Effects of Bacillus subtilis on Degradation of Cellulose
2019-10-22ZhouChunshuangWangZheLiZhiyuangWenXuepengandGaoXuejun
Zhou Chun-shuang, Wang Zhe, Li Zhi-yuang, Wen Xue-peng, and Gao Xue-jun
Key Laboratory of Agricultural Biological Functional Gene of Heilongjiang Province, Northeast Agricultural University, Harbin 150030, China
Abstract: Bacillus subtilis is a representative probiotic widely used in food, medicine, livestock and other industries. In this experiment a strain of Bacillus subtilis was isolated and identified, and its ability to degrade cellulose was also measured. The results showed that Bacillus subtilis had strong capacity to degrade cellulose (62.3% was degraded) and weak capacity to degrade hemicellulose (17.2% was degraded), while it could hardly degrade lignin. The total protein amount in the fermentation medium with cellulose-rich substrate reached by 9.4% after Bacillus subtilis fermentation, compared with that without cellulose-rich substrate.Furthermore, the amounts of Met, Lys and Leu reached by 31.4%, 42.2% and 4.9%, respectively. At 36 h of fermentation, the activity of cellulase reached the highest, and at this time the activity of the enzyme was obtained at 7.19 U · mL-1. The mRNA expression level of the cellulase gene was detected by qPCR, and the experimental group with cellulose substrate was about 2.5 times more than that of the non-cellulose substrate control group. These above results demonstrated that this strain of Bacillus subtilis had a strong ability to degrade cellulose, and synthesize more proteins and restrictive essential amino acids. This study revealed that Bacillus subtilis was a new alternative to ferment cellulose substrates to produce commercial feed or feed additives.
Key words: Bacillus subtilis, cellulose, cellulase activity, biodegradation, limiting essential amino acid
Introduction
Agricultural by-products, such as wheat bran and straw rich in cellulose, hemicellulose and lignin, amount to several hundred million tons in China. It is a rich natural renewable resource with great potentials for development (Yuanet al., 2008; Ji, 2015). Many fungi and bacteria have the ability to produce cellulose,hemicellulose and lignin degradation enzymes(Chandel and Silva, 2013), which can be used for the degradation and utilization of agricultural byproducts. The probiotic fermentation product consists of beneficial microorganisms and metabolites that can be directly used by the animal.Bacillus subtilisis a representative probiotic (Huo, 2016). It is a grampositive aerobic bacteria producing endogenetic anti reverse spore, which has the characteristics of high temperature resistance and being easy storage, and can enhance the immunity of livestock and improve its survival. Therefore, it is widely used in the animal husbandry industry (Wanget al., 2015).Bacillus subtilisis not only one of the 46 microbial strains that can be directly fed by the U.S. Food and Drug Administration and the U.S. Feed Control Officials Association, but also a feed-grade microbial strain approved by the Ministry of Agriculture of the People's Republic of China (Zhuet al., 2014). In many reports both at China and abroad, the currently discovered probiotics which are capable of degrading lignocellulose composed of cellulose, hemicellulose and lignin are mainlyBacillus(Xuet al., 2005;Ventorinoet al., 2015; Liet al., 2015).Bacillus subtilishas the ability to secrete a variety of enzymes (Liet al., 2007; Peixotoet al., 2011; Liuet al., 2005), including cellulase, and also has a strong environmental adaptability (Luoet al., 2010; Yuet al., 2010).
Another important advantage of probiotics added to feeds as an additive is that probiotics can increase the levels of certain limiting essential amino acids, during the fermentation process, while the limiting essential amino acid content has a huge benefit on livestock production. Very little has been reported on this. This study reported a strain ofBacillus subtilisfermenting lignocellulose substrates and rose the protein and limiting essential amino acid amounts in the fermentation medium.
Materials and Methods
Materials
Experimental strains
TheBacillus subtilisstrain used in the experiment was isolated from a probiotic drug preparation.Lactobacillus Bulgaria,Bifidobacteria long,Bifidobacterium infantile,Streptococcus thermophilus,Lactobacillus acidophilusandBacillus licheniformiswere preserved by the Key Laboratory of the Ministry of Science and Education of the Northeast Agricultural University.
Medium
LB liquid medium: peptone 10.0 g, sodium chloride 10.0 g, yeast powder 5.0 g, distilled water was added to 1 L. Solid medium plused 2.0% agar. Cellulose samples: the pasture samples collected in the field were thoroughly dried at 80℃ and pulverized into a powder using an ultra-high speed pulverizer, then sterilized at 120℃ for 1 h and thoroughly dried at 80℃ ultimately. Cellulose sample medium: based on LB medium, added 1% cellulose sample.
Laboratory apparatus
PCR instrument (T-Gradient 96 Thermoblock Modul,Biometra, Germany); gel imaging system (GDS-8000,UVP, USA); vacuum filter; C18Japan SHIMADZU Inertsil ODS-3 stainless steel separation column (4.6 mm×250 mm, 5 μm); Japan SHIMADZU SPD-10AV high performance liquid chromatography (HPLC);fluorescence quantitative PCR (ABI7300, ABI, USA);type 722 visible spectrophotometer (Shandong Gaomi,China). Other instruments were routine laboratory instruments in the laboratory.
Experimental reagents
Reagents for Kjeldahl: copper sulfate, potassium sulfate, sulfuric acid, boric acid, 2 mol · L-1hydrochloric acid solution, 72% concentrated sulfuric acid solution, boric acid solution (20 g · L-1), sodium hydroxide solution (400 g · L-1), standard titrated solution (0.05 mol · L-1) or hydrochloric acid standard titrated solution (0.05 mol · L-1), methyl red ethanol solution (1 g · L-1), methylene blue ethanol solution(1 g · L-1) and brominocresol green ethanol solution(1 g · L-1).
HPLC: L-Met, L-Lys, L-Leu standards (chromatographic purity, Sigma, Germany), 0.5 mol · L-1sodium carbonate-sodium bicarbonate buffer (pH 9.0); 0.01 mol · L-1sodium acetate buffer containingφ=40%acetonitrile (pH 6.4), 0.01 mol · L-1sodium acetate buffer containingφ=35% acetonitrile (pH 6.4), 0.01 mol · L-1sodium acetate buffer solution containingφ=23%acetonitrile (pH 6.4); 30 mg · mL-1CDNB (2, 4-dinitrochlorobenzene) solution (the solution should be used just as soon as prepared).
The required reagents for the determination of cellulose content: neutral detergent, etc., were prepared basing on the methods reported by Goering and Van Soest (1971).
Other reagents: DNA extraction kit, agarose gel DNA recovery kit, RNA extraction kit, reverse transcription kit (all were from Tiangen Reagent Company,Beijing, China); cellulase activity detection kit(Solaiba, Beijing, China) and real-time PCR assay kit(TaKaRa, Japan).
Experimental methods
Isolation and purification of strain
The strain ofBacillus subtiliswas separated from a probiotic drug preparation by the flat line method. The samples were crushed and mixed with aseptic water to form the suspension. The inoculation ring was used to inoculate the suspension on the solid culture medium plate. The incubation temperature was 37℃ and the incubation time was 2-3 days. Then a single colony was picked for gram stain and observed under a microscope. Referring to "Berger's Bacteria Identification Manual", the colonies with typical characteristics were re-vaccinated on a new plate.Experiments were repeated several times after that a single form of colonies had been separated and only one typical form of bacteria was observed under microscope. Then the bacterium colony was inoculated into the LB liquid medium, and sample was taken after the medium became turbid, next gram stain was conducted, then microscopic examination was performed and the sample was frozen at -80℃.
Identification of strain
Specific primers was used to conduct PCR analysis for identification of the species of this bacterial. PCR was performed usingBacillus subtilis-specific primers(Caoet al., 2008) (specific primers to amplify GyrA sequence ofBacillus subtilisDNA swollen enzyme A subunit). Upstream primer: 5'-CGTAGAGCCACTTG AGCG-3', downstream primer: 5'-CTGCCGTTACA GTTCCTT-3', amplified length was 256 bp. Reaction conditions of PCR: 94℃ pre-denaturation for 5 min,then 94℃ denaturation for 30 s, 55℃ annealing for 30 s, 72℃ extension for 30 s, 30 cycles, then 72℃final extension 10 min. The amplified product was assayed by 1% agarose gel electrophoresis and recovered using an agarose gel for DNA recovery kit (manufactured by Tiangen Reagent Co., Beijing,China). The recovered product was sequenced by a testing company.
Determination of growth curve
The growth curve of bacteria was determined by turbidimetric method. Briefly, the isolated strain was inoculated into 500 mL LB liquid medium and then packed into 50 labeled tubes in 5 mL volume each,and cultured at 37℃. At every given time, optical density (OD) values of samples in three test tubes were detected, thus the growth curve was drawn.
Fermentation experiments and sample collection
Fermentation experiments were divided into three groups: (1)Bacillus subtiliswas added to MRS medium adding cellulose sample (1%), (2)Lactobacillus delbrueckiiwas added to LB medium fermentation adding cellulose as the control group, (3)Bacillus subtiliswas added to the experimental substrate fermentation without cellulose as the control group.Five parallel samples were set for each group, and the volume of each parallel fermented medium was 100 mL. Bacteria were cultured at 37℃, 120 r · min-1,shaking for 96 h. The fermentation broth after 96 h fermentation was stirred and the residue of cellulose sample was filtered through several layers of gauze to obtain this sample from the fermented liquor. The fermentation broth was used to detect the total protein amounts and furthermore the amounts of Met, Lys and Leu were determined by HPLC.
Determination of amounts of cellulose, hemicellulose and lignin
The amounts of the three components cellulose,hemicellulose and lignin in the samples were analyzed by the modified high performance liquid chromatography method (Jianget al., 2015), Wanget al.(1987) system analysis method and Van Soest method (1967). (1) The residues of the filtered cellulose were placed into a 100 mL iodine volumetric flask, and 50 mL of neutral detergent was added,and then the solution was incubated at 100℃ for 1 h. Then, it was filtered with a sand funnel, and the residues were washed with distilled water and acetone three times and then dried to a constant weight. The weights of the obtained residues were weighed and recorded as W1. (2) The residues from the previous step were collected into a 100 mL iodine volumetric flask and 50 mL of 2 mol · L-1hydrochloric acid solution was added. The incubation was performed at 100℃ for 50 min. and samples were filtered through a sand funnel. The resulting residues were washed with distilled water and acetone three times and then dried to constant weight, weighed and the mass of the resulting residues were recorded as W2. (3) The residues from the previous step were placed into a 50 mL beaker, and 5 mL of 72% sulfuric acid was added, and samples were hydrolyzed at room temperature for 3 h. Then, 45 mL of distilled water was added,and samples were further placed overnight at room temperature, and filtered using a sand funnel. The resulting residues were washed with distilled water and acetone three times and then dried to constant weight,weighed, and the resulting residue mass were recorded as W3. (4) The residues from the previous step were ashed in a muffle furnace at 550℃ to obtain ash, and the weights of the ash were measured and recorded as W4. Data analysis: cellulose content=W1-W2; hemicellulose content=W3-W2; lignin content=W4-W3.Determination of the total protein amounts
The total protein amounts of the fermentation broth were tested by Kjeldahl method. Twenty mL fermentation broth was taken into the digestive tube,while 20 mL distilled water was added as reagent blank. Then, 0.2 g copper sulfate, 6 g potassium sulfate and 20 mL concentrated sulfuric acid were added into each tube simultaneously. In the digestion furnace, the solution was heated to eventually become blue, green and transparent. After cooling, these solution were added into 100 mL volumetric flask. Ten mL of the mixture was added to the Kjeldahl nitrogen apparatus to carry out the reaction, and then the amount of the ammonia gas produced by the collected reaction was calculated by titration with 0.05 mol · L-1hydrochloric acid solution to calculate the amount of the protein contained in the fermentation broth.
Determination of Met, Lys and Leu amounts in fermentation broth by HPLC analysis
(1) Sample preparation
Preparation of samples for determination of amounts of amino acids in fermentation broth: 1 mL of fermentation broth was taken and hydrolyzed according to the GB/T-18246-2000 acid hydrolysis method. After hydrolysis, samples were vacuumized, evaporated to dry, dissolved in 2 mL three distilled water, and then fixed to 50 mL volume. Derivatization of Met standard sample: derivative method was according to previous reports (Huanget al., 2009; Li and Shi, 1995), CDNB was added five times of the amount of Met, Lys or Leu,and mixed with Met, Lys or Leu in a micro-whirl pool mixer, then the mixture was set in a water bath at 80℃constant temperature for 1 h reaction. After cooling,1 mol · L-1hydrochloric acid was used for the adjustion to pH 7.0. To make a standard derivative curve, Met,Lys or Leu at 100, 80, 60, 40, 20, 10 and 5 μg · mL-1were used, respectively.
Derivatization of Leu standard sample: derivative method was similar to Met's depicted above. Zero point 4 g of L-Leu standard was weighed, and 20%formic acid solution was used, and the volume was set to 10 mL. Standard liquid 1, 0.75, 0.50, 0.25, 0.125 and 0.025 mL of this 40 mg · mL-1Leu was then added with water to 10 mL. CDNB was added five times to the amount of Leu, mixed with Leu in a microwhirl pool mixer, and the solution pH was adjusted to about 7. Other steps were the same as Met's above.Derivatization from the fermentation broth: 1 mL of the fermented sample solution was taken, and 1.5 mL of CDNB solution was added, and other steps were the same as above.
(2) Chromatographic conditions
Detection of Met amount: mobile phase contained 650 mL 0.01 mol · L-1sodium acetate buffer (pH 5.2)and 350 mL chromatographic grade acetonitrile;detection wavelength: 350 nm; column temperature:20℃; flow rate: 0.8 mL · min-1; injected volume:20 μL; the derivatized reagent was filtered through a 0.22 μm membrane and injected.
Detection of Lys amount: mobile phase contained 770 mL 0.01 mol · L-1sodium acetate buffer (pH 5.2) and 230 mL chromatography grade acetonitrile.Determination of Leu: mobile phase contained 600 mL 0.01 mol · L-1sodium acetate buffer (pH 5.2)and 400 mL of chromatography grade acetonitrile and other steps were the same as above.
Determination of cellulase activity in fermented liquid
Samples of culture medium withBacillus subtilisfermentation substrate were used as the experimental group and the sample of the culture medium withBacillus subtilisfermentation without cellulose substrate as the control group. The cellulase activity in different fermentation time was determined by cellulase activity assay kit. The unit of enzymic activity was defined as: 1 mL enzyme solution produces 1 μg glucose per minute as one unit of enzymic activity.
RNA reverse transcription and fluorescence quantitative PCR
Sample of culture medium withBacillus subtilisfermentation substrate was used as the experimental group and the sample of the culture medium withBacillus subtilisfermentation without cellulose substrate as the control group. RNA extraction kit was used to extract total RNA ofBacillus subtilisat different fermentation time, then reverse transcription kit was used to reverse transcribe RNA into cDNA.A specific primer was designed for the gene of interest ofBacillus subtilisendo-β-1, 4-glucanase gene sequence, upstream primer: 5'-TGAACTGGAAG CGTGATATTA-3', downstream primer: 5'-CATAA AAATGAAGTGCGTACATAAC-3'. The mRNA expression of endo-β-1, 4-glucanase gene at different time was detected by SYBR dye method. The 16SrRNA ofBacillus subtiliswas used as the internal reference to detect the expression of the endo-β-1, 4-glucanase gene, upstream primer: 5'-GCGTGAGTGATGAAGG TTT-3', downstream primer: 5'-GCCGTGGCTTTC TGGTTA-3'. Reverse transcription and fluorescence quantitative PCR procedures were performed with reference to the instructions of the manufacturer. TheCtvalue of the endo-beta-1, 4-glucanase gene and the reference gene was automatically analyzed by the qPCR 7300 System Software. The 2-ΔΔCtmethod was used to calculate the relative expression levels of the genes, the expression level in the control group was set as 1, and the proportion of the expression amounts in the experimental group to the control group was used for data analysis.
Statistical method
The data were expressed as mean value±SD, and all the tests were repeated at least three times. Statistical analyses were performed using one-way analysis of variance by IBM SPSS Statistics 21 software. The statistical significance was declared atp<0.05 andp<0.01.
Results
ldentification of strain
The strain isolated was identified by PCR withBacillus subtilisspecific primers. A single bright specific band was found in the 256 bp position, and other control strains had no significant bands (Fig. 1).The identification results showed that a strain ofBacillus subtiliswas isolated.

Fig. 1 Identification of a Bacillus subtilis strain
Determination of growth curve
The growth curve ofBacillus subtilisis shown in Fig. 2.The bacterium reached a logarithmic growth phase at 9-11 h.
Cellulose, hemicellulose and lignin amounts before and after fermentation of cellulose-rich substrates
Cellulose, hemicellulose and lignin amounts were detected after 96 h fermentation. After fermentation withBacillus subtilis, the content of cellulose was decreased from 20.7%±2% to 7.8%±4.1%, and the degradation rate was 62.3% (Fig. 3a). The hemicellulose content was decreased from 33.8%±1.6%to 28.0%±2.6%, and the degradation rate was 17.2%(Fig. 3b). There was no significant change in the content of lignin before and after fermentation(7.8%±0.7% before fermentation and 8.2%±1.9% after fermentation) (Fig. 3c). Fermentation withLactobacillus delbrueckiidid not affect the amounts of these three lignocellulose. These above data suggested thatBacillus subtilishad a significant ability to degrade cellulose, while it had a weak ability to degrade hemicellulose and could not degrade lignin.

Fig. 2 Growth curve of Bacillus subtilis for 35 h

Fig. 3 Changes of cellulose, hemicellulose and lignin contents in cellulose culture for 96 h
Determination of the total protein amounts in fermentation broth
After fermentation of lignocellulose substrate-rich medium byBacillus subtilisfor 96 h, the protein content was increased from 48.8%±0.5% to 58.2%±1.1%, which was increased by 9.4%±0.8%. The corresponding protein content ofLactobacillus delbrueckiiwas increased from 48.8%±0.5% to 54.9%±1.9% and increased by 6.1%±1.2% (Fig. 4). These experimental results suggested thatBacillus subtilissynthesized more proteins thanLactobacillus delbrueckiiby using cellulose substrates.
Changes of Met, Lys and Leu amounts in fermentation broth
The peak time of Met, Lys and Leu detected by HPLC was 16.641, 25.932 and 17.370 min, respectively.Standard curves were generated based on the peak areas of each concentration of the Met, Lys and Leu standards, and the regression equation was given.R2was 0.9992, 0.9997 and 0.9995, respectively, and the linear relationship was good.
Bacillus subtiliswas grown well in cellulose substrate medium, and the contents of Met, Lys and Leu after fermentation were increased from 0.156,1.330 and 1.871 to 0.205, 1.891 and 1.963 mg · mL-1,and increased by 31.4%, 42.2% and 4.9%, respectively.WhenBacillus subtiliswas grew in common LB liquid medium without cellulose substrate, the content of Met, Lys and Leu after fermentation was 0.184, 1.419 and 1.874 mg · mL-1, which was only increased by 17.9%, 6.7% and 0.16%, respectively (Fig. 5). These results showed that the addition of cellulose substrates in the medium significantly increased the ability ofBacillus subtilisto produce Met, Lys and Leu.
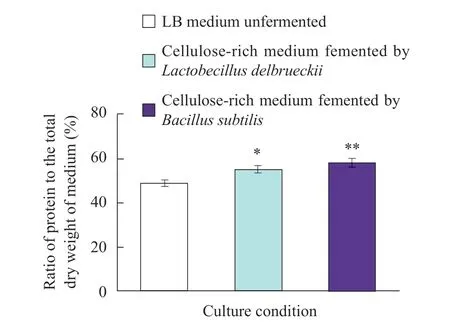
Fig. 4 Proportion of the total protein to dry weight of LB medium after fermentation with cellulose fermentation medium for 96 h
Determination of cellulase activity in fermentation broth
The activity of cellulase was measured at different fermentation time (12, 24, 36, 48, 60 and 72 h). The enzymic activity in the control group ofBacillus subtiliswithout cellulose experimental substrate was very low at all these time points. LB liquid medium with cellulose substrate was fermented byBacillus subtilis. With the increase of fermentation time, the highest value was reached at 36 h, and the enzymic activity was 7.19 U · mL-1(Fig. 6). These experimental results showed that the cellulase activity ofBacillus subtiliswas induced by cellulose substrate, and suggested that the degradation products of cellulose substrate could inhibit the expression ofBacillus subtiliscellulase.

Fig. 5 Amounts of several essential amino acids after fermentation with Bacillus subtilis by HPLC detection

Fig. 6 Cellulase activity changes in cellulose-containing medium at different fermentation time
Analysis of mRNA expression of cellulase gene endo-β-1, 4-glucanase
Real-time fluorescence quantitative PCR reaction was performed to analyze the mRNA expression of cellulase gene endo-β-1, 4-glucanase. Data were analyzed by qPCR7300 System Software. The relative expression of this gene inBacillus subtilisin 24 , 36 and 48 h experimental groups were calculated after the experimental data were adjusted by the quantitative values of the internal reference gene 16S rRNA(Fig. 7). Results showed the highest relative expression was obtained at 36 h, which was expressed about 2.5 folds higher comparing to LB liquid medium. At the meantime, the relative expression level were increased by about 0.9 times than that of control group. This change of mRNA expression showed a similar trend with cellulase activity.

Fig. 7 Relative mRNA expression of cellulase gene endo-β-1, 4-glucanase at 24, 36 and 48 h fermentation time
Discussion
The experimental results showed thatBacillus subtilishad the ability to degrade cellulose (degradation rate of 62.3%) and to degrade hemicellulose (degradation rate of 17.2%). It could increase the total protein amounts in the fermentation products from cellulosecontaining fermentation medium, and also increase the amounts of Met, Lys and Leu in the fermentation products by 31.4%, 42.2% and 4.9%, respectively.The highest cellulase activity was observed at 36 h of fermentation, and the enzymic activity was 7.19 U · mL-1. The mRNA expression level of cellulase gene endo-β-1, 4-glucanase was detected by qPCR,and its mRNA level was consistent with its cellulase activity at different fermentation points. These results demonstrated thatBacillus subtiliscould degrade cellulose and synthesize amino acids and proteins by expressing cellulase gene.
At present, the mechanism of bacterial degradation of cellulose was still unclear. Several lines of reports indicated that some aerobic bacteria could degrade cellulose through a free enzyme system (Makiet al.,2009; Gomez and Saadeddin, 2013). The main way for bacteria (mainly anaerobic bacteria) to degrade cellulose was to adhere cellulose on the surface of cells, and the cellulose was destroyed by cellulase secreted by bacteria (Yanget al., 2015). The degradation of lignin by bacteria was not as efficient as the mould, and the lignin could resist the effect of the general hydrolase because of its dense structure,thus the degradation efficiency of this complex aromatic compound by the bacteria was very low(Shiet al., 2013; Zhang and Yu, 2014). The structure of hemicellulose was highly branched and more easily decomposed, but lignin often formed complex intertwined structures with hemicellulose, which to some extent hindered the hydrolysis of hemicellulase(Cosgrove, 2005).
In this study, it was obsterved that the contents of cellulose and hemicellulose in the medium containing cellulose fermented byBacillus subtiliswere both decreased with the increase of fermentation time.Bacillus subtiliscould produce cellulase to degrade cellulose. With the increase of fermentation time, the activity of cellulase showed a parabolic trend. At 36 h, the enzymic activity reached the highest value. The decrease in enzymic activity might be due to changes in pH or the degeneration of the enzyme caused by cell metabolism during the fermentation(Garciaet al., 2015).Lactobacillus delbrueckiiwas used as a negative control. The cellulose content in the medium fermented by this bacterium was almost unchanged before and after fermentation. It had not been reported thatLactobacillus delbrueckiicould degrade cellulose, which was consistent with the results of this study. The key to cellulose degradation lied on the comprehensive utilization of cellulase systems. Cellulase was mainly composed of endoglucanase (EC 3.2.1.4), exoglucanase(EC 3.2.1.91) andβ-glucosidase (EC 3.2.1.21) (Chenet al., 2016). It showed that the expression levels of endo-β-1, 4-glucanase gene in the experimental group added with cellulose substrate were significantly higher than those in the control group without cellulose substrate. The control group had very low enzymic activity at each time. These experimental results suggested thatBacillus subtilisdegraded cellulose by expressing cellulase genes, and cellulases were induced by the cellulose substrate. The results were consistent with the previous reports thatBacillus subtilisexpressed cellulase genes in the presence of a cellulose substrate thereby degrading cellulose (Fenget al., 2017; Budihalet al., 2016).
In theBacillus subtilisfermentation product added with the cellulose sample, both the limiting essential amino acid and the total protein were significantly increased. The total protein content ofBacillus subtilisbroth product was also significantly higher than that ofLactobacillus delbrueckiibroth that lacked the ability to degrade cellulose as a negative control. These experimental results suggested thatBacillus subtilismight also transfer nitrogen from the original cellulose sample into the fermentation broth and convert it into protein, which increased the total protein level in the fermentation broth. It speculated that cellulose degradation products might provide a carbon-containing backbone or energy to stimulate the production of amino acids and proteins (Chen, 2014).
Conclusions
In summary, this new isolatedBacillus subtiliscould degrade cellulose-rich substrates and synthesize amounts of proteins and limiting essential amino acids. The cellulose and hemicellulose degradation efficiency reached 62.3% and 17.2% with little lignin degradation. Besides the cellulose degradation,Bacillus subtiliscould even produce 9.4% protein amount in the cellulose-rich substrate. For the limiting essential amino acids, Met, Lys and Leu reached by 31.4%, 42.2% and 4.9%, respectively. At 36 h during fermentation, cellulase activity obtained the highest value of 7.19 U · mL-1and mRNA expression was improved nearly 2.5 times higher with cellulose-rich substrate than without. It indicated the importance of usingBacillus subtilisas the cellulose degrader and energy producer. However, it was worth noting the further researches on how to induceBacillus subtilisto express cellulose degrading enzymes more efficiently or introduce more efficient exogenous degrading enzymes through gene technology for agriculture and animal husbandry in the future.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Differential Responses of Phytophthora sojae to Seed Exudates of Host Soybean and Non-host Maize
- Identification of QTL and Analysis QTL with Tolerance to Sclerotinia sclerotiorum in Soybean
- Screening of Transgenic Soybean Materials for Salt Tolerance
- Development, Reproduction and Body Size Variation of Aphis glycines Matsumura Fed on Different Plants
- Effects of Dietary Fat Levels on Growth, Nutrient Digestibility,Nitrogen Utilization and Fur Quality of Growing-furring Blue Foxes
- Phylogeny and Homologous Recombination Occurring in Classical Swine Fever Viruses
