Development, Reproduction and Body Size Variation of Aphis glycines Matsumura Fed on Different Plants
2019-10-22LiuJianWangSujiBaiBingGaoBoandFanYanjie
Liu Jian, Wang Su-ji, Bai Bing, Gao Bo, and Fan Yan-jie
College of Agriculture, Northeast Agricultural University, Harbin 150030, China
Abstract: Soybean aphid, Aphis glycines Matsumura is one of the most important pests in soybean. Life cycle of A. glycines is characterized as heteroecious and holocyclic. The primary hosts of A. glycines are Rhamnus spp. and the secondary hosts include cultivated soybean, Glycine max (L.) Merr, and wild soybean, Glycine soja Sieb. & Zucc. In this study, A. glycines were fed on Trifolium repens L. and Metaplexis japonica (Thunb.) Makino for three generations and their development, reproduction and body sizes were studied. These data were compared to the control fed on the known hosts, G. max and G. soja. These newly deposited offspring by the 3rd generation were transferred back onto G. max and these differences in their development, reproduction and body sizes were also studied. It showed that A. glycines all could survive, develop and reproduce well, when they were fed on T. repens and M. japonica for three generations, but there were significant differences in their nymph stage, adult longevity and fecundity, life table parameters and body sizes. When these offspring were transferred back onto G. max which were newly deposited by the 3rd generation aphids fed on T. repens and M. japonica, they could survive, develop and reproduce well, but there were significant differences in their nymph stage, adult longevity, intrinsic rate of increase and body sizes. It provided important information for studies on host adaptability of A. glycines on T. repens and M. japonica and to make clear the potential that this aphid involved into host biotypes on these plants.
Key words: Aphis glycines Matsumura, Trifolium repens L., Metaplexis japonica (Thunb.) Makino
Introduction
Soybean aphid,Aphis glycinesMatsumura (Hemiptera:Aphididae), is one of the most important pests in soybean, which is native to Asia. In the summer of 2000,A. glycineswere established in North America(Ragsdaleet al., 2011). It can cause direct damage to plants by sucking fluids from leaves and stems (Liu and Zhao, 2007; Wuet al., 2004). It is also capable of transmitting a variety of plant viruses (Burrowset al., 2005; Daviset al., 2005; Wanget al., 2006).Additionally, black sooty mold fungus growing on honeydew produced byA. glycinesfeeding can lead to photosynthesis inhibition of soybean. Yield reductions fromA. glycinesare approximately 20%-30% in years with normal damage (Liu and Zhao, 2007).
Life cycle ofA. glycinesis characterized as heteroecious (use of the primary and the secondary hosts) and holocyclic (sexual morphs produce over-wintering eggs on primary hosts) (Liu and Zhao, 2007; Wuet al., 2004). In Asia,Rhamnus davuricuusPallas(Wanget al., 1962) andRhamnus japonicaMaximowicz (Takahashiet al., 1993) serve as primary hosts forA. glycines. In North America,Rhamnus catharticaL.,Rhamnus alnifoliaL' Heritier andRhamnus lanceolataPursh are identified as primary hosts (Voegtlinet al., 2004; Voegtlinet al., 2005). The primary hosts play an important role in the establishment of aphid populations, since sexual aphids must produce eggs on these plants which will survive the whiter. In spring,nymphs hatch and become apterous fundatrices, which in turn produce alate viviparous females that migrate to the secondary hosts (Liu and Zhao, 2007; Wuet al.,2004).
The secondary hosts ofA. glycinesinclude cultivated soybean,Glycine max(L.) Merr, and wild soybean,Glycine sojaSieb. & Zucc (Wanget al., 1962).A. glycinescan also colonize some other species of the fabaceae family, such asMedicago sativaLinn.andPhaseolus coccineusLinn. (Hillet al., 2004).Trifolium pratenseL. It is determined thatA. glycinescan likely utilize horsenettle,Solanum carolinenseL.,as a host (Clarket al., 2006). The latest studies showed thatA. glycinescould develop and reproduce on Japanese metaplexis,Metaplexis japonica(Thunb.)Makino (Chenet al., 2015; Sunet al., 2015) and white clover,Trifolium repensL (Chenet al., 2017).
In this study,A. glycineswere fed onM. japonicaandT. repensfor three generations and their development, reproduction and body sizes were studied. These data were compared to the control fed on the known hosts,G. maxandG. soja. These newly deposited offspring by the 3rd generation were transferred back ontoG. maxand the differences in their develop-ment, reproduction and body size were also studied. It provided important information for studies on host adaptabilities ofA. glycinesonM.japonicaandT. repensand to make clear the potential that this aphid involved in host biotypes on these plants.
Materials and Methods
Aphid source and host plant
Soybean aphids were taken from a soybean field in Northeast Agriculture University (NEAU), Harbin,Heilongjiang Province, Northeast China (126.72°E, 45.74°N). The colony was maintained onG. max(variety Heinong 51), in a growth chamber at (25±1)℃, 70%±5% relative humidity and a 14L : 10D h photoperiod with artificial light of 12 000 LX. Soybeans were grown in a growth chamber at (25±1)℃with six to ten seeds per pot in 10 cm×10 cm (diameter by height) plastic pots under the same humidity and photoperiod as described above. Seedlings 15-20 cm tall at V2 growth stage (Fehr and Caviness,1997) were used for experiments.T. repens(variety Rivendel) andM. japonicawere collected from a lawn in Northeast Agricultural University (NEAU) and were transplanted into a 50 m2experiment plot. Seeds ofG. sojawere collected from a field near Limin Town,Harbin (126.61°E, 45.87°N) and were also planted in this plot. These plants ofT. repens,M. japonicaandG. sojawere used for experiments.
Nymph stage of A. glycines fed on different hosts for three generations
About 200 apterous adult aphids from the stock colony were transferred onto ten pots of soybeans(approximately 20 aphids per pot). These plants were placed in a growth chamber at (25±1)℃, 70%±5% RH and a 14 : 10 (L : D) h photoperiod for a 24-h reproduction period, after which, all the adults were removed. Using a small brush, newly deposited nymphs were individually removed from the plants.Detached leaves ofG. max,G. soja,T. repensandM. japonicawere cut into 3.0 cm diameter leaf discs using a hole-punch instrument. Solid agar media were prepared in 45 mL, 4 cm×4.5 cm (diameter×height)glass beakers. Each nymph was placed on the reverse side of a leaf disc adhered on the surface of medium.To do this procedure successfully, a small drop of water was spread onto the agar surface before the disc was pressed onto the agar medium. The beaker was then placed upside-down onto a plastic Petri dish(5 cm in diameter) (leaf disc method) (Chenet al.,2017). For each host treatment, 50 nymphs (denoted as G1cohort) were tested. When they developed into adults, 50 of the newly deposited offspring at the second day (G2cohort) were tested which were also fed on these plants, respectively. When the G2developed into adults, another 50 of their nymphs produced at the second day (G3cohort) were tested.Beakers were placed in growth chambers at (23±1)℃, 70%±5% RH and a 14 : 10 (L : D) h photoperiod.Individual nymphs in these three generation cohorts were checked daily for ecdysis and survivorship.Leaves and mediums were replaced every 5-7 days,when the old leaves became yellowish or upon observation of fungal growth.
Adult longevity and fecundity of A. glycines fed for three generations
Adults used for longevity and reproduction experiments developed from nymphs of these three generation cohorts mentioned above and were maintained in the same manner as the immature.Nymphs deposited by each female were counted and removed daily. Adult longevity was recorded daily until death of soybean aphids. Leaves and medium were replaced every 5-7 days, when leaves became yellowish or upon observation of fungal growth.
Body size of A. glycines fed on different hosts for three generations
When adults ofA. glycineswere dead in the trial on adult longevity and reproduction, they were all preserved in 95% alcohol solution. Body lengths and body widths ofA. glycineswere measured by optical microscope and micrometer. The shape ofA. glycinesbody back was regarded as standard elliptical. Body sizes ofA. glycineswere estimated by elliptic area equation: surface area=body length×body width×π/4(π≈3.14) (Chenet al., 2015).
Development, reproduction and body size of A. glycines transferred back to G. max
These newly deposited nymphs by the G3aphids in trial on development and reproduction ofA. glycinesfed for three generations were used for this study.Each 50 nymphs onT. repensandM. japonicawas transferred back toG. maxand tested at (23±1)℃,70%±5% RH and a 14 : 10 (L : D) h photoperiod using leaf disc method. Nymphs were checked daily for ecdysis and survivorship.
When these nymphs mentioned above developed into adults, their longevity and fecundity, intrinsic rate of increase and body sizes were tested. These adults were maintained in the same manner as the immature insects. Nymphs deposited by each female were counted and removed daily. Adult longevity was recorded daily, until death of soybean aphids. When these adults were dead, they were preserved in 95%alcohol solution and then their body lengths and body widths were measured by optical microscope and micrometer. Body sizes of these adults were estimated by elliptic area equation mentioned above.
Data analysis
Raw data of all the individuals fed on four species of plants for three generations were analyzed, according to the age-stage, two sex life table theory (Chi, 1988).Nymph duration, adult longevity and adult fecundity and their means and standard errors were calculated using the computer program TWOSEX-MSChart (Chi,2017). Differences of these parameters ofA. glycinesamong different hosts and generations were analyzed by analysis of variance (PROC GLM) and Tukey's honest significant difference (HSD) tests. Analyses were conducted with the SAS 8.1 (SAS Institute, 2000).Intrinsic rate of increase (r), net reproductive rate(R0), the mean generation time (T) and finite rate of increase (λ) ofA. glycinesfed on different plants for three generations were calculated, and their means,standard errors and significance of difference were estimated with the bootstrap technique (Efron and Tibshirani, 1993) included in the TWOSEX-MSChart program (Chi, 2017). Because bootstrap analysis used random resampling, a small number of replications would generate variable means and standard errors. To reduce the variability of the results, 100 000 bootstrap iterations were used. The paired bootstrap test was used to examine the differences of the life table parameters among different hosts and generations(Efron and Tibshirani, 1993).
When nymphs deposited by the 3rd generation were transferred back ontoG. max, these differences in nymph stage, adult longevity, adult fecundity and body size ofA. glycineswere analyzed byu-test(SAS Institute, 2000). Differences in intrinsic rate of increase ofA. glycinestransferred back ontoG. maxwere estimated with the bootstrap technique.
Results
Survial of A. glycines fed on different hosts for three generations
WhenA. glycineswere fed onG. max,G. soja,T. repensandM. japonicafor three generations, they all could survive well (Fig. 1).
Nymph stages of A. glycines fed on different hosts for three generations
There were significant differences in nymph stage ofA. glycinesfed on different hosts for three generations(df=11, 527;F=27.54;P<0.0001).
Nymph stage ofA. glycinesfed onT. repensfor one generation was as long as that onG. max, and it was longer than that onG. soja. Nymph stage ofA. glycinesfed onM. japonicafor one generation was longer than that onG. maxandG. soja. WhenA. glycineswere fed on these four plants for two generations, there were no significant differences among their nymph stages.Nymph stage ofA. glycinesfed onT. repensfor three generations was as long as that onG. maxandG. soja,which were all shorter than that onM. japonica(Table 1).

Table 1 Nymph stage (mean±SE) of A. glycines fed on four species of plants for three generations
Adult longevity and fecundity of A. glycines fed on different hosts for three generations
There were significant differences in adult longevity(df=11, 527;F=9.87;P<0.0001) and adult fecundity(df=11, 527;F=36.37;P<0.0001) ofA. glycinesamong different hosts and generations.
Adult longevities ofA. glycinesfed onT. repensandM. japonicafor one generation were both as long as those onG. soja, but they were shorter than those onG. max. Adult fecundity ofA. glycinesfed onT. repensfor one generation was as long as that onG. max, but it was shorter than that onG. soja. Adult fecundity ofA. glycinesfed onM. japonicafor one generation was shorter than that onG. maxandG. soja.Adult longevities ofA. glycinesfed onT. repensandM. japonicafor two generations were both as long as that onG. maxandG. soja. Adult fecundity ofA. glycinesfed onT. repensfor two generations was as big as that onG. maxandG. soja. Adult fecundity ofA.glycinesfed onM. japonicafor two generations was as big as that onG. soja, but it was smaller than that onG. max. WhenA. glycineswere fed onG. max,G. soja,T. repensandM. japonicafor three generations, there were no significant differences among their adult longevities. Adult fecundities ofA. glycinesfed onT. repensandM. japonicafor three generations were both as big as those onG. max, and they were shorter than those onG. soja(Table 2).
Life table parameters of A. glycines fed on different hosts for three generations
Intrinsic rate of increase, net reproductive rate, the mean generation time and finite rate of increase ofA. glycinesfed onG. max,G. soja,T. repensandM. japonicafor three generations are listed in Table 3. There were no significant differences in values ofr,R0andλofA. glycinesfed onT. repensandM. japonicafor one generation, which were all smaller than those onG. maxandG. soja. Values ofTofA. glycinesfed onT. repensfor one generation was as big as those onG. maxand it was bigger than that onG. soja. Values ofTofA. glycinesfed onM. japonicafor one generation was bigger than those onG. maxandG. soja. Values ofrandλofA. glycinesfed onT. repensfor two generations were both bigger than those onG. maxandG. soja. Values ofrandλofA. glycinesfed onM. japonicafor two generations were as big as those onG. soja, but they were smaller than those onG. max. Values ofR0andTofA. glycinesfed onM. japonicafor two generations were both smaller than those onG. max,G. sojaandT. repens. Values ofr,R0andλofA. glycinesfed onT.repensfor three generations were as big as those onG. max, but they were all smaller than those onG. soja. Values ofr,R0andλofA. glycinesfed onM. japonicafor three generations were all smaller than those onG. max,G. sojaandT. repens. There were no significant differences in mean generation time ofA. glycinesfed onG. max,G. sojaandT. repensfor three generations, which were all smaller than that onM. japonica.

Table 2 Adult longevity and fecundity of A. glycines fed on four species of hosts for three generations
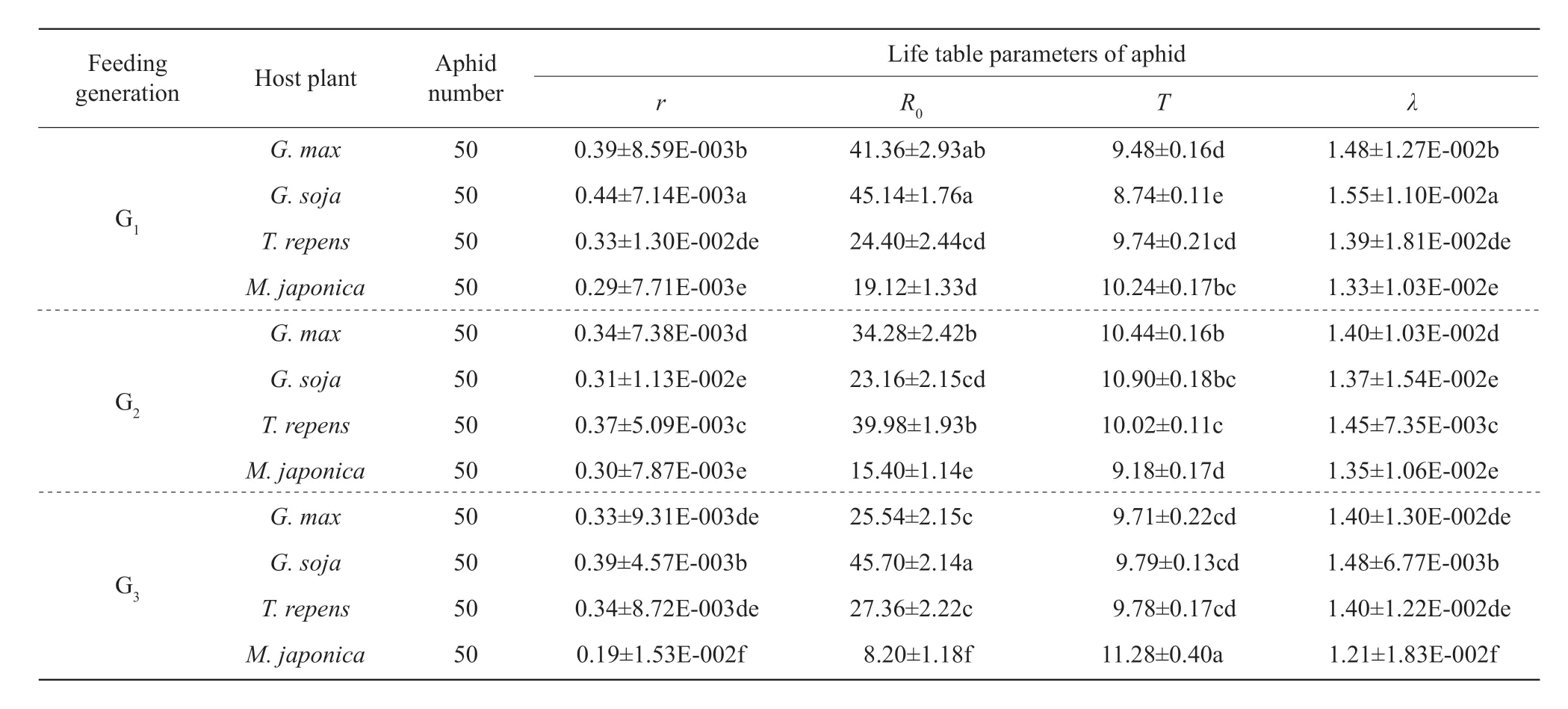
Table 3 Life table parameters (mean±SE) of A. glycines fed on four species of plants for three generations
Body size of A. glycines adult fed on different hosts for three generations
WhenA. glycineswere fed onG. max,G. soja,T. repensandM. japonicafor three generations, there were significant differences in their body sizes of adults(df=11, 432,F=27.98 andP<0.0001).
Body size ofA. glycinesadult fed onT. repensfor one generation was big as those onG. maxandG. soja.Body size ofA. glycinesadult fed onM. japonicafor one generation was as big as that onG. max, which were both smaller than that onG. soja. Body size ofA. glycinesadult fed onM. japonicafor two generations was as big as that onG. maxandG. soja, which were all smaller than that onT. repens. Body size ofA. glycinesadult fed onM. japonicafor three generations was as big as that onG. max, which were both smaller than that onG. sojaandT. repens(Table 4).
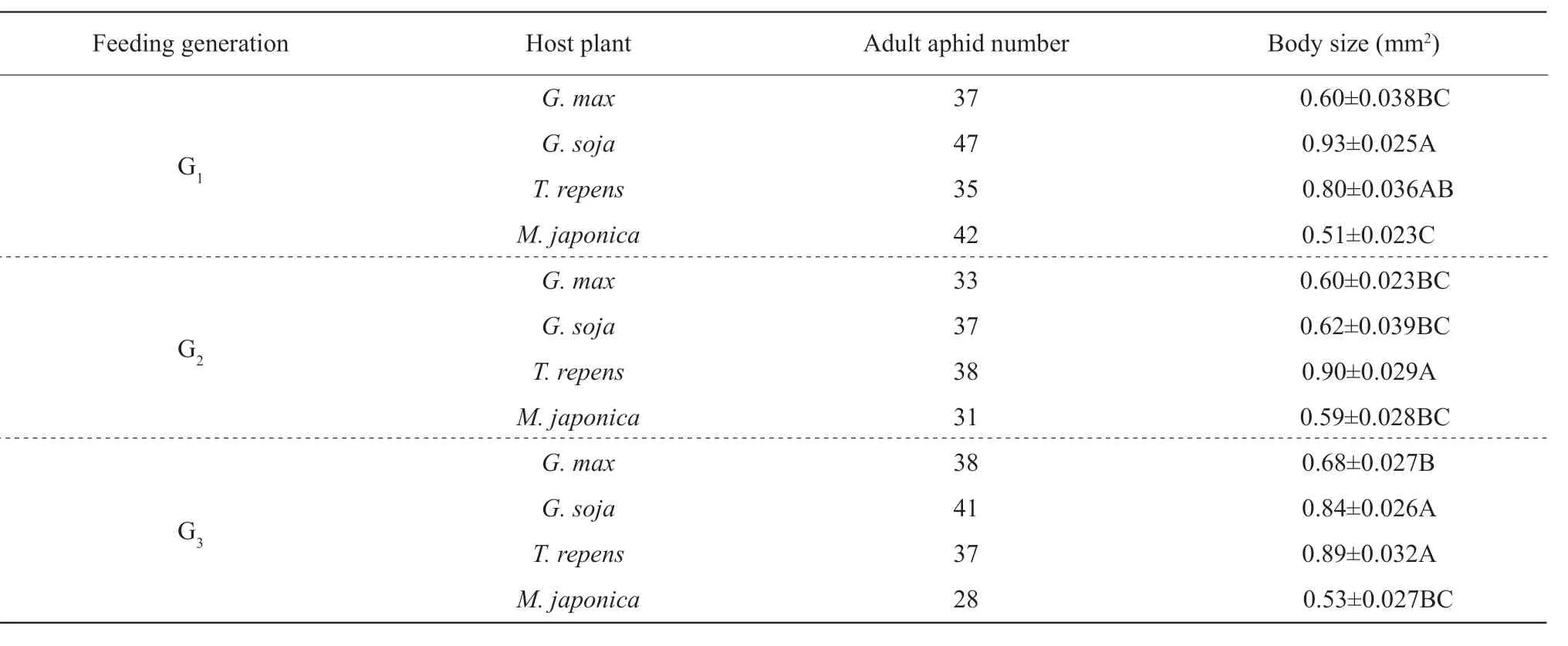
Table 4 Body sizes of A. glycines adult fed on four species of hosts for three generations
Development, reproduction, body size of A. glycines transferred back onto G. max
Soybean aphid all could survive well, when they were transferred fromT. repensandM. japonicaback ontoG. max(Fig. 2). There were significant differences in nymph stage (u=3.12,P<0.01), adult longevity(u=2.23,P<0.05), intrinsic rate of increase (paired bootstrap test,P<0.05) and body size (u=3.32,P<0.01)ofA. glycinestransferred back ontoG. max, but there were no significant differences in their adult fecundity(u=0.78,P>0.05) (Table 5).

Fig. 2 Survival rate of A. glycines transffered from T. repens and M. japonica back onto G. max

Table 5 Developmental reproductive and morphological parameters (mean±SE) of A. glycines transferred from initial plants to G. max
Discussion
In Asia, primary hosts ofA. glycineswereR. davuricuus(Wanget al., 1962) andR. japonica(Takahashiet al.,1993). The confirmed secondary hosts ofA. glycinesincludedG. maxandG. soja(Wanget al., 1962). The latest studies showed thatA. glycinescould develop and reproduce onM. japonica(Chenet al., 2015; Sunet al., 2015) andT. repens(Chenet al., 2017). These conclusions were drawn from results ofA. glycinesfed on hosts for one generation (Chenet al., 2015;2017) or a study on molecular identifying method(Sunet al., 2015). To further study host adaptability ofA. glycinesfed on these novel plants, life table ofA. glycineswas constructed and their development and reproduction were studied. It showed that nymphs ofA. glycinescould successfully develop into adults and reproduce well, when they were fed onT. repensandM. japonicafor three generations.
In northeast China,R. davuricuswas abundant in eastern and western mountainous area (Chen, 1982)and soybeans were planted in middle plain regions.Traveling from primary hosts to the secondary hosts was a long distance. IfA. glycinesfeed onT. repensorM. japonicafor a short time during the autumn migration, these plants would serve as a "halfway energy station", which would be useful, during the migration course. If this hypothesis holds true, these plants would be identified as intermediate hosts ofA. glycines. AlthoughT. repenscould not survive the cold winter in northeast China, the plant remains viable in tropical and subtropical regions. In these regions,soybean aphid could likely survive onT. repensrather thanR. davuricusduring the winter. If this hypothesis holds true,T. repenswould be identified as a winter primary host ofA. glycines. In these regions whereT. repenswas widely planted as a landscape plant, it was still unknown whetherA. glycineswould become more prominent.
WhenA. glycineswere fed onT. repensandM. japonicafor three generations, their offspring deposited by the 3rd generation could still develop and reproduce onG. max. It showed thatA. glycinesdid not involve into host biotypes on these plants after feeding three generations. But there were significant differences in some life parameters and body size ofA. glycines, when they were transferred fromT. repensandM. japonicaback ontoG. max. To further studies on soybean aphid's host adaptability, researches onA. glycinesfed onT. repensandM. japonicafor more generations were required.
In this study,A. glycineswere fed on detached leaves ofT. repensandM. japonicain the laboratory.If studies could be conducted using living plants in the field, the question, such as whetherT. repensandM. japonicawere hosts ofA. glycinesin northeast China probably could be answered.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Differential Responses of Phytophthora sojae to Seed Exudates of Host Soybean and Non-host Maize
- Identification of QTL and Analysis QTL with Tolerance to Sclerotinia sclerotiorum in Soybean
- Screening of Transgenic Soybean Materials for Salt Tolerance
- Effects of Bacillus subtilis on Degradation of Cellulose
- Effects of Dietary Fat Levels on Growth, Nutrient Digestibility,Nitrogen Utilization and Fur Quality of Growing-furring Blue Foxes
- Phylogeny and Homologous Recombination Occurring in Classical Swine Fever Viruses
