Evaluation of the safety and effectiveness of direct oral anticoagulants and low molecular weight heparin in gastrointestinal cancer-associated venous thromboembolism
2019-10-23AlejandroRecioBoilesSumanaVeeravelliJessicaVondrakHaniBabikerAaronScottRachnaShroffHitendraPatelEmadElquzaAliMcBride
Alejandro Recio-Boiles,Sumana Veeravelli,Jessica Vondrak,Hani M Babiker,Aaron J Scott,Rachna T Shroff,Hitendra Patel,Emad Elquza,Ali McBride
Alejandro Recio-Boiles,Hani M Babiker,Aaron J Scott,Rachna T Shroff,Emad Elquza,Department of Medicine,Hematology and Medical Oncology,University of Arizona Cancer Center,Tucson,AZ 85724,United States
Sumana Veeravelli,Jessica Vondrak,Department of Medicine,Internal Medicine Residency Program,University of Arizona,Tucson,AZ 85725,United States
Hitendra Patel,UC San Diego Health Moores Cancer Center,La Jolla,CA 92093,United States
Ali McBride,University of Arizona College of Pharmacy,Tucson,AZ 85725,United States
Abstract
Key words: Direct oral anticoagulants; Low molecular weight heparin; Gastrointestinal cancer; Venous thromboembolism; Cancer associated thrombosis; Clinical risk
INTRODUCTION
Venous thromboembolism (VTE) is a common occurrence in cancer patients,specifically in those patients with advanced disease.Cancer-associated VTE (CAVTE)has a three-fold higher risk of recurrent VTE,poorer prognosis and carries with it a significant morbidity and mortality burden as compared to those patients without malignancy[1,2].The goal of anticoagulation therapy is to prevent VTE recurrence while balancing the risk of bleeding events.Two major randomized controlled clinical trials(RCTs) (CLOT[3]and LITE[4]) demonstrated improved outcomes without secondary safety signals for low molecular weight heparin (LMWH),although 3 other RCTs(CANTHANOX[5],ONCENOX[6]and CATCH[7]) showed equivocal outcomes compared to vitamin K antagonists (VKA) (Table1)[3-7].The CLOT trial is the only RCT with mortality benefit at 12 mo in the non-metastatic subgroup (HR = 0.50,95%CI:0.27-0.95).Several guidelines including the American Society of Oncology (ASCO)[8],the European Society of Medical Oncology[9],the American College of Chest Physicians[10],and the National Cancer Comprehensive Network[11],recommend LMWH-based therapy over warfarin as the preferred VTE treatment in cancer patients.
Gastrointestinal cancer (GICA) is associated with a higher incidence of VTE compared to other solid tumors[12].The VTE incidence (per 100 person-years/percent of patients) based on GI site is gastroesophageal 50/7-14,pancreatic 20/5-60,colorectal and anal 13.7/3-10,and hepatobiliary cancer 4.6/2-15[13].Moreover,recurrent VTE and major bleeding (MB) complications during anticoagulation treatment of GICA patients are increased as compared to other cancer types.(HR =5.1,95%CI:2.3-11.3 and 1.3,95%CI:0.3-5.6,VTE and MB respectively)[14].Several therapies contribute to increased bleeding while on chemotherapy as noted with angiogenesis inhibitors (e.g.,bevacizumab[15]and chemotherapy-induced thrombocytopenia (e.g.,gemcitabine)[16].Recurrent VTE and MB complications due to secondary VTE prophylaxis remain a salient issue in treating patients with CAVTE with VKA and LMWH.
Direct oral anticoagulants (DOACs) have been shown to be non-inferior to warfarin for VTE treatment (Table2)[17-26].Four major phase III trials with pooled-analysis of patients with active cancer and VTE suggest that apixaban,rivaroxaban,dabigatran,and edoxaban have independently shown similar efficacy to LMWH plus VKA while having less associated MB[27-30].LMWH remained the preferred treatment regimen for CA-VTE until 2018 when it was challenged by edoxaban in a positive non-inferior open-label RCT for primary-VTE recurrence outcome albeit at the expense of higher MB rate and increased bleeding events in patients with upper GICA[31].A recent study evaluated rivaroxaban to dalteparin to cancer patients with VTE that yielded a 6-mo non-significant VTE recurrence rate and the safety profile for major bleeds in both arms were not significant.However,noticeable MB was seen with upper GI malignancies and these patients were subsequently excluded from trial enrollment[32].Cancer-associated VTE in GI malignancy remains a challenging clinical scenario with a lack of data for utilization of DOACs in the setting of primary treatment and secondary prophylaxis,thus the need for a safer DOAC in patients with active GICAVTE remains an open question.
Our objective was to retrospectively evaluate cancer patient risk factors,effectiveness,and safety of DOACs and LMWH in patients with active GICA-VTE at The University of Arizona Cancer Center (UACC).
MATERIALS AND METHODS
A retrospective chart review of patients receiving DOACs and LMWH with GICA and symptomatic or incidental VTE treated at UACC between November 2013-February 2017 was performed.We obtained prior to initiating research an Institutional Review Board approval Protocol Number:1508054987 and further obtained a waiver of personal health information authorization [45 CFR 164.512(i)(2)(ii)]:As the use or disclosure of protected health information involves no more than minimal risk to the individuals and the research could not practicably be conducted without the waiver.GI malignancy included any Gastro-esophageal or junction cancer (squamous and adenocarcinoma),pancreatic cancer (adenocarcinoma),neuroendocrine tumors,colorectal cancer (including appendix and cecum),anal cancer,and hepatobiliary cancer (pathology-confirmed).Any treatment for cancer,prior to,current with,or posterior to CAVTE diagnosis and any stage including recurrent or metastatic cancer were included.Acute symptomatic or incidental deep vein thrombosis (DVT) or pulmonary embolism (PE) diagnosed by venous duplex ultrasound,computed tomography (CT) with intravenous (IV) contrast,ventilation/perfusion (V/Q) scan and/or PE pulmonary angiography protocol by CT or magnetic resonance imaging with IV contrast was required.Primary endpoints included recurrent DVT,nonfatal PE,or fatal PE.Adverse events such as MB included a Hg drop of ≥ 2 g/dL,transfusion of ≥ 2 units of pack of red blood cells (PRBC),bleeding in a critical site,or bleeding contributing to death[33].
Patients who were prescribed 6 mo or more of DOACs [rivaroxaban and apixaban)or LMWH (enoxaparin)] at UACC by retrospective review of medical records were included.Only patients receiving Federal Drug Administration (FDA)-approved VTE dosage were included:Rivaroxaban (Xarelto FDA-approved November 2,2012) at 15 mg BID for 3 wk,then 20 mg daily and Apixaban (Eliquis FDA-approved August 21,2014) at 10 mg BID for 7 d,then 5 mg twice daily,and Enoxaparin (Lovenox) at 1 mg/kg/dose every 12 h or 1.5 mg/kg once daily).Crossover of up to a month ofLMWH to DOACs was allowed.
2017年7月以来,按照中央环保督察反馈意见和整改方案,湖南省委书记、省长先后21次主持召开与中央环保督察整改工作有关的会议,9次深入整改一线调研督导,20次对环保督察整改工作做出批示,督促地方加快解决突出环境问题。各地坚持高位推进,集中资源,倒排工期,全力推进整改攻坚,加快解决了一批长期未得到有效解决的突出问题。
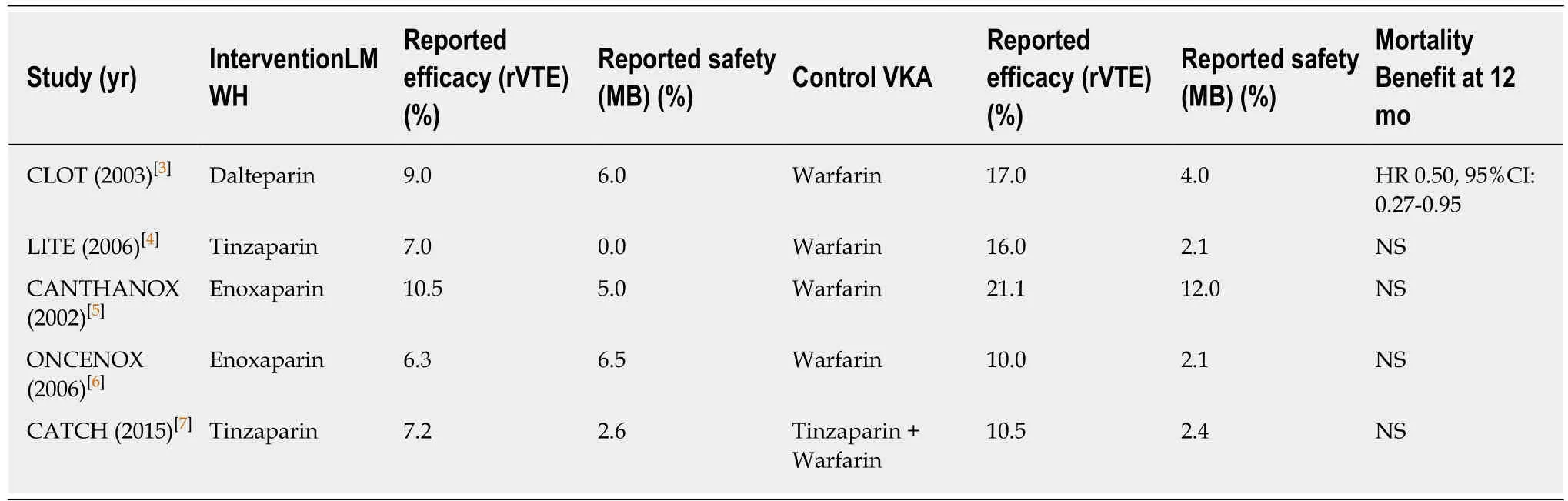
Table1 Clinical trials for low molecular weight heparin primary efficacy and secondary safety compared to vitamin K antagonist
Patients were excluded if DOACs or LMWH were prescribed for any other reason not related to GI-CAVTE (e.g.,atrial fibrillation,VTE prophylaxis),when anticoagulation was contraindicated (e.g.,active bleed,high bleeding risk,thrombocytopenia,palliative,and hospice care) and in patients with other malignancy not related to a gastrointestinal site.Dabigatran was excluded due to an N of one patient.Edoxaban,tinzaparin,and dalteparin were not prescribed at UACC.Betrixaban was not included due to FDA approval on June 23,2017,after the conclusion of the review.
GICA subgroup extracted from clinical trial delineations followed:Active cancer,defined as cancer diagnosed at any stage +/- 6 mo of VTE diagnosis.The Chi-squared test was used for overall and the Fisher exact test for pairwise comparisons of the proportions of patients experiencing VTE and MB events.Odds ratios (OR) were used to compare the relative of the occurrence of the outcome given exposure to the risk factor.The 95%CI was used to estimate the precision of the OR.Results were determined to be “statistically significant” when this value was less than or equal to 0.05.
RESULTS
A total of 144 patients were prescribed anticoagulation,in which 106 fulfilled inclusion criteria and 38 were excluded non-malignant indication (atrial fibrillationn= 13),palliative and hospice treatment interruption (n= 8),VTE prophylaxis (n= 2),other anticoagulation (VKAn= 7 and IVC filtern= 1),other concurrent malignancy(n= 5),and outside records (n= 2).Our analysis included patients on Apixaban (n=29),Rivaroxaban (n= 37) and Enoxaparin (n= 40).
Patients median age was 66.5 years old (range 37-83) at GICA diagnosis and 67 years old (range 37-83) at CAVTE event,and compromised of 62% males and 80%Caucasian (Table3).The population was typical for those presenting with GI-CAVTE,with 70% having recurrent or metastatic disease,predominately composed of pancreatic cancer (40.5%),with a 30% predictive High Khorana Score (≥ 3 points),and with 43.5% on active chemotherapy.The VTE distribution was 65%,15%,and 20% for DVT,PE and DVT/PE,respectively.Identifiable risk factors for VTE were seen in 6 patients with recent surgery/hospitalization and 8 patients with diagnosed catheterrelated VTE.Approximately,patients were 20% current smokers (n= 10),40% on active antiplatelet therapy (n= 53) and 7.5% had previous VTE (n= 9).Sixty-four percent of patients completed anticoagulation therapy (range 1 to 40 mo).Patients had similar baseline characteristics compared to Hokusai (Dalteparinn= 140) (Raskobet al[31],2018),AMPLIFY (Apixabann= 81) (Agnelliet al[27],2015),and pooled-EINSTEIN(Rivaroxabann= 71) (Prinset al[28],2014) (Supplemental Table1).
Recurrent VTE at 6 mo was noted in 7.5% (n= 3),6.8% (n= 2) and 2.7% (n= 1) of patients on enoxaparin,apixaban and rivaroxaban,respectively (allP= NS,P= 0.0623 for rivaroxabanvsLMWH,for rivaroxaban vs apixabanP= 0.1659,for apixabanvsLMWHP= 1.000).VTE historical cancer subgroups comparison to Hokusai (11.3%),AMPLIFY (3.7%),and EINSTEIN (2.8%) showed no significant difference (allP= NS).MB at 6 mo were 5% (n= 2) for enoxaparin,6.8% (n= 2) for apixaban and a significantly higher 21.6% (n= 8) for rivaroxaban (overallP= 0.048;vsLMWHpairwiseP= 0.0423; all otherP= NS).Historical CAVTE major bleed rate comparison was significantly different for rivaroxaban reported as 2.8% in the EINSTEIN trial (P=0.0027),and not different as reported in the Hokusai (4%),and AMPLIFY (2.3%),respectively.

Table2 Clinical trials for direct oral anticoagulants reported recurrent venous thromboembolism and reported mayor bleed outcomes compared to cancer subgroup
Rivaroxaban had one recurrent non-fatal PE event and a significantly worse safety profile with 3 major bleed requiring PRBC,2 critical bleeding sites (subarachnoid hemorrhage and retroperitoneal),and 3 fatal bleeds [hemopericardium (n= 2) and upper GI bleed] (P= 0.0423),whereas Apixaban had 2 recurrent DVT and 2 major bleeds.LMWH had 1 recurrent non-fatal bleed,2 DVT,1 major bleed and 1 fatal bleed[altered mental status presumed intracranial bleed,the family declined further investigation].Including those events beyond 6 mo,21.1% of DOACs patients had recorded bleeding events [2 additional major bleeds for apixaban and 2 for rivaroxaban (including another intra-operative fatal bleed following an urgent small bowel resection)] compared to 10% events of LMWH patients (2 more major bleeds)with non-significant difference (P-value 0.5610).
Significant predictors of a primary or secondary outcome for all anticoagulation therapies included:active systemic treatment (OR = 5.1,95%CI:1.3-19.3,P= 0.016),high Khorana Score (≥ 3 points) (OR = 5.5,95%CI:1.7-17.1,P= 0.003),active smoker(OR = 6.7,95%CI:2.1-21.0,P= 0.012),pancreatic cancer (OR = 6.8,95%CI:1.9-23.2,P=0.002),and stage IV disease (OR = 9.9,95%CI:1.2-79.1,P= 0.03)(Table4).Those who suffered a primary or secondary outcome were 17.4 times more likely to die within a month period,compared to those who didn’t experience an event (CI:4.7-63.4,P=0.0001).Antiplatelet therapy may have affected on four major bleeds (2 rivaroxaban and 2 enoxaparin),although was not a significant risk factor as 11 patients completed therapy without any outcome event (P= 0.479).
DISCUSSION
Review of anticoagulation in cancer treatment
The treatment of VTE in cancer patients aims at reducing mortality and morbidity and improving quality of life,but there are potentially life-threatening challenges - namely hemorrhagic risk and the high rate of recurrence.Until the mid-2000s,the standard treatment for acute CAVTE consisted of initial therapy with LMWH or unfractionatedheparin followed by a transition on long-term therapy with an oral anticoagulant with a VKA as the standard of care.The first study (CLOT trial) to challenge this paradigm showed that a specific LMWH,namely dalteparin,was more effective than oral anticoagulation in reducing the risk of recurrent thromboembolism in cancer patients,with a HR 0.48 (95%CI:0.30-0.77;P= 0.002) over the 6-mo study period.There wereno differences between groups regarding bleeding rates (14%vs19%;P= 0.09) or mortality rates at 6 mo (39%vs41%;P= 0.53)[34].After a plethora of supportive research,LMWH became the new standard of care with significantly lower primary recurrent VTE events balanced by an improved secondary MB profile,although not cancer-site specific.

Table3 Baseline characteristics of the study population
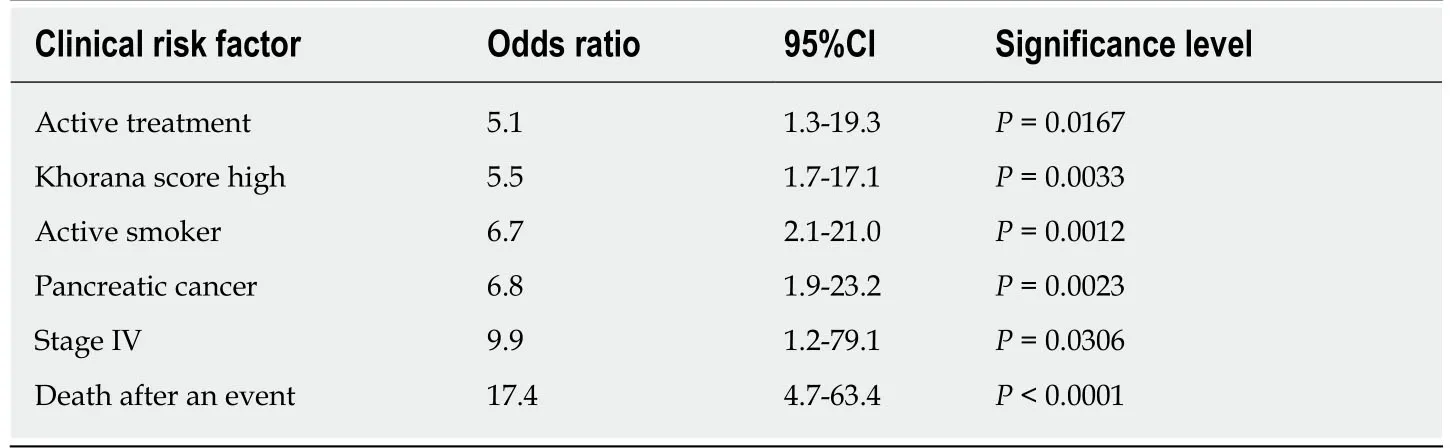
Table4 Clinical risk factors of a primary or secondary outcome for all anticoagulation therapies
Effectiveness and safety of DOAC vs LMWH
The utilization of DOACs in cancer patients provides another form of primary and secondary VTE prophylaxis,which must be weighed,based on safety profiles in our study population.In 2019 a systemic review and network meta-analysis,extracted data for “active cancer patient subpopulation” from major DOACs RCT have reported similar rates of VTE recurrence (HR = 0.74,95%CI 0.54-1.01) and MB (HR = 1.78,95%CI 1.11-2.87) in DOACs as compared to LMWH,although lower to VKA[35].To our knowledge,we present the first and largest retrospective analysis with long-term outcome data of DOACs in patients with GICA and VTE,which showed a nonsignificant risk of recurrent VTE and worse safety profile compared to rivaroxaban,vsapixaban or enoxaparin,by indirect comparison[27-29].Our reported safety profile is consistent with the clinical practice experience literature among non-valvular atrial fibrillation patients on DOACs indirect comparison,whereas apixaban appears to have a lower risk of bleeding than rivaroxaban and any other DOACs[35].Furthermore,rivaroxaban matched to other DOACs patients had a significantly higher risk of MB(HR = 1.82,95%CI:1.36-2.43) compared to apixaban patients[36].The most recently published systemic review and meta-analysis which included the only 2 published RCTs on this topic to date[31,32]showed that DOACs have a higher incidence of 6-mo major bleeds compared to LMWH for CAVTE (RR:1.74 (95%CI:1.05-2.88)[37].
There are significant safety concerns for MB with edoxaban[31],and rivaroxaban[32]in the treatment of GICA-VTE,despite demonstrated non-inferiority in recurrent VTE efficacy to LMWH.Edoxaban was non-inferior for clinically relevant nonmajor bleeds(HR = 1.38; 95%CI:0.98),however it had a significant risk for major bleeds (HR = 1.77,95%CI:10.3-3.04,P= 0.04),where upper GI bleeds were associated with half of all major bleeds events and were mainly in patients with GICA with (17 of a total 33 events,P= 0.02 for interaction in the safety population)[31].In our cohort,4 out of 10 upper GI major bleed events occurred while on DOACs through the 6-mo interval.Although,safety profile for major bleeds on the rivaroxaban study was not significant,a noticeable difference in MB events was identified in patients with upper GI malignancies and consequently a midterm safety analysis elected to exclude their enrollment[32].It is worth noting that in the Select-D trial,major bleeds occurred more frequently in GICA than in all other included malignancies (13vs4,P= 0.0102) in both arms 5/6 (83%) events on dalteparin and 8/11 (73%) events in rivaroxaban.Surprisingly,pancreatic cancer had no major bleeds contrary to our experience (8/10 rivaroxaban bleeds).Moreover,Mcbaneet al[38]have presented at 2018 American Society of Hematology preliminary data with regards of oral apixaban therapy associated with significant low MB and VTE recurrence compared to dalteparin in treatment of CAVTE although unknown patient characteristics at present (ADAMVTE trial pending publication).Cancer-associated VTE in GI malignancy remains a challenging clinical scenario with a lack of data for utilization of DOACs in the setting of primary treatment and secondary prophylaxis,thus the need for a safer DOAC in patien ts with active GI-CAVTE remains an unmet need.
Risk of VTE/Bleed treatment failure
Our study had similarly high numbers of patients with GI-CAVTE,particularly with cancers of the pancreas (40.5%),consistent with the predictive Khorana risk score for VTE based model by cancer site (Khorana Score stomach and pancreatic cancer site OR = 4.3,95%CI:1.2-15.6)[39].We hypothesize that a high Khorana score may also predict anticoagulation treatment failure and worse outcomes.Interestingly,during the development of,a clinical predictor of recurrent VTE Ottawa score,the derivation population sample recognized GICA and Stage IV cancer to be at an increased risk for efficacy failure,like our report,although only Lung Cancer and Stage I were included in the validation tool due to a potential statistical limitation[40].Similar to our cancer center exploratory DOAC cohort,another population-based study has documented Stage IV pancreatic cancer as the strongest predictor of VTE recurrence and bleeding among active cancer patients on LMWH followed by VKA (HR = 6.38,95%CI:2.69-15.13)[41].
There is a gap in knowledge of predictive variables for MB in active cancer patients that was addressed by the RIETE group’s bleed risk stratification of the general population which included all cancer (OR = 1.7,95%CI:1.4-2.2) among others clinical risk items,receiving LMWH plus VKA[42].Kamphuisenet al[43]on behalf of the CATCH trial presented the first pre-specified second analysis were a metastatic stage,older age (> 75 years old) and intracranial lesions described on clinical risk considerations in CAVTE with LMWH (tinzaparin) for major bleed.We found that active systemic treatment and active smoker significantly contributed to treatment failure,regardless of the therapy modality or packs per year smoked,respectively (Table4).Antiplatelet therapy in “real world” appeared not to contribute to an excess bleed,although we do not recommended combination use unless there is a premising cardiovascular indication.Due to LMWH treatment failure (recurrent VTE or MB) and other patient referred inconveniences (e.g.,subcutaneous administration),an unmet need remains to be filled by emerging,effective,safe and more convenient therapies like DOAC.
Limitations
A major limitation of this study is the small sample size of the GICA tumor types,which resulted in wide confidence intervals.Another limitation is the retrospective nature of our analysis,which is unable to capture clinically relevant non-major bleeds due to a lack of detailed documentation to further delineate unscheduled contacts physician recommendations,interruptions in treatment,motives for discontinuation or transition,and patients’ preference or discomfort with their treatment.Current clinical trials from multi-center participation will maximize sample size and appropriately power comparison of DOACs with LMWH in which the primary safety outcome should be the rate of major bleed (e.g.,apixabanvsdalteparin,ADAM-VTE;NCT02585713,pending publication)[38].Furthermore,GI-CAVTE is a high-risk subpopulation deserves additional prospective clinical analysis of the efficacy and safety of DOACs.In addition,evaluation of clinical predictors that may influence the risk of VTE recurrence and MB could include GICA as a high-risk group.At the present time,ASCO clinical practice guidelines update prefers LMWH on patients with an increase risk for bleeding (e.g.,GI malignancy) due to the increased of the reported major bleeds events with DOACs when treating existing CAVTE,until data from ongoing trials and real-world practice provides more safety information[44].
ARTICLE HIGHLIGHTS
Research background
Venous thromboembolism (VTE) is a common occurrence in cancer patients,specifically in those patients with advanced disease.The goal of anticoagulation therapy is to prevent VTE recurrence while mitigating the safety side effects of therapy,mainly major bleed (MB).Gastrointestinal (GI) cancers are associated with a high incidence of thromboembolic events and an even higher risk of bleeding events while on active chemotherapy.Recurrent VTE efficacy and MB safety complications due to secondary VTE prophylaxis remain a noticeable limitation in treating patients with cancer-associated VTE (CAVTE) with vitamin K antagonist and low molecular weight heparin (LMWH).Direct oral anticoagulants (DOACs),a newer set of agents with easier access and administration for CAVTE,have promising effectiveness outcomes although there is a safeness hesitance to utilize these agents in select subsets of high-risk cancer patients.
Research motivation
The current role of DOACs in cancer patients is still unfolding and current treatment guidelines recommend them as a preferred option.Since the advent of DOACs,our clinical practice has noticed an unusual safety profile often having to be addressed by changes in administration,holding of therapy,cessation of therapy or switching to another treatment regimen.We wanted to analyze the efficacy and safety outcome of our own institutional real-world experience with DOAC’s in the GI cancer setting.
Research objectives
The goal of our study was to evaluate our institutional outcomes of DOACs and LMWH in patients with active GICA-VTE at The University of Arizona Cancer Center based on safety and efficacy reported events.
Research methods
Subjects were extracted from a retrospective chart review of GI cancer patients treated at our comprehensive cancer center for incidental or symptomatic VTE with either DOACs or LMWH.Outcomes events,recurrent VTE and MB,were recorded from patients with an active GI malignancy and concurrent anticoagulation therapy at and beyond 6 mo.
Research results
Patients on apixaban (n= 29),rivaroxaban (n= 37) and LMWH (n= 40) met inclusion criteria.Recurrent VTE at 6 mo was noted in 7.5% (n= 3),6.8% (n= 2) and 2.7% (n= 1) of patients on LMWH,apixaban and rivaroxaban,respectively (allP= NS).MB at 6 mo was 5% (n= 2) for LMWH,6.8% (n= 2) for apixaban and 21.6% (n= 8) for rivaroxaban (overallP= 0.048;vsLMWHP= 0.0423; all otherP= NS).Beyond six-months,MB rates were 21% and 10% for DOACs and LMWH (P= NS),respectively,while maintaining efficacy.Significant predictors of any outcome for all anticoagulation therapies included:active systemic treatment (OR - 5.1,95%CI:1.3-19.3),high Khorana Score (≥ 3 points) (OR = 5.5,95%CI:1.7-17.1),active smoker (OR = 6.7,95%CI:2.1-21.0),pancreatic cancer (OR = 6.8,95%CI:1.9-23.2),and stage IV disease (OR = 9.9,95%CI:1.2-79.1).
Research conclusions
Rivaroxaban compared to apixaban and LMWH had a significantly higher risk of major bleeding on GICA-VTE patients with equivocal efficacy.
Research perspectives
Our study shows similar efficacy of LMWH as compared to apixaban and rivaroxaban.Nonetheless,the safety profiles of these new DOACs have to lead to the preferred use of apixaban,which had lower bleeding events in the high-risk GI cancer patient population.
ACKNOWLEDGEMENTS
The University of Arizona Hematology and Medical Oncology Fellowship program for supporting their trainees’ research interests and goals.
猜你喜欢
杂志排行
World Journal of Gastrointestinal Oncology的其它文章
- Acylcarnitine:Useful biomarker for early diagnosis of hepatocellular carcinoma in non-steatohepatitis patients
- MicroRNA-320a suppresses tumor progression by targeting PBX3 in gastric cancer and is downregulated by DNA methylation
- Targeted agents for second-line treatment of advanced hepatocellular carcinoma
- Gastric submucosa-invasive carcinoma associated with Epstein-Barr virus and endoscopic submucosal dissection:A case report
- Abnormally expressed circular RNAs as novel non-invasive biomarkers for hepatocellular carcinoma:A meta-analysis
- Retrospective Cohort Study Fat clearance and conventional fixation identified ypN0 rectal cancers following intermediate neoadjuvant radiotherapy have similar long-term outcomes
