全国23个城市水源水中邻苯二甲酸酯代谢物浓度调查
2019-10-23丁梦雨康启越张释义赵繁荣张海峰胡建英
丁梦雨,康启越,张释义,赵繁荣,张海峰,杨 敏,胡建英*
全国23个城市水源水中邻苯二甲酸酯代谢物浓度调查
丁梦雨1,康启越1,张释义1,赵繁荣1,张海峰2,杨 敏2,胡建英1*
(1.北京大学城市与环境学院,地表过程分析与模拟教育部重点实验室,北京 100871;2.中国科学院生态环境研究中心,环境水质学国家重点实验室,北京 100085)
采用UPLC-MS/MS 方法对中国 23 个城市的90个自来水厂141个水源水样中5 种常用PAEs的8种代谢产物进行检测.结果发现,所有自来水水源水中均检出了MPAEs,邻苯二甲酸单正丁酯(MnBP)检出浓度最高,为74.7ng/L.水源水中邻苯二甲酸单乙酯(MEP),邻苯二甲酸单异丁酯(MiBP)和MnBP浓度与邻苯二甲酸二乙酯(DEP),邻苯二甲酸二异丁酯(DiBP)和邻苯二甲酸二正丁酯(DnBP)浓度分别呈显著相关,表明两者可能是同源.邻苯二甲酸二(2-乙基己基)酯(DEHP)二级代谢产物所占DEHP一级、二级代谢产物浓度和 (∑DEHP)为4.0% ± 5.6%,和天然水体中DEHP的微生物降解结果类似,水源水中的MPAEs可能来自PAEs在自然水体中的微生物降解.
邻苯二甲酸双酯(PAEs);邻苯二甲酸单酯(MPAEs);饮用水水源水
邻苯二甲酸酯(PAEs)是一种被广泛使用的增塑剂,多用于PVC塑料、化妆品和儿童玩具等产品中[1]. PAEs和高分子材料以非共价键相连,因此容易释放到环境中[2],国内外众多研究表明水源水中广泛存在PAEs[3-10].据报道,我国2016年邻苯二甲酸酯类增塑剂生产量为200万t,占全部增塑剂产量的60%[11].我国制定了邻苯二甲酸二丁酯(DBP)和邻苯二甲酸二(2-乙基己基)酯(DEHP)的地表水环境标准限值分别为3μg/L和8μg/L[12].
作为一种典型的内分泌干扰物,PAEs能导致精液质量下降、精子凋亡、自然流产、儿童肥胖、过敏症状、哮喘、高血压、注意力表现差和DNA损伤等[13-17].PAEs在体内快速代谢为邻苯二甲酸单酯(MPAEs),且其毒性被认为主要是由MPAEs造成的[18].动物实验表明暴露MPAEs会引起多种毒性,如邻苯二甲酸单(2-乙基己基)酯(MEHP)能抑制人类绒毛外滋养细胞侵蚀[19],降低卵母细胞的发育能力[20]、导致排卵停止[21]、诱导免疫系统细胞凋亡[22]等.此外,邻苯二甲酸单正丁酯(MnBP)还能损害人类精子的功能[23].
PAEs通过人体代谢产生的MPAEs最终通过生活污水排入自然水体,因此水体中可能残留这些代谢产物.此外,自然水体中的微生物也能降解PAEs而生成MPAEs[24],目前已在河流、湖泊、海水、生活废水等水环境中检出MPAEs[9,24-27],但是未有文献报道水源水中是否存在MPAEs.由于饮用水是人类暴露污染物质的一个主要途径之一,考虑到MPAEs的毒性,所以有必要对全国水源水中MPAEs的浓度进行全面的调查,获得其浓度水平及其空间分布.
本研究对我国23个城市的90个自来水厂水源水中5种常用PAEs(DMP, DEP, DiBP, DnBP和DEHP)的8种代谢产物(MMP, MEP, MiBP, MnBP, MEHP, MEHHP, MEOHP和MECPP)进行了监测,研究了其时空变化.为了比较,同时检测了水源水中上述5种PAEs的浓度水平.水源水中均检出MPAEs,这一结果为我国水源地MPAEs的风险评估提供暴露信息.
1 材料与方法
1.1 样品的采集
2015年5月~2018年1月于全国23个城市的90个自来水厂水源地采集141个水源水样品,采样点分布如图1所示,采样情况见表1.为了去除余氯,采样时在水样中添加L-抗坏血酸.

图1 全国自来水厂水源水中PAEs (a)和MPAEs (b)浓度空间分布
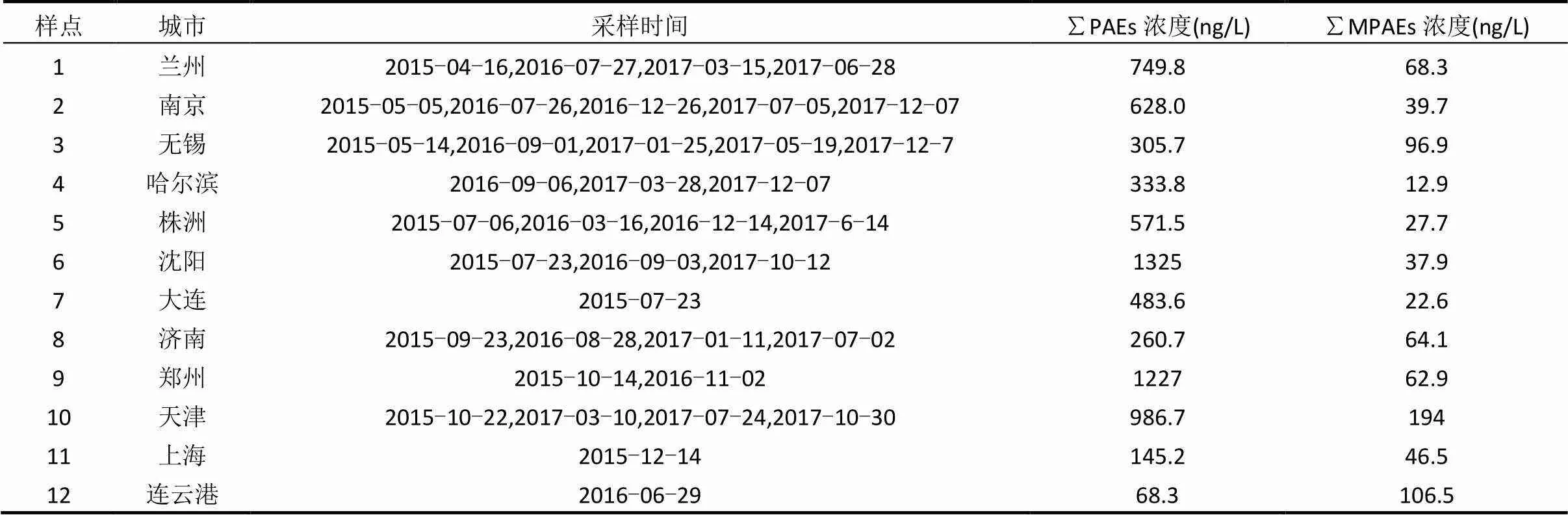
表1 采样城市、时间及其不同城市的PAEs和MPAEs总浓度水平

续表1
1.2 试剂与材料
5种PAEs,包括邻苯二甲酸二甲酯(DMP),邻苯二甲酸二乙酯(DEP),邻苯二甲酸二异丁酯(DiBP), DnBP和DEHP的标准品和氘代同位素内标均购于Labor Dr. Ehrenstorfer (Augsburg,德国), 8种MPAEs标样:邻苯二甲酸单甲酯(MMP),邻苯二甲酸单乙酯(MEP),邻苯二甲酸单异丁酯(MiBP),MnBP, MEHP购买自AccuStandard (New Haven, CT,美国),邻苯二甲酸单(2-乙基-5-羟基己基)酯(MEHHP)和邻苯二甲酸单(2-乙基-5-羰基己基)酯(MEOHP)购于TRC (Toronto, 加拿大),邻苯二甲酸单(2-乙基-5-羧基戊基)酯(MECPP)购于Cambridge Isotope Laboratories (Andover, MA,美国).6种同位素取代的内标MMP-13C4, MEP-13C4,MnBP-13C4, MEHP-13C4, MEHHP-13C4和MECPP-13C4均购于剑桥同位素实验室(Andover, MA,美国).
甲醇和乙腈(LC/MS级),正己烷(农药级)均购于费希尔化工(New Jersey,美国).乙醚(农药级)购于霍尼韦尔实验室,氢氧化铵(28%)来自阿尔法伊萨尔(Heysham,英国),甲酸(HPLC级)和乙酸(HPLC级)来自迪克玛技术公司(California,美国),超纯水经Milli-Q超纯水装置(Millipore,Bedford,MA,USA)制备(电导率>18.2MΩ·cm).所用材料包括HLB固相萃取柱(200mg/6CC,Waters,美国),MAX固相萃取柱(150mg/6CC,Waters,美国),0.45μm玻璃纤维滤膜(Waters,美国).
1.3 样品前处理
用预先在450℃烘焙4h的玻璃纤维滤膜过滤水源水后,取1L用来分析PAEs,加入5种PAEs同位素内标各125ng,用事先活化过的Waters HLB固相萃取柱进行富集.HLB固相萃取柱活化条件为10mL乙醚,5mL甲醇,5mL超纯水活化,流速控制在5~10mL/min.水样全部通过后,用高纯氮气流吹干HLB柱,然后用5mL乙醚:甲醇(/95:5)溶液进行洗脱.最后洗脱液用高纯氮气吹至近干后溶于0.5mL正己烷.
取0.5L用来分析MPAEs,加入6种同位素内标各12.5ng,用Waters MAX固相萃取柱进行富集.MAX固相萃取柱依次用10mL甲醇预淋洗,5mL超纯水活化,水样全部通过后,用5mL超纯水、5mL 5%氢氧化铵和5mL甲醇淋洗MAX柱,用5mL甲酸:甲醇(/5:95)溶液进行洗脱.洗脱液用高纯氮气吹至近干后溶于0.5mL甲醇.样本进入仪器定量分析前保存于-20℃[24].由于PAEs在环境中无处不在,为了消除过程空白,前处理过程中没有使用塑料或橡胶容器;整个实验过程中使用了农药级或LC-MS级的有机溶剂,高纯度氮气(>99.999%),所使用玻璃器皿在马弗炉中400℃烘烤4h以上.
1.4 仪器分析
本研究采用美国安捷伦科技有限公司的Agilent 6890N气相色谱-5975C质谱联用仪器对PAEs进行分析.采用Waters ACQUITY UPLCTM仪器(Waters, Milford, MA,美国)与 Waters Micromass串联四级杆质谱联用仪对MPAEs进行分析.具体分析条件和参数采用本实验室之前报道过的方法[24].
1.5 质量保证与质量控制(QA/QC)
本研究中对于样品中目标物质的定性主要依据:(1)与标样相比保留时间相差在2%以内;(2)与标样相比,2个选择离子峰面积之比相差在20%以内.目标物质的定量选用丰度最高及背景干扰最小的MRM 选择离子,同时用内标校正前处理和基质干扰引起的损失,并用以消除仪器波动的影响.为了评估空白和基质效应,每一组样品跟随1个过程空白,2个基质加标样品作为质量控制. DMP, DEP, DiBP, DnBP和DEHP的检出限分别为5, 2, 3, 7和8ng/L,5种物质的回收率分别为101、102、94、97和71%. MMP, MEP, MiBP, MnBP, MEHP, MEOHP, MEHHP和MECPP的检出限分别为3, 0.6, 1, 2, 3, 0.01, 0.01和0.01ng/L,10种物质的回收率为82%、84%、89%、94%、95%、84%、85%和96%.
1.6 数据处理和分析

2 结果与分析
2.1 全国自来水厂水源地邻苯二甲酸酯浓度
由表2可见,全国141个自来水厂水源水水样中的DiBP, DEHP, DMP, DnBP和DEP5检出率分别为91.5%, 87.9%, 87.2%, 84.4%和81.6%.其中DnBP浓度最高,平均浓度为(425.1±1225)ng/L (

表2 全国水源水中检出的邻苯二甲酸酯及其代谢物的检出率和浓度水平
注:LOD为检出限.
2.2 全国自来水厂水源地邻苯二甲酸酯代谢产物浓度
由表2可见,水源水中MEHP, MnBP, MiBP, MMP和MEP这5种一级代谢产物的检出率分别为94.3%, 92.9%, 88.7%, 88.7%和58.2%,其中MnBP浓度最高,平均浓度为(74.7±372)ng/L.每种一级代谢产物的浓度都低于所对应的PAEs.水源水中MMP和MiBP浓度符合对数正态分布(>0.05),几何平均浓度分别为(6.01±4.12)ng/L和(4.47±4.27)ng/L.使用ProUCL软件对其余3种物质的浓度数据处理后进行对数正态分布检验,MEP和MEHP的浓度符合对数正态分布(>0.05),几何平均浓度分别为(0.720±6.25)ng/L和(10.1±2.41)ng/L.水源水中五种MPAEs浓度均高于加拿大海水浓度(MMP:0.42~20.1ng/L; MEP: 4.41~38.8ng/L; MnBP: 50.9~107.8ng/L; MEHP: 45.5~57.2ng/L)[25];除MEHP外,其余物质的浓度均高于日本Tama河流水体浓度(MnBP:
PAEs在人体内能够很快代谢成为MPAEs, 因此MPAEs会通过生活污水的排放进入水环境中;另外,在自然水体中也会通过微生物降解生成MPAEs.为了进一步探究MPAEs的可能来源,研究了水源水中PAEs和MPAEs浓度相关性,其中DEP和MEP (=0.52,<0.01), DiBP和MiBP(=0.68,<0.01), DnBP和MnBP(=0.85,<0.01)均显著正相关(图2a-c).表明这3种MPAEs可能来自生活污水的排放或者是微生物降解产物.考虑到水源地得到严格保护,所以PAEs在自然水体中的微生物降解可能是MPAEs的重要来源.结合前面二次代谢物占∑DEHP的比例结果,推测水源水中MPAEs的来源可能是环境中PAEs的生物降解.
MPAEs是PAEs的生物活性物质,且已有动物实验表明,低浓度MEHP (20~1000nmol/L)会影响牛卵母细胞的发育,20nM MEHP能够降低卵母细胞的发育能力[20]; MEHP能影响线粒体膜电位,促进活性氧的产生和半胱天冬酶的激活,在环境浓度下诱导免疫系统细胞凋亡[22].因此人群可能通过饮用水途径暴露这些具有潜在生殖和免疫毒性的PAEs代谢产物而引起潜在健康风险.

2.3 全国自来水厂水源地邻苯二甲酸酯及其代谢产物浓度时空变化
由图1(b)所见,山东滨州水源水中五种单酯(MMP, MEP, MiBP, MnBP和MEHP)的总浓度(∑MPAEs)最高,为995ng/L,保定其次(560ng/L),哈尔滨的总浓度最低(12.8ng/L).同样,滨州采集的地下水样本中∑MPAEs浓度高达1712ng/L,特别是MnBP,浓度为1703ng/L,这与滨州地下水中高浓度DnBP的检出情况相似.从全国分布来看,除深圳之外,PAEs和MPAEs的分布情况相似.通过对城市之间PAEs和MPAEs的相关性研究发现,除MEHP和DEHP外,MMP和DMP(=0.69,<0.01),MEP和DEP (=0.81,<0.01),MiBP和DiBP(=0.58,<0.01). MnBP和DnBP(=0.93,<0.01)均显著相关,进一步表明MPAEs的空间分布与PAEs的空间分布类似.
对哈尔滨、济南、兰州、南京、天津、无锡和株洲这7个城市的水源水分丰水期(5~10月)和枯水期(12月~次年3月)进行采样,MPAEs浓度季节性变化如图3所示.通常枯水期污染物浓度被认为高于丰水期的.对于MPAEs,天津枯水期∑MPAEs高于丰水期,但是其他城市丰水期∑MPAEs浓度和枯水期的相仿.这可能和水源类型有关,天津是水库水水源,而其他城市是河流水水源.

图3 水源水中MPAEs浓度的季节变化
枯水期:12月-次年3月;丰水期:5月-10月
3 结论
3.1 全国自来水厂水源水141个样本中都检出了MPAEs, MnBP浓度最高,算术均值为74.7ng/L,水源水中MPAEs风险有待进一步评估.
3.2 水源水中MEP, MiBP和MnBP浓度与DEP, DiBP和DnBP浓度分别呈显著相关,表明两者可能是同源.
3.3 DEHP二级代谢产物所占∑DEHP比例较低,认为水源水中的MPAEs,其可能来自PAEs的微生物降解.
3.4 对于天津水库性水源,枯水期水源水MPAEs浓度远高于丰水期,而其他6个以河流作为水源的城市,其浓度不受季节变化.
[1] Wen Z, Huang X, Gao D, et al. Phthalate esters in surface water of Songhua River watershed associated with land use types, Northeast China [J]. Environmental Science and Pollution Research, 2017,25(8):1-11.
[2] Schettler T. Human exposure to phthalates via consumer products [J]. International Journal of Andrology, 2006,29(1):134-139.
[3] Liu X, Shi J, Bo T, et al. Occurrence of phthalic acid esters in source waters: a nationwide survey in China during the period of 2009~2012 [J]. Environmental Pollution, 2014,184:262-270.
[4] Loraine G A, Pettigrove M E. Seasonal Variations in Concentrations of Pharmaceuticals and Personal Care Products in Drinking Water and Reclaimed Wastewater in Southern California [J]. Environmental Science & Technology, 2006,40(3):687-695.
[5] Mackintosh C E, Maldonado J A, Ikonomou M G, et al. Sorption of Phthalate Esters and PCBs in a Marine Ecosystem [J]. Environmental Science & Technology, 2006,40(11):3481-3488.
[6] Net S, Dumoulin D, El-Osmani R, et al. Case study of PAHs, Me-PAHs, PCBs,Phthalates and pesticides contamination in the Somme River water, France [J]. International Journal of Environmental Research, 2014,8(4):1159-1170.
[7] Selvaraj K K, Sundaramoorthy G, Ravichandran P K, et al. Phthalate esters in water and sediments of the Kaveri River, India: environmental levels and ecotoxicological evaluations [J]. Environmental Geochemistry and Health, 2015,37(1):83-96.
[8] Sibali L L, Okonkwo J O, Mccrindle R I. Determination of selected phthalate esters compounds in water and sediments by capillary gas chromatography and flame ionization detector [J]. Journal of Environmental Science and Health Part A Toxic/Hazardous Substances & Environmental Engineering, 2013,48(11):1365-1377.
[9] Suzuki T, Yaguchi K, Suzuki S, et al. Monitoring of phthalic acid monoesters in river water by solid-phase extraction and GC-MS determination [J]. Environmental Science & Technology, 2001,35(18): 3757-3763.
[10] Vethaak A D, Lahr J, Schrap S M, et al. An integrated assessment of estrogenic contamination and biological effects in the aquatic environment of the Netherlands [J]. Chemosphere, 2005,59(4):511- 524.
[11] 中国产业信息网.2018年中国增塑剂行业发展现状及发展前景分析 [EB/OL].https://www.chyxx.com/industry/201806/653004.html,2018- 06-26/2019-03-03. China Industrial Information Network. Analysis of development status and prospect of plasticizer industry in China, 2018 [EB/OL]. https: //www.chyxx.com/industry/201806/653004.html.2018-06-26/2019-03- 03.
[12] GB 3838-2002 地表水环境质量标准 [S]. GB 3838-2002 Environmental Quality Standard for Surface Water. [S].
[13] Arbuckle T E, Davis K, Boylan K, et al. Processed data for CHMS 2007–2009: Bisphenol A, phthalates and lead and learning and behavioral problems in Canadian children 6–19years of age [J]. Data in Brief, 2016,8:784-802.
[14] Bloom M S, Whitcomb B W, Chen Z, et al. Associations between urinary phthalate concentrations and semen quality parameters in a general population [J]. Human Reproduction, 2015,30(11):2654-57.
[15] Huen K, Calafat A M, Bradman A, et al. Maternal phthalate exposure during pregnancy is associated with DNA methylation of LINE-1and Alu repetitive elements in Mexican-American children [J]. Environmental Research, 2016,148:55-62.
[16] Mu D, Gao F M, Fan Z L, et al. Levels of phthalate metabolites in urine of pregnant women and risk of clinical pregnancy loss [J]. Environmental Science & Technology, 2015,49(17):10651-10657.
[17] Wang Y X, Zeng Q, Sun Y, et al. Phthalate exposure in association with serum hormone levels, sperm DNA damage and spermatozoa apoptosis: A cross-sectional study in China [J]. Environmental Research, 2016,150:557-565.
[18] Mittermeier A, Völkel W, Fromme H. Kinetics of the phthalate metabolites mono-2-ethylhexyl phthalate (MEHP) and mono-n-butyl phthalate (MnBP) in male subjects after a single oral dose [J]. Toxicology Letters, 2016,252:22-28.
[19] Gao F M, Hu W X, Li Y, et al. Mono-2-ethylhexyl phthalate inhibits human extravillous trophoblast invasion via the PPARγ pathway [J]. Toxicology and Applied Pharmacology, 2017,327:23-29.
[20] Kalo D, Roth Z. Low level of mono(2-ethylhexyl) phthalate reduces oocyte developmental competence in association with impaired gene expression [J]. Toxicology, 2017,377:38-48.
[21] Lovekamp-Swan T, Davis B J. Mechanisms of Phthalate Ester Toxicity in the Female Reproductive System [J]. Environmental Health Perspectives, 2002,111(2):139-145.
[22] Rosado-Berrios C A, Christian Vélez, Zayas B. Mitochondrial permeability and toxicity of diethylhexyl and monoethylhexyl phthalates on TK6human lymphoblasts cells [J]. Toxicology in Vitro, 2011,25(8):2010-2016.
[23] Xie F C, Chen X D, Weng S Q, et al. Effects of two environmental endocrine disruptors di-n-butyl phthalate (DBP) and mono-n-butyl phthalate (MBP) on human sperm functions in vitro [J]. Reproductive Toxicology, 2019,83:1–7.
[24] Jiang J Q, Mu D, Ding M Y, et al. Simultaneous determination of primary and secondary phthalate monoesters in the Taihu Lake: Exploration of sources [J]. Chemosphere, 2018,202:17-24.
[25] Blair J D, Ikonomou M G, Kelly B C, et al. Ultra-Trace Determination of Phthalate Ester Metabolites in Seawater, Sediments, and Biota from an Urbanized Marine Inlet by LC/ESI-MS/MS [J]. Environmental Science & Technology, 2009,43(16):6262-6268.
[26] González-Marino I, Rodil R, Barrio I, et al. Wastewater-Based Epidemiology as a New Tool for Estimating Population Exposure to Phthalate Plasticizers [J]. Environmental Science & Technology, 2017, 51(7):3902-3910.
[27] Du P, Zhu Z L, Huang H M, et al. Estimating population exposure to phthalate esters in major Chinese cities through wastewater-based epidemiology [J]. Science of The Total Environment, 2018,643: 1602-1609.
[28] 贺 涛,白小舰,陈 隽,等.饮用水源地塑化剂类污染物环境健康风险评估[J]. 中国环境科学, 2013,33(1):26-31. He T, Bai X J, Chen J, et al. Environmental health risk assessment of plasticizer contaminants in drinking water source [J]. China Environmental Science, 2013,33(1):26-31.
[29] Colacino J A, Haris T R, Schecter A. Dietary Intake Is Associated with Phthalate Body Burden in a Nationally Representative Sample [J]. Environmental Health Perspectives, 2010,118(7):998-1003.
National survey of phthalate metabolites in drinking source water of 23 cities in China.
DING Meng-yu1, KANG Qi-yue1, ZHANG Shi-yi1, ZHAO Fan-rong1, ZHANG Hai-feng2, YANG Min2, HU Jian-ying1*
(1.Laboratory for Earth Surface Proess, Ministy of Education, College of Urban and Environmental Sciences, Peking University, Beijing 100871, China;2.State Key Laboratory of Environmental Aquatic Chemistry, Research Center for Eco- Environmental Sciences, Chinese Academy of Sciences, Beijing 100085)., 2019,39(10):4205~4211
Eight metabolites of 5 PAEs were determined in 141 drinking source water samples from 90 drinking water supply plants of 23 cities in China using UPLC-MS/MS method. MPAEs were detected in all drinking source water samples, and the average concentration of mono-n-butyl phthalate (MnBP) was the highest (74.7ng/L) among target MPAEs. The concentrations of monoethyl phthalate (MEP), mono-iso-butyl phthalate (MiBP) and MnBP in source water were significantly correlated with those of diethyl phthalate (DEP), di-iso-butyl phthalate (DiBP) and di-n-butyl phthalate (DnBP), respectively, suggesting common source for MPAEs and their corresponding PAEs. The percentage of secondary metabolites relative to∑DEHP (total concentrations of primary and secondary metabolites of DEHP) (4.0% ± 5.6%) in source water was comparable to that from the microbiological degradation of DEHP in aqueous environment, suggesting that these metabolites in drinking source water were mainly from the microbiological degradation of PAEs in aqueous environment.
phthalates (PAEs);mono phthalates (MPAEs);drinking source water
X832
A
1000-6923(2019)10-4205-07
丁梦雨(1994-),女,浙江绍兴人,北京大学城市与环境学院硕士研究生,主要从事饮用水和人体中邻苯二甲酸类物质的浓度调查及风险评估.
2019-03-10
科技部政府间国际科技创新合作项目(2016YFE0117800);水体污染控制与治理科技重大专项(2018ZX07502001)
* 责任作者, 教授, hujy@urban.pku.edu.cn
