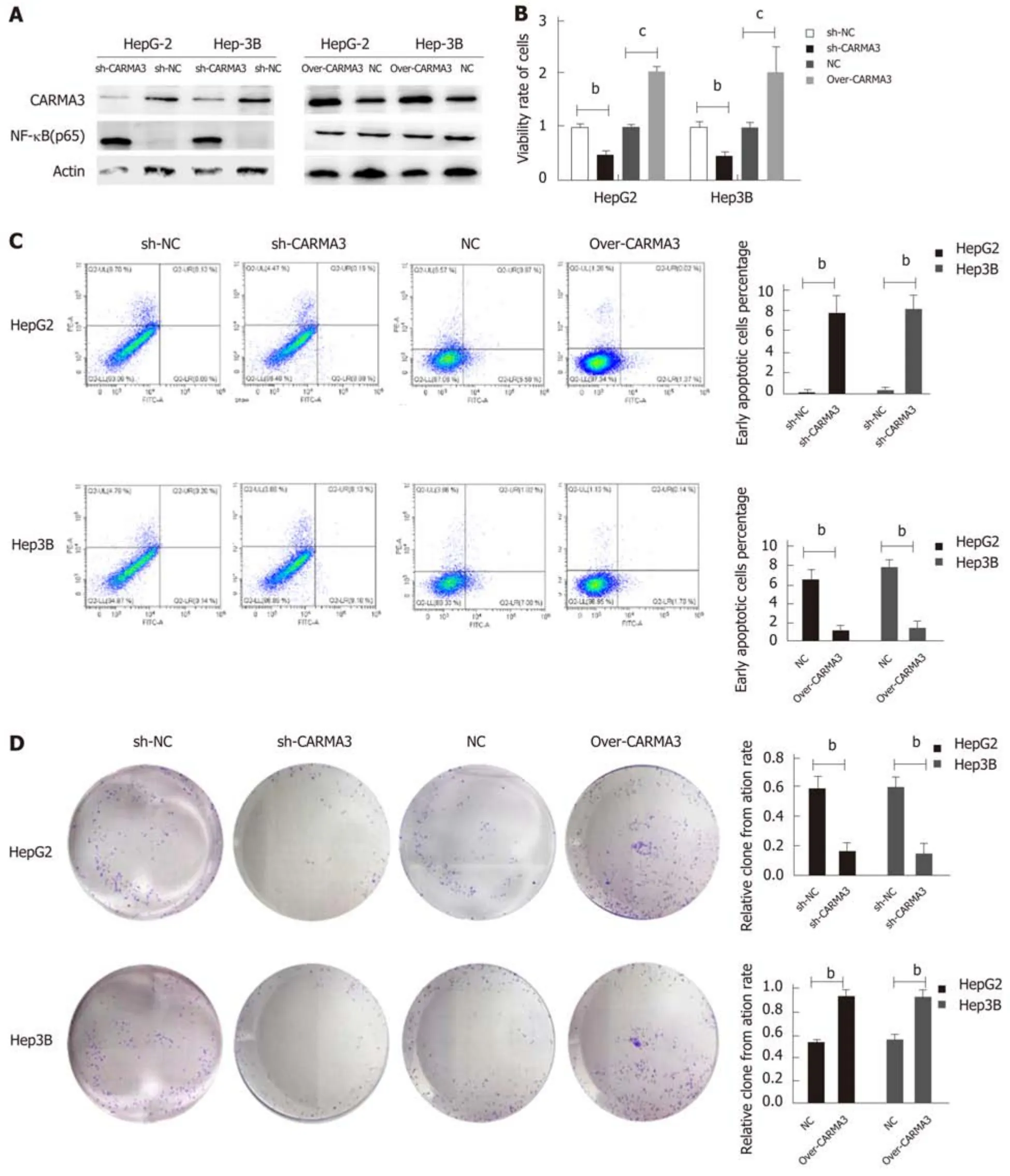
Figure 3 Regulation of CARMA3 expression has effects on capacities of proliferation, colony formation, and apoptosis in hepatocellular carcinoma cells.
ARTICLE HIGHLIGHTS
Research background
Hepatocellular carcinoma (HCC) is one of the deadliest malignant tumors in the world. The incidence rate of HCC continuously rises over the last decades. The therapeutic effects are still unsatisfied.
Research motivation
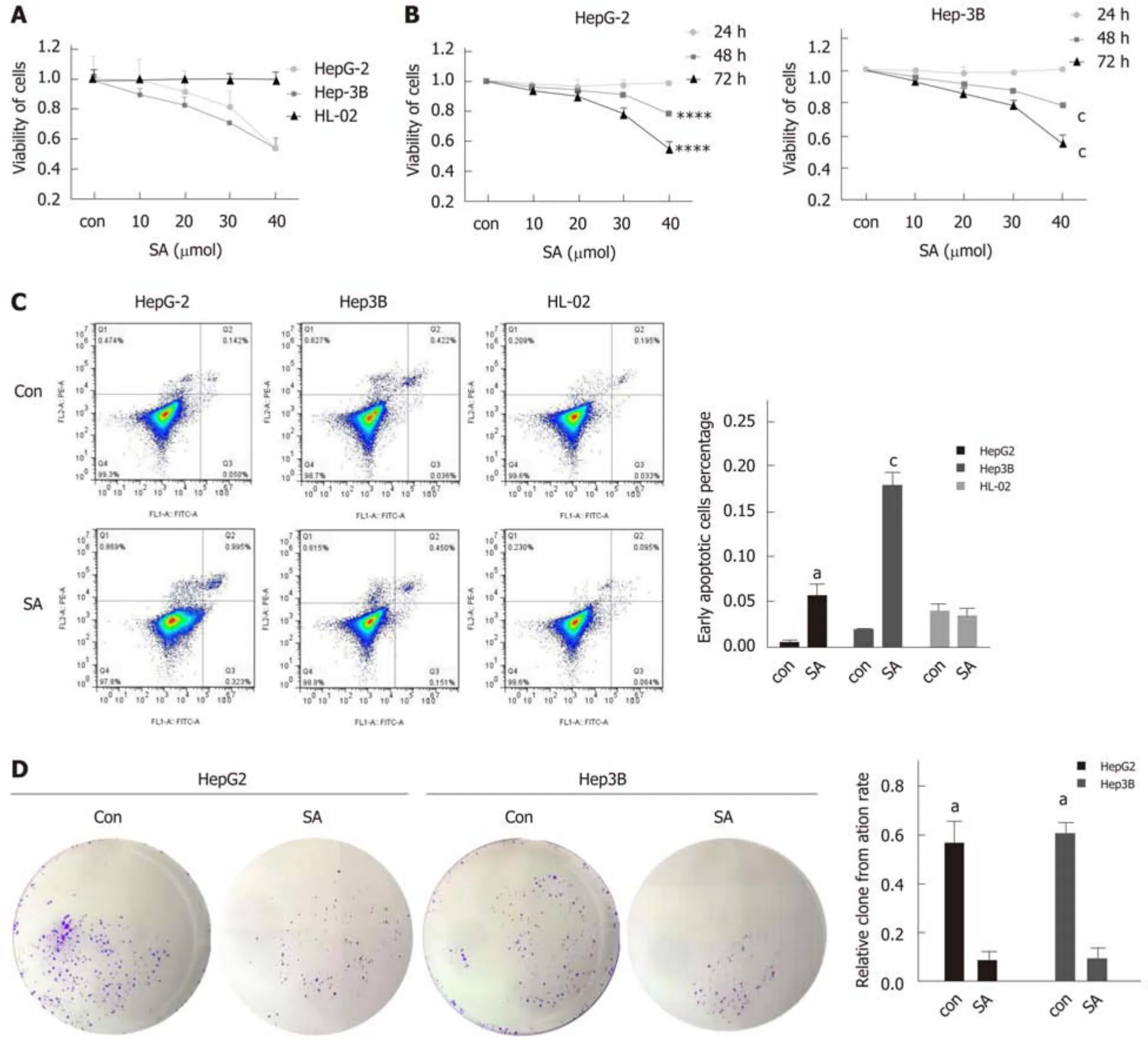
Figure 4 Sodium aescinate inhibits the proliferation and colony formation and induces early apoptosis in hepatocellular carcinoma cells. A: Sodium aescinate (SA) significantly inhibited the proliferation of hepatocellular carcinoma (HCC) cells but had no effects on liver cells; B: Cell viability of HCC cells treated at different doses of SA and for different durations; C: SA induced early apoptosis in HCC cells; D: SA suppressed colony formation capacity in HCC cells (aP < 0.05, cP< 0.001 vs control).
As an oncogenic factor, CARMA3 has been explored in various types of tumors. The role of CARMA3 in the tumorigenesis of HCC and its potential therapeutic application have not been fully identified.
Research objectives
To investigate the biological function of CARMA3 in the progression of HCC, and the potential therapeutic effects of sodium aescinate (SA) in HCC.
Research methods
TMA slides with paraffin-embedded HCC samples from 100 patients were employed in this study. Expression of CARMA3 in HCC tissues was detected by immunohistochemistry (IHC). The biological function of CARMA3 in HCC was investigated by increasing or decreasing endogenous CARMA3 expression in HepG2 and Hep3B cells using plasmid transfection in vitro. The proliferation and colony formation assays and apoptosis detection kits were used to investigate the role of CARMA3 and SA in HCC cells. Western blot and immunofluorescence assays were used to detect the expression of targeted proteins.
Research results
IHC analysis showed that CARMA3 was increased in HCC tissues compared with adjacent non-cancerous liver tissues. High CARMA3 expression in HCC predicted less overall survival time and disease-free survival time in HCC patients. Knockdownof CARMA3 expression inhibited proliferation and colony formation, and induced early apoptosis in HCC cells. Increasing endogenous CARMA3 expression in HCC cells promoted cell growth and suppressed apoptosis. SA inhibited the growth of HCC cells and decreased the expression of CARMA3 and its targeted protein nuclear factor kappa-B (NF-κB). No anti-proliferation or pro-apoptosis effect was observed in human hepatocytes treated with SA.
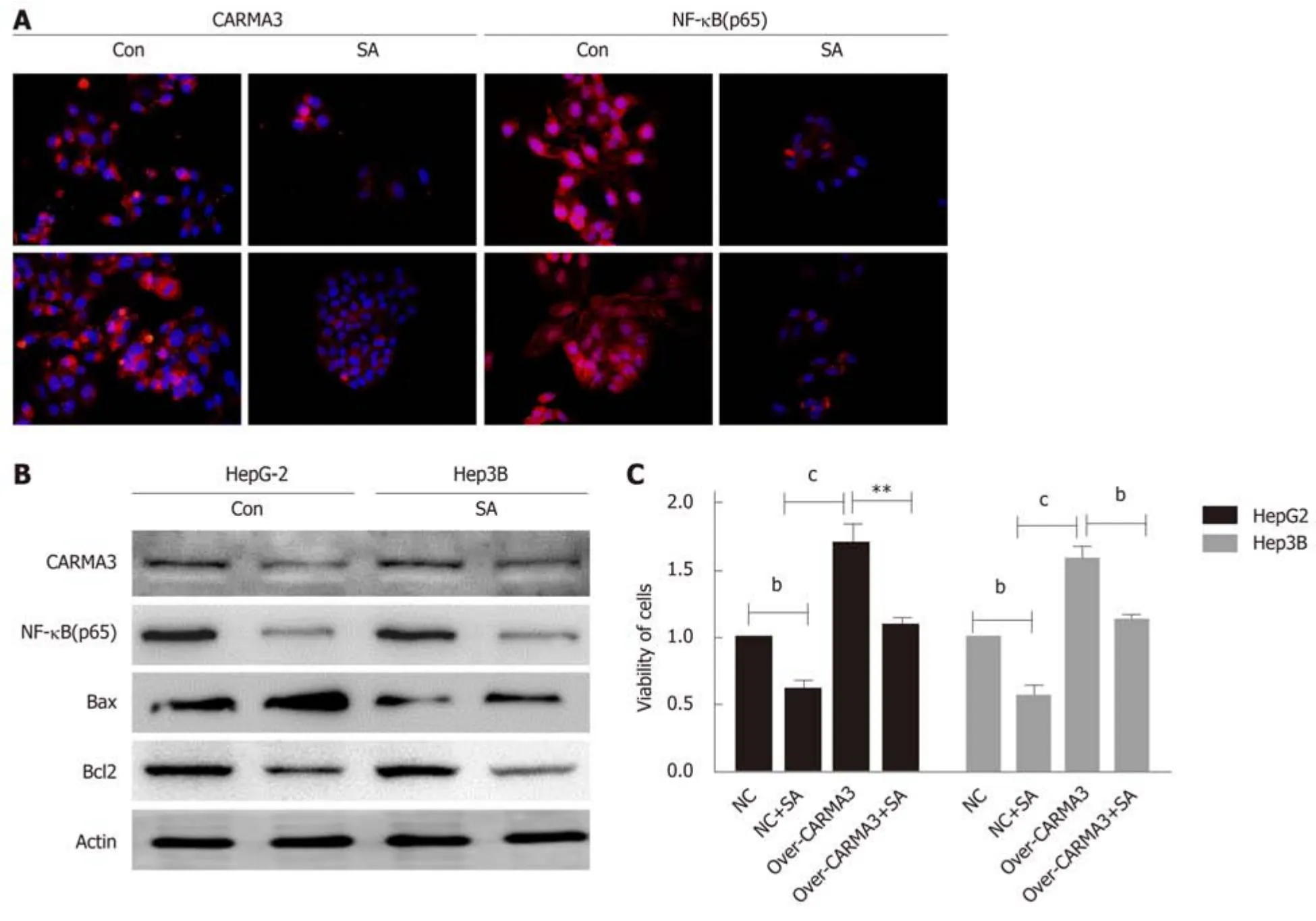
Figure 5 Sodium aescinate inhibits CARMA3/nuclear factor kappa-B signaling. A and B: Expression of CARMA3 and NF-kB (p65) detected in HCC cells after treatment with sodium aescinate (SA) by immunofluorescence (magnification, ×400); C: Cell viability of high CARMA3 expressing hepatocellular carcinoma (HCC)cells and control HCC cells either treated with SA or saline. (bP < 0.01, cP < 0.001 vs control).
Research conclusions
Expression of CARMA3 increases in HCC tissues and correlates with a poor prognosis in HCC patients. CARMA3 acts pro-tumorigenic effects by enhancing HCC growth and inhibiting apoptosis partly through activation of CARMA3/NF-κB. SA inhibits HCC growth by targeting CARMA3/NF-κB.
Research perspectives
Our work gave an insight of the mechanism of CARMA3 in the pathogenesis of HCC and explored its potential therapeutic application for HCC, which provide a promising target for HCC treatment.
ACKNOWLEDGEMENTS
We thank Dr. Xiong and Dr. Wang for helping us to deal with the experiment of FACS for the detection of apoptosis.
杂志排行
World Journal of Gastroenterology的其它文章
- Chinese guidelines on management of hepatic encephalopathy in cirrhosis
- Sexual health and fertility for individuals with inflammatory bowel disease
- High mobility group box-1 release from H2O2-injured hepatocytes due to sirt1 functional inhibition
- Zinc-α2-glycoprotein 1 attenuates non-alcoholic fatty liver disease by negatively regulating tumour necrosis factor-α
- Clostridium butyricum alleviates intestinal low-grade inflammation in TNBS-induced irritable bowel syndrome in mice by regulating functional status of lamina propria dendritic cells
- Laparoscopy-assisted pylorus-preserving gastrectomy for early gastric cancer: A retrospective study of longterm functional outcomes and quality of life
