Endoscopic management of esophageal cancer
2019-10-23OsmanAhmedJafferAjaniJeffreyLee
Osman Ahmed,Jaffer A Ajani,Jeffrey H Lee
Osman Ahmed,Jeffrey H Lee,Department of Gastroenterology,Hepatology and Nutrition,University of Texas MD Anderson Cancer Center,Houston,TX 77030,United States
Jaffer A Ajani,Department of Medical Oncology,University of Texas MD Anderson Cancer Center,Houston,TX 77030,United States
Abstract
Key words: Esophageal cancer; Endoscopy; Resection; Ablation; Stent; Barrett’s esophagus
INTRODUCTION
Esophageal cancer (EC) is an overarching term generally used to describe two separate malignancies,esophageal squamous cell carcinoma (SCC) and esophageal adenocarcinoma.Esophageal SCCs arise in the squamous epithelium (generally in the mid-proximal esophagus but can occur throughout the esophagus) whereas esophageal adenocarcinomas arise in the columnar epithelium and are generally found in the distal esophagus.
The epidemiology of EC is evolving.Although ECs only make up one percent of all new cancer cases in the United States,they make up 2.6% of all cancer deaths.The overall incidence of all types of EC has remained steady over the past two decades with an estimated 17000 new cases annually in the United States.The 5-year survival rate of EC varies according to how advanced the tumor is at diagnosis.Those with localized disease have a 5-year survival rate of 45.2%,while those with distant metastases have a 5-year survival rate of only 4.8%[1,2].
Due to the aggressive nature of the disease and the high mortality rate,it is imperative to identify patients early in the course of the disease.Currently,only 19%of cases are staged as localized disease at diagnosis.The benefit of localized disease is that it opens up a whole array of treatment options including the use of endoscopic therapy.The increasing recognition of risk factors associated with metaplasia and dysplasia has led to an increased interest in screening and surveillance programs.For example,the role of gender,obesity and gastroesophageal reflux disease in the development of Barrett’s esophagus (BE) has allowed for the development of screening and surveillance guidelines,which has then lead to treatment guidelines for pre-cancerous and early cancerous lesions[3,4].
Although endoscopy was initially limited to the diagnosis of EC,recent advancements have allowed the modality to play a growing role in the management of the tumor.The development of advance camera technology has allowed better recognition of the disease,while simultaneously the introduction of novel endoscopic techniques and instruments has allowed endoscopists to treat pre-cancerous lesions and even early ECs.
In this review,the current indications for endoscopy in the management of EC are reviewed.The pre-endoscopic management work-up,endoscopic options for curative therapy,the role of endoscopy in managing complications of surgery as well as how endoscopy can play an essential part in the palliative management of EC are described.
PRE-ENDOSCOPIC MANAGEMENT INVESTIGATIONS
The first step in performing endoscopic management in patients with EC is to recognize the setting where it is appropriate.Multiple studies have demonstrated improved outcomes and less complications in high-volume centers,and therefore consideration should be given to referring patients to centers with experience when endoscopic curative management is an option[5,6].Certain guidelines recommend that endoscopic resection (ER) of early EC only be done in high-volume centers.Similarly,a multi-disciplinary approach,with involvement of surgery,oncology and pathology is critical as the diagnosis of dysplasia can be controversial with poor intra- and interobserver agreement[8].A second opinion,ideally from a gastrointestinal pathologist,should be sought if there are doubts about the presence of dysplasia.Additionally,a multi-disciplinary approach will allow for more flexibility and options for the patients and assist in managing any potential complications.
Once a patient has been referred for endoscopic management of pre-cancerous lesions or early EC,it is vital to establish the stage and characteristics of the tumor.This is done through a combination of endoscopic investigations,as well as potentially other modalities to ensure the tumor has not progressed.In terms of endoscopy,careful examination of the lesion is essential prior to any decision regarding endoscopic therapy.After washing the esophagus to remove any food,liquid or debris,careful examination of affected areas with white-light endoscopy should be performed.Recent studies have demonstrated that high-definition endoscopy is superior to standard definition in assessing mucosal changes in patients with BE (Figure1)[9].
In addition,although there has been an increase in the use of adjuncts to whitelight imaging,their evidence in the diagnosis of EC is still controversial with the exception of narrow-band imaging (NBI).NBI is a technique that allows increased highlighting of mucosa and the mucosal vasculature (Figure2).A meta-analysis on the use of NBI to identify high-grade dysplasia (HGD) in patients with BE demonstrated a pooled sensitivity of 0.96 (95% confidence interval:0.93-0.99) and a pooled specificity of 0.94 (95% confidence interval:0.84-1.0).The meta-analysis included eight studies with 446 patients and a total of 2194 lesions.Based on these studies,there has been an increasing use of NBI to identify high-risk lesions].
Similar to NBI,there has been extensive investigation into the use of chromoendoscopy.Chromoendoscopy is the use of selective dyes to highlight specific features on the mucosa and potentially increase the contrast between normal mucosa and abnormal mucosa.The most commonly used dye in chromoendoscopy is methylene blue,which is thought to selectively stain intestinal metaplasia.A previous randomized control trial on the use of methylene blue as compared to random 4-quadrant biopsies showed that although there was no increased detection of dysplasia,the use of methylene blue led to a smaller requirement for the number of biopsies[11].On the other hand,a separate randomized control trial showed that methylene blue detected less dysplasia compared to random 4-quadrant biopsies].Finally,a systematic review and meta-analysis was performed in 2009 and included nine studies with a total of 450 patients.The study demonstrated no incremental yield in the use of chromoendoscopy as compared to standard 4-quadrant biopsies].Subsequently,current guidelines do not recommend the routine use of chromoendoscopy when assessing esophageal lesions for advanced or high-risk features.
When inspecting a lesion with white-light endoscopy or NBI,there are certain features that should be carefully sought for in the mucosa as they will likely change therapy.When examining BE,it is important to document landmarks including any potential hiatal hernia,the location of the gastroesophageal junction,the top of the gastric folds,the location of the squamo-columnar junction and the length of columnar mucosa both circumferentially and the maximal longitudinal length.One commonly used classification for reporting BE is the Prague classification,which documents circumferential and maximal longitudinal length and has been found to have high validity and inter-observer agreement[14,15].
In addition,it is critical to document any nodularity found and the location of the nodularity as it will likely require separate management from the remainder of the BE.Nodules are also suggestive of advanced lesions requiring therapy.In addition to nodules,other high-risk features that portend to malignancy include the presence of ulceration or structuring[16].Careful examination should be done in the 12 o’clock to 6 o’clock (or the right hemisphere) as these have higher rates of EC in BE[17].
Although a careful examination of a lesion using white-light endoscopy is the gold standard,there have been previous studies looking into adjunctive methods to determine resectability.One potential option was the use of endoscopic ultrasound(EUS).EUS would allow the clinician to determine the depth of the lesion as well as any potential locoregional lymph nodes.Initially,the thought was that EUS could provide the ability to determine whether any invasive cancer was present and therefore assist in determining which lesions endoscopic therapy should be avoided.Although initial results were promising,they have not been followed up by similar outcomes in subsequent studies[18].
A systematic review and meta-analysis examining the role of EUS found that EUS only had a 65% concordance for T-staging when compared to surgical or endoscopic mucosal resection (EMR) based pathology[19].A follow-up meta-analysis found better results but was limited due to the heterogeneity between studies].A more recent study examined the same utility of EUS in pre-malignant lesions and found poor correlation with a sensitivity of 50% and a specificity of 93%[21,22].Interestingly,previous studies have found that EUS-guided mini-probe based examinations have better sensitivity than radial echoendoscopes].Due to all the previous studies,the use of EUS to determine resectability is limited and should not be done to guide management decisions in patients with pre-malignant lesions.
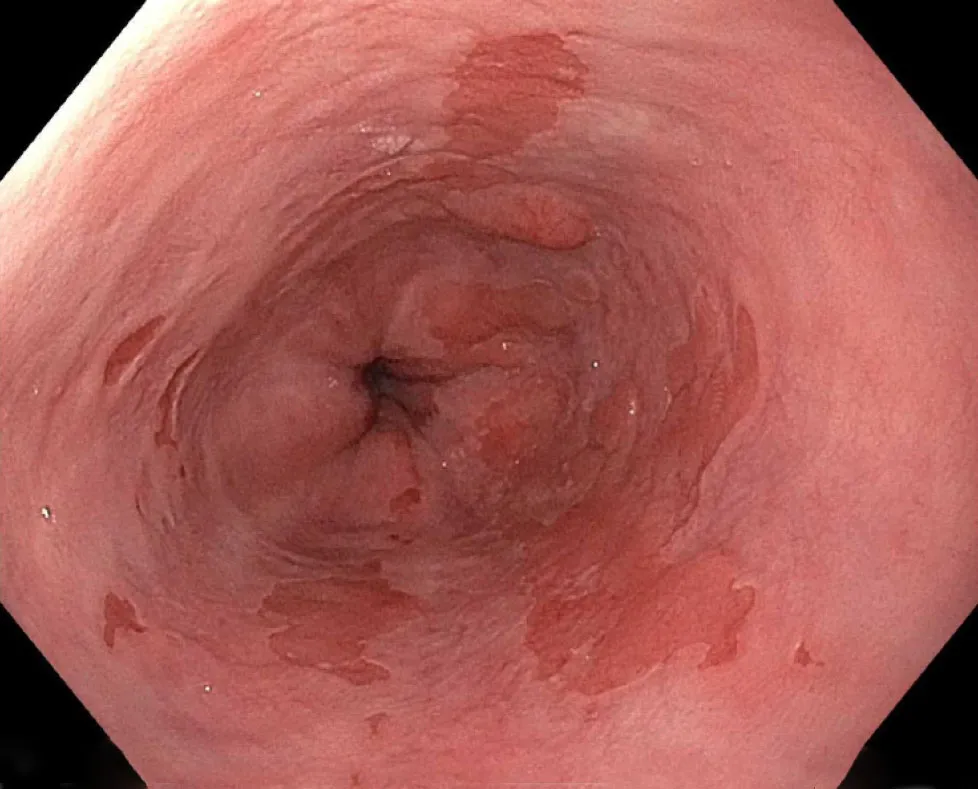
Figure1 Barrett’s esophagus with nodularity.
Nevertheless,EUS can provide a helpful role in patients with early EC.Although EUS has difficulty staging cancers,it can be a useful tool in both identifying and sampling lymph nodes (Figure3).EUS generally has been found to over-stage T2 malignancies and therefore caution should be taken before labelling a lesion as unresectable[24].When it comes to lymph nodes,EUS was found to have fairly high sensitivity and specificity as compared to positive electron-transmission scans and has the added benefit of being able to sample nodes through a fine needle aspiration or biopsy[25,26].
In general,when approaching a patient for potential endoscopic management,it is important to ensure that care is provided in a center with expertise not only in endoscopy but also in surgery,pathology and radiology.The most important investigation is a careful examination during upper endoscopy both with white light endoscopy and NBI.Although adjunctive investigations have so far not yielded fruit,consideration can be given to performing EUS if there is concern for locoregional invasion.
When it comes to the endoscopic management of EC,it can generally be divided into two categories,curative and palliative therapy.Curative therapy is generally reserved for early ECs limited to the mucosa with no lymph node involvement.In this section,we will review the common methods for endoscopic management,as well as upcoming frontiers.
ER
ER is the mainstay of endoscopic management of early ECs.ER can be performed in two ways,by EMR or by endoscopic submucosal dissection (ESD).ER can be performed for both adenocarcinomas and SCCs.In adenocarcinoma patients,the spectrum of disease where ER can be performed generally includes pre-malignant low-grade dysplasia in a patient with BE up to in some cases stage T1b adenocarcinoma (as per the TNM staging of tumors).For SCCs,ER can be performed in patients with early EC that is staged as T1 or intramucosal.
EMR is generally performed by two distinct methods:the cap-assisted method and the ligation-assisted method.The cap-assisted method,also known as the “suck and cut” method involves suctioning the mucosa into a cap-fitted endoscope and then using a snare to cut the mucosa.The snare is pre-opened prior to suctioning and generally comes as part of a pre-assembled ensemble kit.In the ligation-assisted method,or multi-band ligator method,the upper endoscope is fitted with an apparatus similar to a variceal band ligator,and the mucosa is suctioned and has a band placed around it.Subsequently,a snare is passed,and the mucosa upheld by the band is resected (Figure4).
The evidence comparing the two methods of EMR showed that they are generally comparable.In a randomized control trial comparing the techniques,the ligationassisted method was shown to be quicker with smaller resection specimens compared to the cap-assisted method.However,both techniques had similar maximal thickness in their resection specimens and similar adverse event rates (Figure5)[27].Previous studies that compared the two techniques in a non-randomized manner also demonstrated similar results[28,29].The use of the lifting and then direct snare technique that is commonly used in the colon is discouraged in the esophagus due to an increased risk of perforation[30].

Figure2 Narrow-band imaging of Barrett’s esophagus.
ESD is a more recent technique that involves careful dissection of the submucosa of the lesion in systematic fashion followed by en bloc removal of the desired tissue.Although the benefit is that it provides en bloc specimen and can give information about the margins of resection,the disadvantage is that it is time consuming and requires a deeper resection potentially leading to increase adverse events.Indeed,in a systematic review and meta-analysis comprising of 15 non-randomized trials comparing ESD to EMR,they found that although ESD had higher curative resection rates and lesser local recurrence rates,it was balanced by more time-consuming procedures and higher rates of bleeding and perforation].Another meta-analysis looking specifically at esophageal neoplasms found no difference between EMR and ESD in terms of margins,lymph node positivity or metachronous cancers but found less recurrence with ESD though balanced by an increased risk of strictures[32].
The one situation where ESD has had positive results (as compared to EMR) is in the setting of SCCs.A previous study examining resection techniques found less recurrence when en bloc resection was performed by ESD in patients with SCC as compared to patients that had piecemeal resection[33].Based on this study,EMR is generally considered sufficient for small lesions (less than 10 mm) if the diagnosis is SCC,but patients with larger lesions should ideally undergo ESD.Overall,current guidelines recommend EMR for resection of BE or early esophageal adenocarcinomas unless the lesions are larger than 15 mm,are poorly lifting or are at risk for submucosal invasion in which case ESD should be performed.For patients with SCC,current guidelines generally recommend ESD though EMR is acceptable in smaller lesions[34].
ENDOSCOPIC ABLATION
Ablative therapy is generally reserved for flat lesions or treatment of BE after ER.There are many ways to perform ablative therapy with the most common being radiofrequency ablation (RFA) (Figure6).Other methods that are less commonly used include photodynamic therapy (PDT) and cryoablation.The main purpose of ablative therapy is to destroy the remaining residual malignant or pre-malignant tissue to prevent recurrence.
RFA is the application of thermal energy that is generated by radiofrequency waves to destroy tissue.It involves contact ablation and can be done in localized areas or in a circumferential manner.The seminal study examining the effects of RFA was published in 2009.It was a multi-center randomized control trial that compared RFA to sham therapy in patients with dysplastic BE.The primary outcome (complete eradication) was followed until 12 mo post-therapy.In the RFA group,when using intention-to-treat analysis,90.5% of patients had complete eradication whereas in the sham group only 22.7% had eradication.The main adverse event related to RFA was the development of chest pain post-treatment[35].Similar results have been shown in the other multi-center studies including European and Asian populations[36,37].
The role of endoscopic therapy in patients with low-grade dysplasia has been controversial,and there has been debate on whether to pursue endoscopic management or only perform careful observation.A previous study examining patients with BE with only low-grade dysplasia found a decrease in the progression of the dysplasia and the development of cancer with the use of RFA[38].Finally,there have been studies on whether RFA should be applied to patients with BE but no evidence of dysplasia.A study looking at the cost-effectiveness of RFA therapy found that treatment of patients with BE without dysplasia did not provide cost-effective therapy[39].
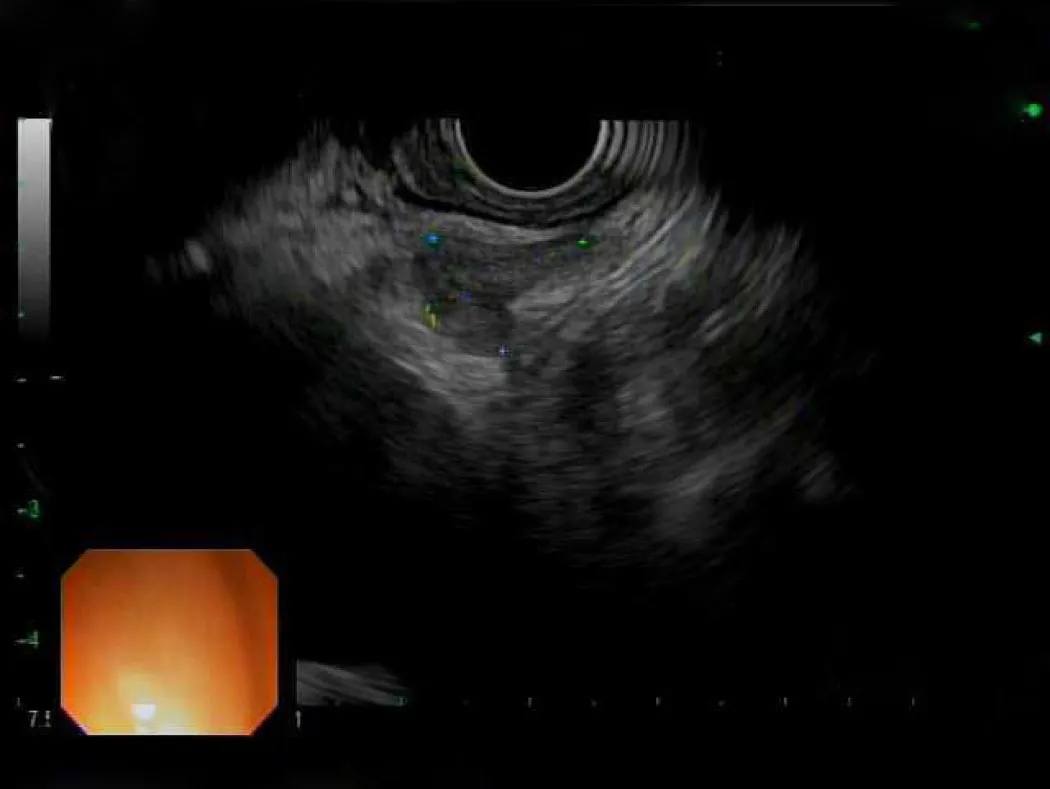
Figure3 Endoscopic ultrasound image of subcarinal lymph node.
Current guidelines generally recommend RFA in patients with dysplasia with nonnodular lesions or intra-mucosal cancer.RFA should also be performed to treat residual BE in patients who have undergone ER.Additionally,although RFA has become well-established in the management of patients with BE or adenocarcinoma,its role in the management of SCC is still developing.Recent studies have showed promise in early SCC with high complete eradication rates and low recurrence rates[40,41].
Other types of ablative therapy include argon plasma coagulation (APC),cryoablation and PDT.APC is widely available,generally due to its use in multiple conditions and diseases and has been widely investigated in the management of BE.In one study examining the role of APC in patients with non-dysplastic BE,complete eradication was successful in 77% of the patients (37/48).The mean number of sessions required was 2.8 (range 1-5) though 9.8% (5/51) had major complications including perforation,hemorrhage and stricture formation[42].Nevertheless,other studies have showed similar positive results with APC[43,44].
Cryoablation of the esophagus has also been studied in the management of premalignant and malignant conditions of the esophagus.The most widely used method is the application of liquid nitrogen therapy.Previous studies have shown high eradication rates in patients with intestinal metaplasia and HGD with minimal adverse events[45].There have also been long-term retrospective studies to determine the sustained ability of cryotherapy.A 5-year follow-up of patients who received cryotherapy revealed complete eradication rates of 93% in HGD and 75% in intestinal metaplasia.The rate of progression to HGD or adenocarcinoma was 1.4% per patientyear in those treated with cryotherapy[46].Cryotherapy has also been studied as rescue or salvage therapy in patients who have had recurrence after initial RFA therapy.The complete eradication of dysplasia rate was 75% in those subsequently treated with cryotherapy,including two patients who initially had intramucosal adenocarcinoma and were both successfully treated[47].
PDT is an ablative process in which a photosensitizer drug is activated by the use of laser light,which leads to mucosal destruction.PDT has evidence in the management of both SCC and esophageal adenocarcinomas.Treatment of either cancer staged as either T1 or T2 showed a complete response rate of 87% with the majority of the complications being either cutaneous photosensitization or esophageal strictures[48].Long-term follow-up has shown sustained response and low rates of recurrence as well[49,50].Comparisons between PDT and APC in the eradication of both BE and dysplasia have showed similar effectiveness though higher costs associated with PDT[51,52].A study comparing RFA to PDT in patients with BE with dysplasia found that RFA had higher complete response rates and was significantly less costly.Though caution must be taken in interpreting these results as the study was nonrandomized with major differences in the baseline characteristics of the two groups[53].
In summary,there are many methods that have evolved to treat flat mucosal lesions with pre-malignant or malignant findings.RFA is generally the most widespread method with increasing evidence of its utility backed by a strong safety record.The development of circumferential balloons as well as through-the-scope segmental pads has made it more user-friendly.In patients who have failed RFA after multiple attempts,consideration should be given to alternative modalities including APC,cryoablation and potentially PDT based on local expertise.
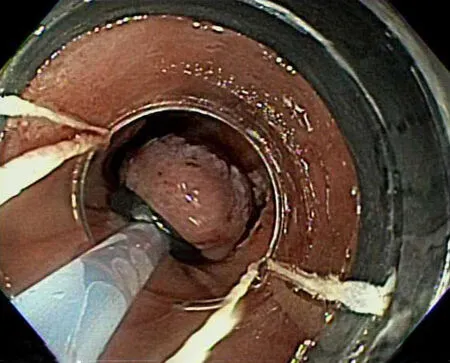
Figure4 Band-ligation method of endoscopic mucosal resection.
ENDOSCOPIC MANAGEMENT OF POST-OPERATIVE COMPLICATIONS
Although an increasing number of patients are being diagnosed with early EC that is amenable to curative resection by endoscopy,a large proportion still progress to surgery.Depending on the features of the tumor and its aggressiveness,the algorithm of neo-adjuvant therapy followed by surgery is generally followed.Nevertheless,endoscopy can play a central role in patients who develop post-operative complications after surgery for EC.The most common complication is the development of a post-operative leak generally at the anastomosis (Figure7).The incidence of post-operative complications can be as high as 22.9% of post-esophageal resection cases[54].The rates of esophageal leaks have been shown to be as high as 7.9%of all esophageal surgeries[55].Prompt recognition and management of esophageal leaks is imperative as the mortality rate associated with leaks can be as high as 35%[56].
Esophageal stent placement is an alternative to a re-operation for an anastomotic leak.Most commonly,a self-expanding metal stent (SEMS) is placed to overlap the site of the leak and allow it to heal.Although SEMS generally come in varying sizes,consideration should be given to place the largest tolerable diameter to prevent migration of the stent as there likely is no narrowing to hold the stent in place.In our practice,we generally use SEMS with a diameter of 23 mm to treat esophageal anastomotic leaks.The securing of the esophageal stent can be done by a variety of methods,including placing a hemostatic clip between the stent and the mucosa or possibly using an endoscopic suturing device to secure the stent in place.The evidence for the role of esophageal stents in the post-operative setting is variable with studies ranging from a technical success (ability to place the stent) rate between 80%to 100% to a clinical success rate (resolution of the leak and removal of the stent) that can be as low as 45%[57,58].The most common complications post-stent placement is pain,stent migration and bleeding[59].
Other methods can be considered for anastomotic leaks including endoscopic clip placement to close the defect.The development of over-the-scope clips have allowed larger defects to be closed endoscopically.Multiple trials on the use of endoscopic clip placement have demonstrated high rates of clinical success and closure.A recent large study examined the role of over-the-scope clips in closure of luminal defects.A total of 188 patients were included of which 108 had fistulas,48 had perforations and 32 had leaks.Successful closure occurred in 90% of patients with perforations,73% with leaks but only 42.9% of patients with fistulas[60].
PALLIATIVE ENDOSCOPIC MANAGEMENT
Once a patient has advanced disease not amenable to curative therapy,the shift of care turns towards palliative management.The role of endoscopy in palliative care is generally the improvement of symptoms especially dysphagia.As patients focus more on end of life care,the need to ensure the ability to take oral contents becomes a matter of quality of life.The main components of endoscopic management in palliative care are dilation,debulking and esophageal stent placement.
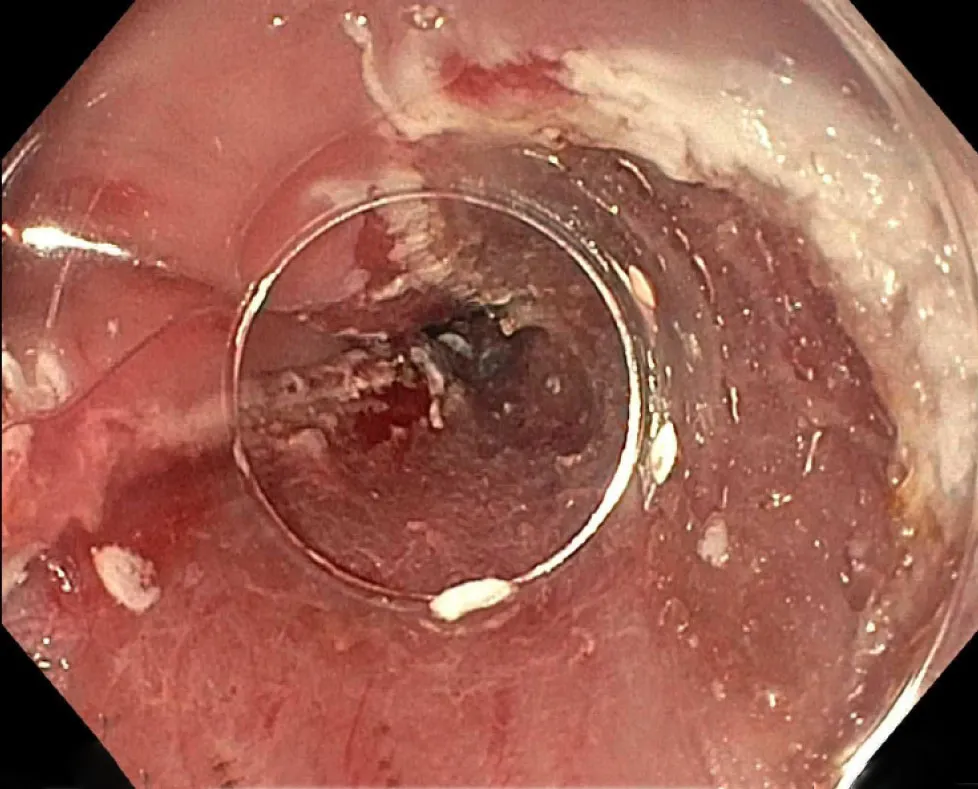
Figure5 Post-endoscopic mucosal resection.
In regard to dilation,the reducing caliber of the esophagus secondary to tumor is the main reason for dysphagia and intermittent periodic dilations are an option to treat the disease (Figure8).Unfortunately,dilation alone rarely provides long-lasting efficacy,and this is compounded by high rates of complications especially perforations[61].Endoscopic debulking therapy can be achieved by the use of laser therapy,PDT or chemical therapy.Chemical therapy,including the use of absolute alcohol,generally only provides transient relief and requires multiple ongoing sessions].PDT has generally been found to be better than laser therapy as shown in randomized comparison trials that have showed similar efficacy between laser therapy (e.g.,Nd :YAG) and PDT but less perforations associated with PDT[63].
Finally,the mainstay of esophageal palliation is the placement of esophageal stents.The most common form of esophageal stents are SEMS,and they can come in covered,partially covered and uncovered forms (Figure9).The evidence for the role of esophageal stents is controversial.Although they have been shown to have durable effectiveness towards dysphagia and lower rates of perforation as compared to dilation alone,they are limited due to patient intolerance of chest pain as well as the risk of stent migration[64].
CONCLUSION
As the epidemiology and presentation of EC evolves,so does the role of endoscopy in its care.No longer relegated to diagnosis only,endoscopy can provide curative therapy in early EC as well as provide therapy for pre-malignant changes.It can also be used to manage complications related to the management of EC specifically postoperative complications.Finally,there is a growing role for endoscopy in the palliative management of EC with an increasing use of debulking therapy as well as the ongoing relief of dysphagia with esophageal stent placements.
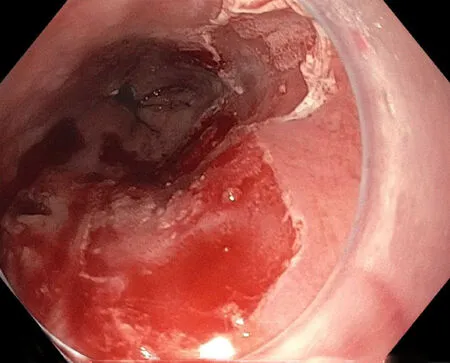
Figure6 Post-radiofrequency ablation.
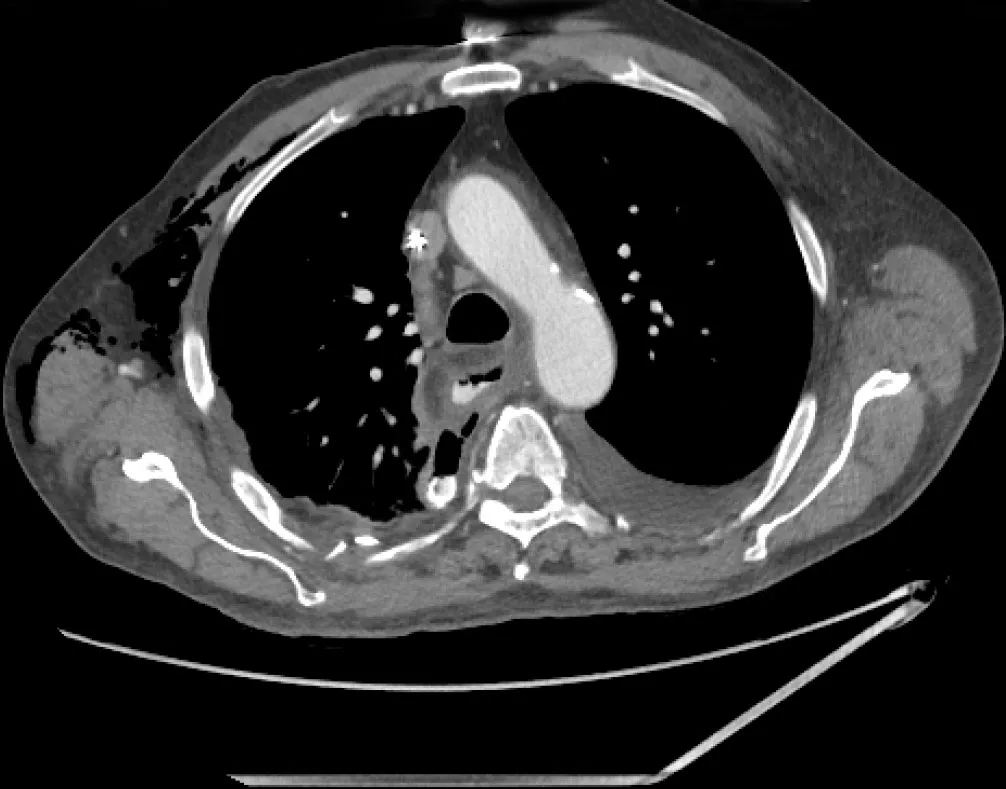
Figure7 Post-operative anastomotic leak.

Figure8 Esophageal balloon dilation.

Figure9 Self-expanding metal stent esophageal stent.
杂志排行
World Journal of Gastrointestinal Oncology的其它文章
- Acylcarnitine:Useful biomarker for early diagnosis of hepatocellular carcinoma in non-steatohepatitis patients
- Evaluation of the safety and effectiveness of direct oral anticoagulants and low molecular weight heparin in gastrointestinal cancer-associated venous thromboembolism
- MicroRNA-320a suppresses tumor progression by targeting PBX3 in gastric cancer and is downregulated by DNA methylation
- Targeted agents for second-line treatment of advanced hepatocellular carcinoma
- Gastric submucosa-invasive carcinoma associated with Epstein-Barr virus and endoscopic submucosal dissection:A case report
- Abnormally expressed circular RNAs as novel non-invasive biomarkers for hepatocellular carcinoma:A meta-analysis
