Up-regulated Wnt1-inducible signaling pathway protein 1 correlates with poor prognosis and drug resistance by reducing DNA repair in gastric cancer
2019-10-28LiHuaZhangYanWangQianQianFanYanKuiLiuLongHaiLiXiaoWeiQiYongMaoDongHua
Li-Hua Zhang, Yan Wang, Qian-Qian Fan, Yan-Kui Liu, Long-Hai Li, Xiao-Wei Qi, Yong Mao, Dong Hua
Abstract BACKGROUND Wnt1-inducible signaling pathway protein 1 (WISP1) is upregulated in several types of human cancer, and has been implicated in cancer progression. However,its clinical implications in gastric cancer (GC) remain unclear.AIM To explore the expression pattern and clinical significance of WISP1 in GC.METHODS Public data portals, including Oncomine, The Cancer Genome Atlas database,Coexpedia, and Kaplan-Meier plotter, were analyzed for the expression and clinical significance of WISP1 mRNA levels in GC. One hundred and fifty patients who underwent surgery for GC between February 2010 and October 2012 at the Affiliated Hospital of Jiangnan University were selected for validation study. WISP1 levels were measured at both the mRNA and protein levels by RTqPCR, Western blot analysis, and immunohistochemistry (IHC). In addition, the in situ expression of WISP1 in the GC tissues was determined by IHC, and the patients were accordingly classified into high- and low-expression groups. The correlation of WISP1 expression status with patient prognosis was then determined by univariate and multivariate Cox regression analyses. WISP1 was knocked down by RNA interference. The 50% inhibitory concentration of oxaliplatin was detected by CellTiter-Blue assay.RESULTS WISP1 levels at both the mRNA and protein levels were remarkably upregulated in GC tissues compared to normal tissues. Moreover, IHC revealed that WISP1 expression was associated with T stage and chemotherapy outcome, but not with lymph node metastasis, age, gender, histological grade, or histological type. GC patients with high WISP1 expression showed a poor overall survival.Multivariate survival analysis indicated that WISP1 was an important prognostic factor for GC patients. Mechanistically, knock-down of WISP1 expression enhanced sensitivity to oxaliplatin by reducing DNA repair and enhancing DNA damage.CONCLUSION Significantly upregulated WISP1 expression is associated with cancer progression, chemotherapy outcome, and prognosis in GC. Mechanistically,knock-down of WISP1 expression enhances oxaliplatin sensitivity by reducing DNA repair and enhancing DNA damage. WISP1 may be a potential therapeutic target for GC treatment or a potential biomarker for diagnosis and prognosis.
Key words: Wnt1-inducible signaling pathway protein 1; Biomarker; Bioinformatics analysis; Chemotherapy outcome; Gastric cancer
INTRODUCTION
Gastric cancer (GC) is one of the most common malignancies[1]. In 2018, the incidence and mortality rates of GC ranked fifth and third, respectively, putting a heavy economic burden on the public health system worldwide, especially in East Asian countries[2]. Cancer incidence and mortality have been increasing in China and consequently a major public health problem in the country[3]. Because patients with early-stage GC are often asymptomatic, most patients are usually diagnosed at an advanced stage[4]. Available treatments are mostly unfavorable and inefficient[5]. Early diagnosis of GC and effective medical treatment are crucial for patient survival and therapeutic outcome[6]. Thus, there is an urgent need to better understand the mechanism of GC progression and development, and to identify promising biomarkers for diagnosis and prognosis.
Wnt1-inducible signaling pathway protein 1 (WISP1), highly homologous to CCN1 and CCN2, is a member of the CCN family of secreted, extracellular matrix (ECM)-associated signaling proteins (CCN intercellular signaling protein). This family of proteins regulate diverse cellular functions, including cell proliferation,differentiation, adhesion, migration, and survival. In recent studies, it was demonstrated that the expression of WISP1 in cancer promotes cell proliferation, and high WISP1 expression correlated with advanced tumors of the brain, breast, colon,and lung. WISP1 reduced p53-mediated apoptosis through activating Akt kinase, and inhibited TNF-induced cell death in cardiomyocytes[7]. In addition, recombinant WISP-1 enhances ECM deposition in human fibroblasts, suggesting that it might play a vital role in matrix remodeling in vivo[8]. In GC cells, WISP1 acts as an oncogene by promoting cell proliferation, migration, and invasion[9]. Although WISP1 has been demonstrated in cancer progression, its prognostic value in GC remains unclear.
In this study, we explored whether WISP1 can be a novel biomarker for prognosis or a therapeutic target in GC. First, public databases and websites, including Oncomine and Coexpedia, were used for predicting the expression of WISP1 in GC. In addition, Kaplan-Meier plotter datasets from The Cancer Genome Atlas (TCGA) were used for analyzing the association of overall survival between WISP1 and GC.Subsequently, WISP1 expression was evaluated at both the mRNA and protein levels in 20 freshly frozen GC tissues and 20 non-cancerous GC tissues. Furthermore,immunohistochemistry (IHC) was used to explore the relationship between WISP1 expression and clinicopathologic parameters, including overall survival. WISP1 was knocked down by RNA interference in MKN45 and AGS cell lines. The 50% inhibitory concentration (IC50) was detected by CellTiter-Blue (CTB) assay to evaluate the effect on oxaliplatin resistance.
MATERIALS AND METHODS
Patients and tissue samples
A total of 150 patients who underwent surgery for GC between February 2010 and October 2012 at the Affiliated Hospital of Jiangnan University (Wuxi, China) were selected for this study. Those patients who postoperatively received oxaliplatin-based or cisplatin-based first-line systematic chemotherapy were enrolled in the present study. Tumor assessment was performed after every two cycles of chemotherapy according to the Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1)criteria, and the assessment was classified as complete response (CR), partial response(PR), stable disease (SD), and progressive disease (PD). This study was approved by the Institutional Research Ethics Committee of Affiliated Hospital of Jiangnan University (Wuxi, China). None of the patients selected received chemotherapy or radiotherapy prior to surgery. According to the American Joint Committee on Cancer,the tumor stage classification was determined by three pathologists who were blinded to the data. The tissues dissected from patients were fixed in formalin immediately after harvesting, and embedded in paraffin for further research. Fresh tissue samples obtained were dissected and immediately stored in liquid nitrogen for PCR and Western blot analysis. All participating clinical doctors and patients provided written informed consent prior to the start of the study.
Bioinformatics analysis
The mRNA levels of WISP1 in GC and normal gastric tissues were mined through Oncomine database (www.oncomine.org) and Coexpedia (www.coexpedia.org)[10].The prognostic value of WISP1 in gastric adenocarcinoma was analyzed through Kaplan-Meier Plotter (kmplot.com/analysis/)[11].
RNA isolation and RT-qPCR
Total RNA was extracted from frozen tissue samples using Trizol reagent (Invitrogen,Carlsbad, CA, United States) according to the manufacturer's guidelines, and reverse transcription was performed using the PrimeScript RT-PCR kit (Takara, Japan)[12]. RTqPCR was carried out on an ABI 7500 RealTime PCR System (Applied Biosystems,United States) using SYBR Green Master Mix (Takara, Japan), and normalized to levels of β-actin. The primers used in this study are: Forward, 5'-GAA GCAGTCAGCCCTTATG-3' and reverse, 5'-CTTGGGTGTAGTCCAGAAC-3' for WISP1; and forward, 5'-CCTGTGGCATCCACGAAACT-3' and reverse, 5'-GAA GCATTTGCG GTGGACGAT-3' for β-actin. All reactions were performed at least in triplicate. The 2-ΔΔCtmethod was used to quantify the relative expression levels of WISP1.
Western blot analysis
Total protein was extracted from GC and paracancerous tissues using RIPA lysis buffer (Pierce, Thermo Scientific, Cramlington, United Kingdom)[13]. Protein concentration was determined with an enhanced bicinchoninic acid assay kit (CWBio,Beijing, China). A total of 40 mg of protein was separated by sodium dodecyl sulfatepolyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride membranes (CWBio, Beijing, China). After blocking with 5% non-fat milk for 1 h at room temperature (RT), the membranes were incubated overnight at 4 °C with primary antibodies directed to WISP1 (Abcam, ab178547), XRCC1 (Abcam, ab9147),γH2AX (Abcam, ab2893), and β-actin (Abcam, ab8226). After washing three times with TBST (Tris-buffered saline with Tween 60), the membranes were incubated with horseradish peroxidase-conjugated secondary antibody at 1:5000 dilution for 1.5 h at RT. Protein bands were visualized using an enhanced chemiluminescence system, and exposure of the membranes to X-ray films (Bio-Rad, Hercules, CA, United States).Densitometric analysis was performed using Image Pro-Plus software (Media Cybernetics, United States). Relative protein expression levels were normalized to βactin.
Immunohistochemistry
Cancerous tissues and adjacent normal tissues were collected from GC patients and subjected to formalin fixation, dehydration, and paraffin embedding. Tissue sections were cut at 5-μm thickness and mounted on glass slides. According to the manufacturer's protocol, IHC staining was performed. In brief, antigen retrieval was performed using a microwave, then the slides were incubated with a primary antibody directed against WISP1 (dilution 1:200, ab178547, Abcam, United States) at 4°C overnight. Sections were washed in TBST three times for 5 min, and incubated with a rabbit secondary antibody at RT for 1 h. Staining was performed using liquid DAB (3,3′-diaminobenzidine) as the substrate. Three pathologists scored the staining intensity and positive ratio of WISP1 staining in a blinded fashion. By analyzing the percentage of positively stained cells, the sections were graded by five levels: 0 (≤5%),1 (6%-25%), 2 (26%-50%), 3 (51%-75%), and 4 (>76%). By analyzing the staining intensity, it was graded as negative (0), weak (1), moderate (2), and strong staining (3).A total score, ranging from 0-12, was generated for each case by multiplying the staining intensity score by the score of the extent of the positive cells[14]. The total score of 0-1 was classified as “-”, 2-3 as “+”, 4-6 as “++”, and 6-12 as “+++”[15]. Subsequently,WISP1 expression was divided into two categories: High (scores 4-12) and low (scores 0-3).
RNA interference
Chemically synthesized WISP1 siRNAs (siRNA-1 and siRNA-2) and matched scramble control siRNAs were purchased from RiboBio Company (Guangzhou,China). Their corresponding sequences are: NM_080838.3 (628-646),GGACATCCATACACTCATT and NM_080838.3 (885-903), GGAA TCCCAATGACATCTT. The siRNAs were transiently transfected into MKN45 and AGS cells by using Lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA, United States) according to the manufacturer's instructions. Western blot and qRT-PCR were used to illustrate the protein and mRNA expression levels of WISPI in MKN45 and AGS cells.
CTB assay
CTB assay was used to assess the viability of cells according to the manufacturer's protocol. Briefly, cells at a density of 5000 cells/well were seeded in 96-well plates and cultured in RPMI 1640 medium supplemented with 10% fetal calf serum, 100 U/mL penicillin, and 100 mg/ml streptomycin (Gibco, Grand Island, NY, United States) at 37 °C in a 5% CO2incubator for 12 h. Then, the medium was replaced with medium containing oxaliplatin at different concentrations[16]. After treatment with oxaliplatin for 48 h, 10 μL of CTB was added to each well and the plates were incubated for 4 h at 37 °C. Fluorescent signals were recorded on a microplate reader (Thermo Labsystems,Helsinki, Finland).
Statistical analysis
Statistical analyses were performed with R version 3.5.3 software. Differences in WISP1 expression between tumor and normal samples were evaluated using the Student's t-test. The associations of WISP1 with clinicopathological features were assessed by the chi-square test or Fisher's exact test when appropriate. The overall survival curve and its significance were determined by Kaplan-Meier survival analysis and log-rank test, respectively. Univariate and multivariate analyses of prognosis were carried out using the Cox proportional hazard regression model. All P-values were two-tailed and considered statistically significant when less than 0.05.
RESULTS
WISP1 is up-regulated in GC
Data from the Oncomine database, Gene Expression Omnibus, Coexpedia database,and TCGA were analyzed by the bioinformatics method to determine the differential expression of WISP1 mRNA between GC and normal tissues. As shown in Figure 1,the WISP1 mRNA expression level was remarkably higher in GC tissues compared to normal gastric tissues (P < 0.05). Meta-analysis of the 17 datasets from five studies on WISP1 mRNA levels in GC versus normal gastric tissues was performed based on the Oncomine database. Data showed that WISP1 was up-regulated in GC. From the Coexpedia database, WISP1 was not only up-regulated in GC, but also positively associated with clinical stage (P < 0.05).
To validate the predictive results, RT-PCR was performed to determine WISP1 mRNA levels in 20 cases of GC and matched normal gastric tissues. Furthermore,Western blot analysis and IHC were performed to determine WISP1 protein levels in 20 cases of GC and normal gastric tissues (Figure 2). Our findings indicated that WISP1 was mainly located in the cytoplasm of GC cells. The highly positive rate of WISP1 staining in GC was 85% (17/20). When compared to matched normal tissues,the expression level of WISP1 protein in GC was dramatically higher (P < 0.001).
WISP1 is associated with T stage and chemotherapy outcome
To determine the protein expression of WISP1 in GC, IHC was used on 150 cases of GC samples. Subsequently, to verify the predictive results on the protein level,immunohistochemical staining was performed and the results were statistically analyzed. Our findings showed that positive staining for WISP1 was predominantly distributed in the cytoplasm of GC. Among those cases of GC, 83 (55.33%) GC tissues showed high WISP1 expression, whereas in the remaining 67 (44.67%) cases, WISP1 expression was low. The Chi-square test was used to determine whether there was a statistically significant difference in WISP1 expression between different groups of GC patients based on the following parameters: Age, gender, tumor differentiation, T stage, N stage, TNM stage, and chemotherapy outcome. The results are presented in Table 1. The differential expression of WISP1 protein (high versus low) was dramatically related to T stage and chemotherapy outcome of GC patients (P < 0.05).
Upregulated WISP1 expression predicts a poor prognosis of GC patients
We next investigated whether overexpression of WISP1 is associated with the prognosis of GC patients. First, through mining the TCGA database and Kaplan-Meier Plotter, we found that compared to patients with low WISP1 mRNA expression, GC patients with high WISP1 mRNA expression had a significantly lower overall survival (P = 0.023, Figure 3). Furthermore, GC patients with high expression of WISP1 protein had a dramatically lower overall survival compared to those with low expression of WISP1 protein (P = 0.0019, Figure 3B). In addition, Cox univariate and multivariate survival analyses were performed on WISP1 expression levels and patients' clinicopathological parameters. Cox univariate analysis showed that TNM stage (III vs I-II), T stage (T3-T4 vs T2-T1), N stage (N1-N3 vs N0), chemotherapy outcome (SD/PD vs CR/PR), and WISP1 (High vs Low) were critical parameters affecting the survival time of GC patients (Table 2). Furthermore, Cox multivariate survival analysis indicated that high expression of WISP1 (High vs Low), TNM stage(III vs I-II), and N stage (N1-N3 vs N0) were predictors of unfavorable prognosis in patients with GC (P < 0.05, Table 2). Taken together, these findings suggested that WISP1 could be a novel biomarker for overall survival.
Inhibiting WISP1 expression reverses drug resistance by inducing DNA damage in gastric cancer cells
In this study, the best interference primers for MKN45 and AGS cells were selected and used in the oxaliplatin resistance assay. The half maximal IC50 was 183.6 ng/mL in MKN45, and the IC50 was 51.28 after silencing of WISP1. In AGS cells, the IC50 was 423.3 ng/mL, and after silencing of WISP1, the IC50 was 140.4 ng/mL. These findings suggested that inhibition of WISP1 expression significantly enhanced oxaliplatin sensitivity. Oxaliplatin is a cytotoxic drug that induces DNA damage in cells, γH2AX is a marker for DNA double-strand breaks, and XRCC1 is a multidrug resistance marker that plays a crucial role in the repair of DNA single-strand breaks. In MKN45 and AGS cells, inhibition of WISP1 significantly increased γH2AX expression, and inhibited XRCC1 expression. This suggested that inhibition of WISP1 reversed resistance by enhancing DNA damage repair.
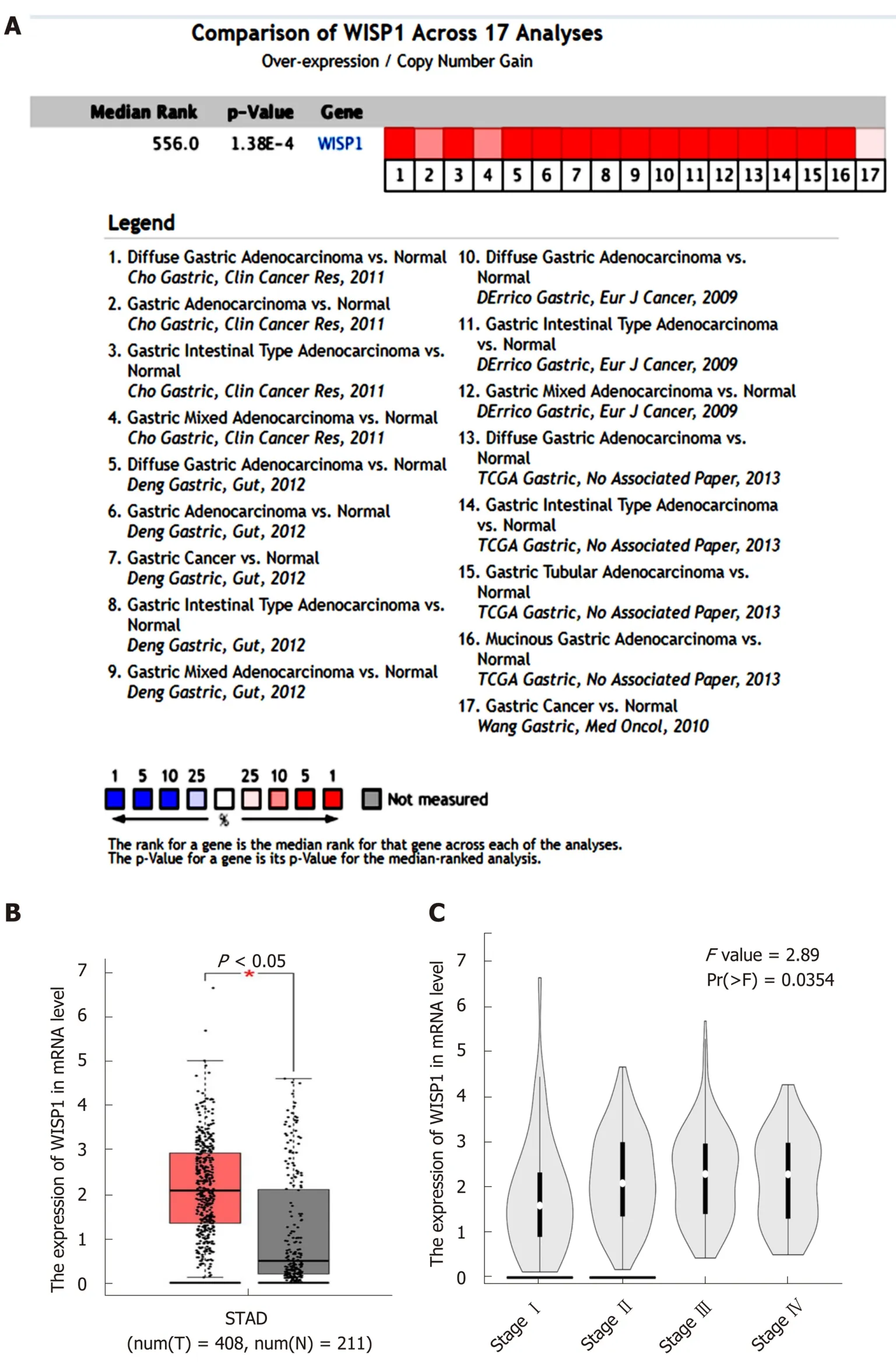
Figure 1 High expression of Wnt1-inducible signaling pathway protein 1 mRNA in gastric cancer predicted based on Oncomine and Coexpedia databases.
DISCUSSION
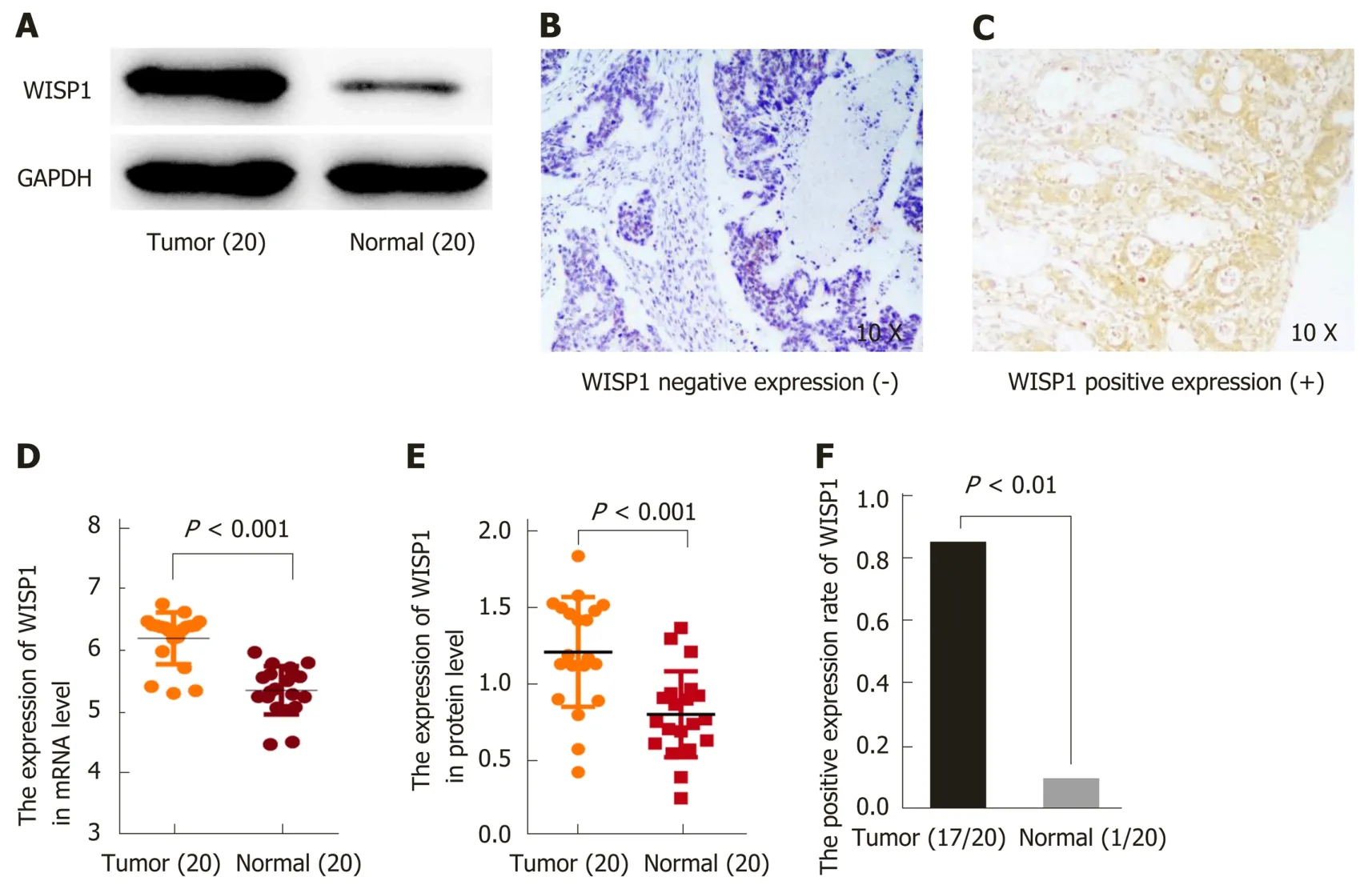
Figure 2 Expression of Wnt1-inducible signaling pathway protein 1 at the mRNA and protein levels is upregulated in gastric cancer patients. A: Western blot analysis showed the expression of Wnt1-inducible signaling pathway protein 1 (WISP1) at the protein level in 20 samples; B: Negative expression of WISP1 in gastric cancer by immunohistochemical staining; C: Positive expression of WISP1 in gastric cancer by immunohistochemical staining; D: Statistical results of WISP1 expression at the mRNA level in 20 samples; E: Statistical results of WISP1 expression at the protein level in 20 samples; F: Statistic results of the immunohistochemical staining for WISP1 at the protein level in 20 samples. WISP1: Wnt1-inducible signaling pathway protein 1.
The molecular mechanisms underlying the carcinogenesis and development of GC have not yet been elucidated. Therefore, GC remain a heavy health issue for decades,and novel anticancer targets and drugs with prognostic value are imperatively needed to explore. WISP1, encoded by the WISP1 gene, is also known as CCN4[7]. The WNT1 inducible signaling pathway (WISP) protein subfamily regulates diverse cellular functions, including cell adhesion, migration, proliferation, differentiation,and survival. WISP1 expression has been shown to promote proliferation, and high WISP1 expression correlates with advanced tumors of the breast, colon, brain, and lung. WISP1 acts as an oncogene by promoting proliferation, migration, and invasion in GC cells[9]. Although the role of WISP1 has been demonstrated in cancer progression, its prognostic value in clinical samples of GC remains unclear.
To address this issue, we first evaluated the expression of WISP1 in GC compared to normal tissues through bioinformatics analysis. The mRNA levels of WISP1 in GC and normal tissues were analyzed using the Oncomine database (www.oncomine.org). The meta-analysis of all datasets from five studies in the Oncomine database demonstrated that WISP1 mRNA levels in GC versus normal gastric tissues were significantly upregulated. Coexpedia is an analysis platform for exploring biomedical hypotheses via co-expression associated with medical subject headings.Coexpedia demonstrated that WISP1 was not only up-regulated in GC, but also positively associated with clinical stage (P < 0.05). Next, to verify these findings in Chinese GC patients, we analyzed the expression of WISP1 at the mRNA level using RT-qPCR. At the protein level, WISP1 expression was detected by Western blot analysis and IHC. Our results confirmed that WISP1 was significantly up-regulated in GC compared to normal tissues at both the mRNA and protein levels. Taken together,these findings strongly showed that WISP1 expression is remarkably upregulated in GC at both the mRNA and protein levels.
Next, we explored the relationship of WISP1 protein expression with the clinicopathological parameters of GC. The Kaplan-Meier plotter is a meta-analysis tool to assess the effect of 54675 genes on survival using 10461 cancer samples[17]. In Kaplan-Meier Plotter analysis for GC, we found that GC patients with high WISP1 mRNA expression had a significantly lower overall survival (P < 0.05, Figure 3A),suggesting that WISP1 might be a novel prognostic biomarker. In addition, IHC was performed to determine WISP1 protein expression in GC tissues. We analyzed the association of WISP1 protein expression and clinicopathological factors of GC patients by the chi-square test. The expression of WISP1 was significantly associated with T stage and chemotherapy outcome. However, no significant differences were found between age, gender, tumor differentiation, N stage, or TNM stage. The clinical prognostic significance of WISP1 expression in patients with GC was investigated using Kaplan-Meier survival analysis, which showed that patients with high WISP1 expression had a significantly poor overall survival when compared to those with lowWISP1 expression. Cox multivariate analysis showed that high expression of WISP1,advanced TNM stage, and N stage were independent unfavorable risk factors in GC.Overall, these findings suggested that WISP1 might be a prognostic biomarker for GC,and might be involved in chemotherapy outcome of GC.
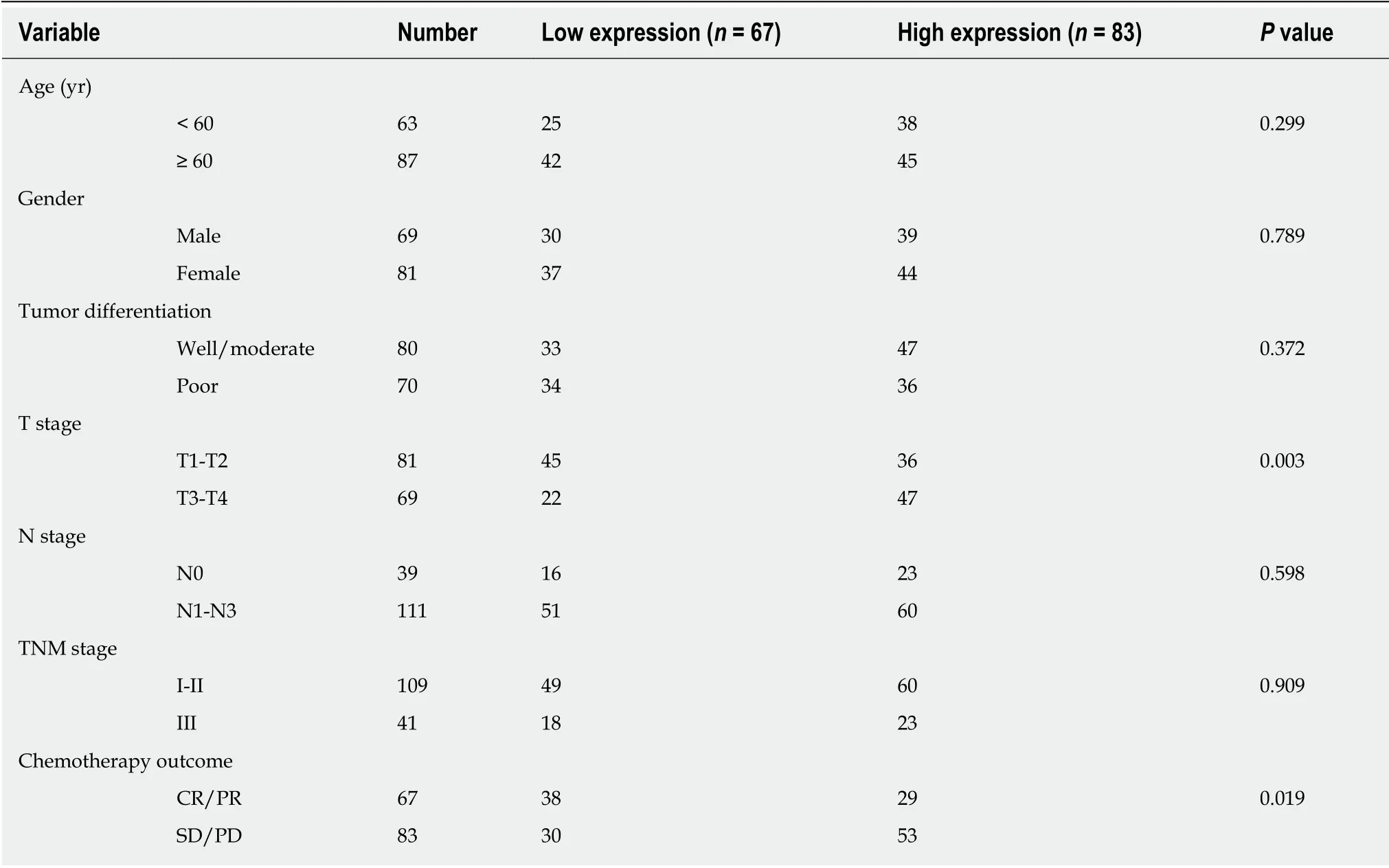
Table 1 Correlation between Wnt1-inducible signaling pathway protein 1 expression and clinicopathological variables in gastric cancer patients
Because WISP1 was associated with the effects of chemotherapy treatment, we speculated that WISP1 might be a primary drug-resistant gene for chemotherapy.Oxaliplatin is commonly used in chemotherapy for GC, which is used for DNA damage, and is cross-linked to DNA, antagonizing its replication and transcription[18,19]. The sensitivity of MKN45 and AGS cells to oxaliplatin was significantly enhanced after inhibiting WISP1 (Figure 4). γH2AX is a sensitive molecular marker of DNA damage and repair[20,21]. XRCC1 is a mediator of single-strand breaks DNA repair[22-24], playing a pivotal role in drug resistance by promoting DNA damage repair in cancer cells. After inhibiting WISP1, γH2AX significantly increased and XRCC1 significantly decreased. These findings suggested that inhibiting WISP1 and enhancing the toxicity of oxaliplatin on GC cells enhanced the sensitivity of oxaliplatin by reducing DNA repair and enhancing cell DNA damage.
This study had some limitations. First, the sample size used in this research was limited and future studies from multiple centers and an increased sample number would be needed. Second, the effect of WISP1 siRNA on the sensitivity of the standard regimen, i.e., 5-FU and cisplatin, warrants further investigation. Third, the detailed molecular mechanisms of WISP1 in GC need further exploration.
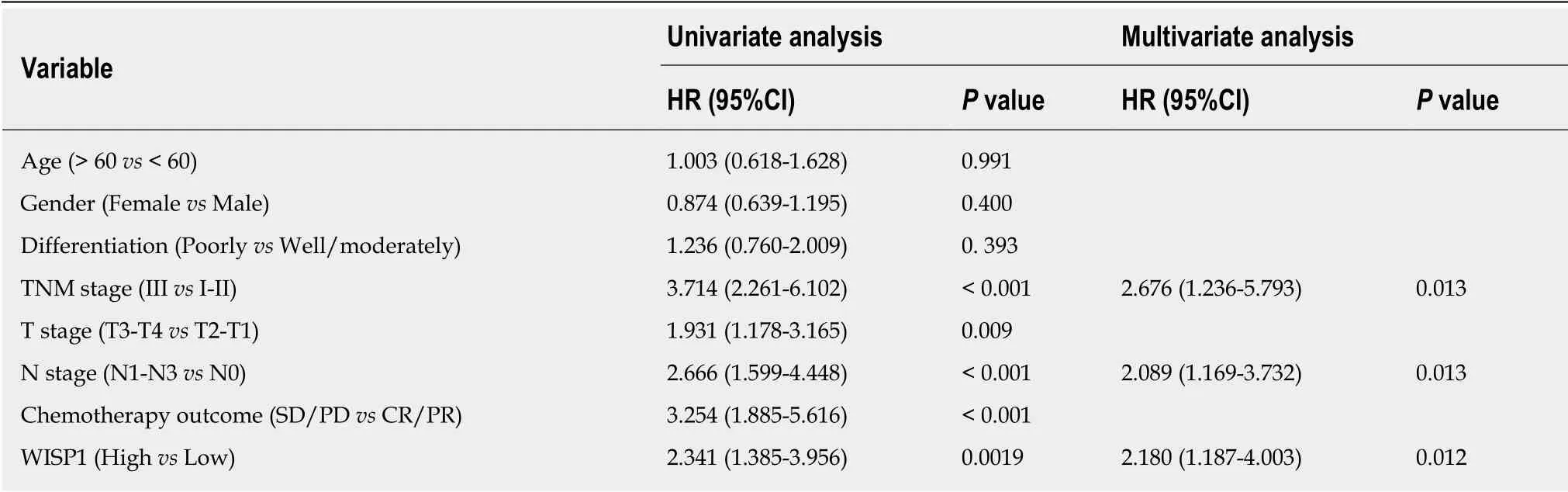
Table 2 Univariate and multivariate analyses of prognostic factors for gastric cancer
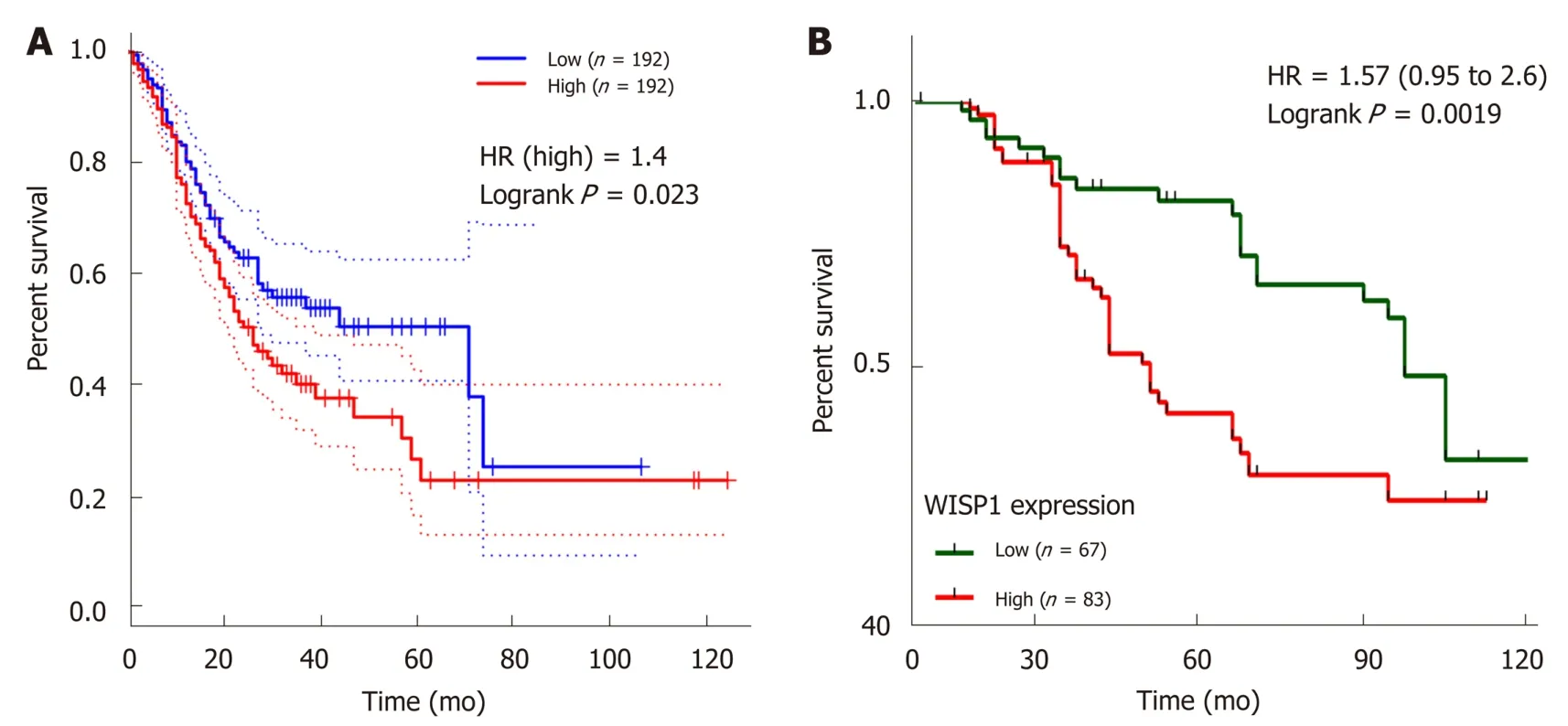
Figure 3 Wnt1-inducible signaling pathway protein 1 expression is associated with overall survival in gastric cancer. A: Kaplan-Meier survival curves plotted with Coexpedia by stratifying patients into high and low Wnt1-inducible signaling pathway protein 1 (WISP1) expression groups based on the median expression value at the mRNA level; B: Kaplan-Meier survival curves generated by WISP1 expression at the protein level. CI: Confidence interval; HR: Hazard ratio; WISP1: Wnt1-inducible signaling pathway protein 1.
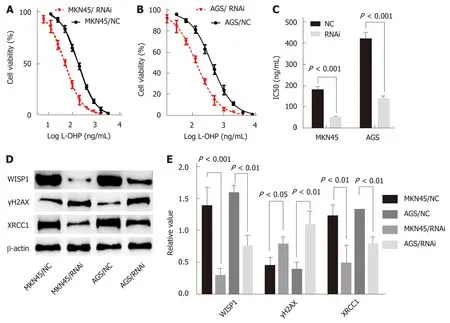
Figure 4 Mechanistic role of Wnt1-inducible signaling pathway protein 1 in gastric cancer cells. A and B: Silencing of Wnt1-inducible signaling pathway protein 1 (WISP1) has effects on cell viability of MKN45 and AGS cells; C: Silencing of WISP1 results in a significant decrease in the IC50 value; D and E: Silencing of WISP1 results in γH2AX overexpression and XRCC1 downregulation in MKN45 and AGS cells. NC: The matching scramble control siRNAs were transfected into cell lines as normal controls; RNAi: The siRNAs were transfected into cell lines as RNA interference; WISP1: Wnt1-inducible signaling pathway protein 1.
ARTICLE HIGHLIGHTS
Research background
Gastric cancer (GC) is the most prevalent gastrointestinal tract malignancy. The prognosis of GC patients remains relatively poor. Through bioinformatics data mining and integrated analysis,we found that Wnt1-inducible signaling pathway protein 1 (WISP1) mRNA was upregulated in GC tissues relative to normal gastric tissues. However, it needs to be further verified clinically.Research motivation There are insufficient reports about the correlation between WISP1 and GC.
Research objectives
The aim of the present study was to explore the expression pattern and clinical significance of WISP1 in GC.
Research methods
Public data portals, including Oncomine, the TCGA database, COEXPEDIA, and Kaplan-Meier plotter were analyzed for the expression and clinical significance of WISP1 mRNA levels in GC.One hundred and fifty patients who underwent surgery for GC between February 2010 and October 2012 at the Affiliated Hospital of Jiangnan University were selected for validation study.WISP1 expression was measured at both the mRNA and protein levels by RT-qPCR, Western blot analysis, and immunohistochemistry (IHC). The correlation of WISP1 expression status with patient prognosis was then determined by univariate and multivariate Cox regression analyses.WISP1 was knocked down by RNA interference. IC50 was detected by CTB assay.
Research results
WISP1 expression at both the mRNA and protein levels was remarkably upregulated in GC tissues compared to normal tissues. Moreover, IHC revealed that WISP1 expression was associated with T stage and chemotherapy outcome, but not with lymph node metastasis, distant metastasis, age, sex, histological grade, or histological type. GC patients with high WISP1 expression showed a poor overall survival. Multivariate survival analysis indicated that WISP1 was an important prognostic factor for GC patients. The IC50 of oxaliplatin in MKN45 and AGS cell lines were significantly reduced after WISP1 knock-down. In
Research conclusions
WISP1 is overexpressed in GC tissues and is associated with a poor prognosis, indicating its potential as a novel prognosis biomarker for GC. Mechanistically, knock-down of WISP1 expression enhances oxaliplatin sensitivity by reducing DNA repair and enhancing DNA damage. WISP1 may be a potential therapeutic target for GC treatment.
Research perspectives
The present study suggested that WISP1 is a novel prognostic biomarker for GC, and the significance of WISP1 as a promising therapeutic target for GC is highlighted.
ACKNOWLEDGEMENTS
The authors would like to thank the members at the Department of Pathology,Affiliated Hospital of Jiangnan University, Department of Pharmaceutical Design and Molecular Pharmacology, School of Pharmacy, Jiangnan University, Cancer Drug Resistance Research Laboratory, Wuxi Medical College, Jiangnan University and Nanchang Hongda Jianghua Educational Foundation.
杂志排行
World Journal of Gastroenterology的其它文章
- Role of ion channels in gastrointestinal cancer
- Targeted therapies in metastatic gastric cancer: Current knowledge and future perspective
- lncRNA-SNHG15 accelerates the development of hepatocellular carcinoma by targeting miR-490-3p/histone deacetylase 2 axis
- Sirtuin 1 alleviates endoplasmic reticulum stress-mediated apoptosis of intestinal epithelial cells in ulcerative colitis
- Hepatitis C virus clearance and less liver damage in patients with high cholesterol, low-density lipoprotein cholesterol and APOE ε4 allele
- Nomogram to predict prolonged postoperative ileus after gastrectomy in gastric cancer
