Inhibitory effects of methanolic Olea europaea and acetonic Acacia laeta on growth of Babesia and Theileria
2019-10-17AmanyMagdyBeshbishyGaberElSaberBatihaOluyomiStephenAdeyemiNaoakiYokoyamaIkuoIgarashi
Amany Magdy Beshbishy, Gaber El-Saber Batiha, Oluyomi Stephen Adeyemi, Naoaki Yokoyama, Ikuo Igarashi✉
1National Research Center for Protozoan Diseases, Obihiro University of Agriculture and Veterinary Medicine, Nishi 2-13, Inada-cho, Obihiro, Hokkaido 080-8555, Japan
2Department of Pharmacology and Therapeutics, Faculty of Veterinary Medicine, Damanhour University, Damanhour 22511, Al Beheira, Egypt
3Medicinal Biochemistry, Infectious Diseases, Nanomedicine and Toxicology Laboratory, Department of Biochemistry, Landmark University, Omu-Aran 251101, Kwara State, Nigeria
ABSTRACT Objective: To evaluate the antipiroplasmic activities of methanolic extract of Olea europaea(MOE) and acetonic extract of Acacia laeta (AAL) against Babesia and Theileria parasites in vitro and evaluate the chemotherapeutic effects of these extracts against Babesia (B.) microti in vivo.Methods: Fluorescence assay using SYBR Green 1 nucleic acid stain was used to detect inhibitory effects of the two extracts as well as the combination effects of the two extracts with diminazene aceturate and atovaquone on four Babesia species and Theileria equi in vitro while for in vivo experiments, 8-weekold female BALB/c mice were injected intraperitoneally with 1× 107 B. microti-iRBCs and treated orally at a dose of 150 mg/kg of both extracts.Results: The half maximal inhibitory concentration (IC50) values of AAL against B. bovis, B.bigemina, B. divergens, B. caballi, and Theileria equi were lower than those of MOE extracts.Toxicity assay on Madin-Darby bovine kidney, mouse embryonic fibroblast (NIH/3T3), and human foreskin fibroblast cell lines showed that MOE and AAL affected only the viability of Madin-Darby bovine kidney cell line with half maximal effective concentrations (EC50) of (794.7±41.9) and (873.9±17.5)µg/mL, respectively. The oral treatments of MOE and AAL at 150 mg/kg inhibited the growth of B. microti in mice by 80.4% and 64.4%, respectively. The MOE and diminazene aceturate combination showed a higher chemotherapeutic effect than that of monotherapy.Conclusions: MOE and AAL have the potential to be an alternative remedy for treating piroplasmosis. Furthermore, the combination therapy of MOE + DA was more potent against B.microti infection in mice than their monotherapies.
Keywords:Olea europaea Acacia laeta Babesia Theileria In vitro In vivo Inhibition
1. Introduction
Piroplasmosis is a tick-borne disease caused by hemoprotozoan parasites of the genus Babesia and Theileria[1]. Babesia (B.)bigemina, B. bovis, and B. divergens infect cattle and cause bovine babesiosis; however, B. bovis is more pathogenic than B. divergens and B. bigemina. Clinical symptoms of bovine babesiosis are high fever, ataxia, anorexia, general circulatory shock, hemoglobinuria,and hemolytic anemia[2], while B. caballi and Theileria (T.) equi infect horses and donkeys and cause equine piroplasmosis. The symptoms of equine piroplasmosis range from acute fever to anemia,jaundice, and sudden death[3]. B. microti and B. divergens mainly affect rodents and cattle, respectively, and sometimes infect humans,causing human babesiosis[4,5].
The search for safer and more effective chemotherapeutic agents against Babesia and Theileria is urgently needed due to toxic effects,limited efficacy of diminazene aceturate (DA) and imidocarb dipropionate and the appearance of drug-resistant parasites[6,7].Furthermore, some Plasmodium strains and Babesia gibsoni showed resistance towards atovaquone (AQ)[8,9].
The traditional medicine system based on the use of medicinal plants continues to play an important role in the health care system.In the last decade, traditional medicine has become popular and alternative to currently available antiparasitic drug candidates in many developing countries, partly due to the long unsustainable economic situation in these countries, the high cost of drugs and increased drug resistance to common diseases such as malaria and bacterial infections[10].
Olea (O.) europaea is a small tree species in the Family Oleaceae.The health properties of components of this polyphenolic plant and their potential use as natural food additives have been the subject of great scientif i c and commercial interest[11]. The leaf extracts of O.europaea have been reported to have anti-Plasmodium activity[12],antimicrobial[13] and anti-HIV, vasomotor, and hypoglycemic effects[14]. The major pharmacological molecules of O. europaea leaf are hydroxytyrosol, tyrosol, caffeic acid, p-coumaric acid, vanillic acid, vanillin, oleuropein, luteolin, diosmetin, rutin, verbascoside,luteolin-7-glucoside, apigenin-7-glucoside, and diosmetin-7-glucoside[15].
Acacia (A.) laeta is a perennial shrub belonging to the Leguminosae Family. Several pharmacological molecules have been isolated from different species of the genus Acacia. The leaves, bark, pods, and root of Acacia plants contain the highest amount of tannin, as well as polyphenolic compounds such as dicatechin, quercetin, gallic acid, robidandiol, hentriacontane, sitosterol, betulin, β-amyrin,kaempferol-3 glucoside isoquercetin, and chlorogenic acid[16].Acacia extracts have been reported for their antioxidant effects, their cytotoxicity against the MCF-7 breast cancer cell line[17], and their antimalarial effect[18]. Isorhamnetin 3-o-neohesperidoside isolated from A. laeta leaves protected the cells against oxidative stress by inhibiting xanthine oxidase and scavenged a superoxide anion with high inhibitory effects against Klebsiella oxytoca, Staphylococcus aureus, and Klebsiella pneumoniae strains[19].
The aforementioned pharmacological properties of O. europaea and A. laeta motivated us to investigate the antipiroplasmic activity of their extracts against the growth of B. bovis, B. bigemina, B.divergens, B. caballi and T. equi using in vitro cultures. Furthermore,we evaluated the chemotherapeutic effect of the two extracts on B.microti infection in mice.
2. Materials and methods
2.1. Chemicals and reagents
To obtain methanolic extract of O. europaea (MOE) and acetonic extract of A. laeta (AAL) extracts, 99.8% methanol, dimethyl sulfoxide(DMSO) (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and 99.5% acetone (Nacalai Tesque, Kyoto, Japan) were used to prepare stock solutions of 100 mg (crude extract)/1 mL DMSO. DMSO was used to prepare stock solutions of 10 mM of DA (Ciba-Geigy Japan Limited, Tokyo, Japan) and AQ (Sigma-Aldrich Japan, Tokyo, Japan).The SYBR Green 1 nucleic acid stain (10 000×; Lonza America,Alpharetta, Georgia, USA) was stored at -20 ℃ and thawed before use.Tris HCl (130 mM; pH 7.5), 10 mM ethylenediaminetetraacetic acid,saponin (0.016%; W/V), and TritonX-100 (1.6%; V/V) were used to prepare the lysis buffer and stored at 4 ℃ for future use.
2.2. Plant extracts
O. europaea and A. laeta leaves were obtained from Delta, North part of Egypt. Identif i cation was conf i rmed and voucher specimen numbers were placed by the members of the Department of Botany and Department of Pharmacology and Chemotherapeutics, Faculty of Science and Faculty of Veterinary Medicine, Damanhour University,Egypt. The voucher specimen numbers of O. europaea [L. Sp. PI.: 8(1753), subsp. europaea, var. europaea, Family Oleaceae] and A.laeta (Acacia laeta R.Br. ex Benth., Family Fabaceae) are A0177101(DPV) and A 0177102 (DPV), respectively. The preparation of the crude extracts were carried out following the method as previously described in our earlier publication[20,21] and the obtained extracts were weighed and the stock solution was prepared by adding 1 mL of DMSO/100 mg of the extract, stored at -30 ℃ to be used in the future.
2.3. Parasites
Bovine and equine parasites were cultivated in purif i ed bovine and equine red blood cells (RBCs) using a microaerophilic stationary phase culture system[22]. Medium 199 was used for B. bovis (Texas strain), B. bigemina (Argentine strain) and T. equi (USDA strain)cultivation, while GIT medium (Sigma-Aldrich, Tokyo, Japan) was used for B. caballi (USDA strain) cultivation. RPMI 1640 medium(Sigma-Aldrich, Tokyo, Japan) was used for B. divergens (Germany strain) cultivation. The media were supplemented with 40% cattle or horse serum and 60 µg/mL of streptomycin, 0.15 µg/mL of amphotericin B, and 60 U/mL of penicillin G (Sigma-Aldrich, St.Louis, MO, USA). For T. equi culture, 13.6 µg/mL of hypoxanthine(MP Biomedicals, Santa Ana, CA, USA) was added as a vital supplement. For in vivo experiments, B. microti (Munich strain)[23]was used to infect 8-week female BALB/c mice (CLEA, Japan).
2.4. Ethics approval
The experiments described in this study were conducted according to the rules of care and animal use in research published by Obihiro University of Agriculture and Veterinary Medicine, Japan. The protocol was approved by the Animal Experimentation Ethics committee at Obihiro University of Agriculture and Veterinary Medicine (animal experiment accession number: 29-016-8).
2.5. Evaluation of the effect of MOE and AAL extracts on cattle and horse RBCs
The hemolytic effect of MOE and AAL extracts on RBCs was determined as described previously[24]. Briefly, the two extracts at 400 µg/mL were used to treat fresh cattle and horse RBCs for 3 h at 37 ℃. The treated RBCs were then used to culture bovine and equine parasites and Giemsa-stained blood smears were prepared daily to determine the parasitemia in treated and untreated RBCs.
2.6. In vitro growth inhibitory effects
In three separate trials, the growth-inhibitory effects were conducted via fluorescence assay in accordance with the previously described protocol[24,25]. The infected RBCs (iRBCs) were diluted with noninfected RBCs to obtain a stock supply of RBCs with 1%parasitemia. In 96 well plates, 2.5 µL (for B. bovis and B. bigemina)or 5 µL (for B. divergens, B. caballi and T. equi) of iRBCs was added to each well in triplicate and mixed with culture medium containing the indicated concentrations, 0.24, 1.2, 6, 30 and 150 µg/mL for MOE and AAL extracts or 0.002 5, 0.012, 0.025, 0.051, 0.25, 0.5 and 1.1 µg/mL for AQ and DA to a total volume 100 µL[20]. The positive control wells containing iRBCs, and DMSO (0.3%), while the wells with noninfected RBCs were used as a negative control.Afterward, the plates were incubated at 37 ℃ for four successive days and on day four, 100 µL of lysis buffer containing 2 × SYBR Green 1 was added to each well. The fluorescence signals were evaluated using a fluorescence plate reader (Fluoroskan Ascent;Thermo LabSystems, Oceanside, California, USA) at excitation and emission wavelengths of 485 and 518 nm, respectively. Gain values were set to 100.
Last year I was put into a lower-level math class at school. The reason I was in this class had nothing to do with my intellect or math skills. I am blind. The school decided1 that it would be better for me to learn at a lower level because it takes me a great deal longer to complete assignments and grasp visual concepts.
2.7. Viability test and morphological changes
Microscopy assay was used to evaluate the viability of parasites treated with MOE and AAL extracts. In three separate trials, 200µL of media containing 0.25×, 0.5×, 1×, 2×, and 4× of the IC50of the two extracts and 20 µL of iRBCs at 1% parasitemia were added to each well in triplicate and cultivated by changing the media daily for four successive days. After 96 h, in new 96-well plates, 6 µL of iRBCs was mixed with 14 µL of fresh RBCs and supplemented with 200 µL drug-free medium. The plates were then incubated at 37 ℃ for an additional eight days. The parasite growth and the morphological changes were detected by microscopy as previously described[24],and micrographs were captured using Nikon Digital Sight® (Nikon Corporation, Tokyo, Japan).
2.8. Cell cultures
Madin-Darby bovine kidney (MDBK), human foreskin fibroblast(HFF), and mouse embryonic fibroblast (NIH/3T3) cell lines were cultured at 37 ℃ in a humidified incubator with 5% CO2. In 75 cm2culture flasks, Minimum Essential Medium Eagle (Gibco, Grand Island, NY, USA) was used to maintain MDBK cell line, while HFF and NIH/3T3 cell lines were maintained in Dulbecco’s Modified Eagle’s Medium (Gibco). Each medium was supplemented with 0.5% penicillin/streptomycin (Gibco), 1% glutamine and 10%fetal bovine serum. 4, 6-diamidino-2-phenylindole dihydrochloride(Sigma-Aldrich Corp., St. Louis, MO, USA) was added to the cells to ensure free mycoplasma contamination. Afterward, the cells were washed twice with Dulbecco’s phosphate-buffered saline and TrypLETM Express (Gibco) was added to allow the cell detachment from the culture flask. Finally, a Neubauer improved C-Chip(NanoEnTek Inc., Seoul, Korea) was used for counting the viable cells after staining with 0.4% trypan blue solution.
2.9. Cytotoxicity assay of MOE and AAL extracts on MDBK,NIH/3T3 and HFF cell lines
The drug-exposure viability assay was conducted according to the previously described protocol[21]. Briefly, 100 microliters of cells were added to each well of 96-well plates at a density of 5 × 104cells/mL and incubated for 24 h at 37 ℃ in a humidified incubator with 5% CO2. Ten microliters of twofold drug dilutions were added in triplicate to a final concentration of 15.8 to 1 500 µg/mL for the MOE and AAL extracts and 100 µM for DA and AQ and the plates were then incubated for another 24 h. The wells containing culture medium were used as a negative control, while the wells containing cells with 0.4% DMSO were used as a positive control. In the next day, 10 µL of Cell Counting Kit-8 (CCK-8) was added, and the plates were further incubated for 3 h. The absorbance at 450 nm was measured using a microplate reader.
2.10. In vitro effect of combination treatment
The combination assay was performed using the fluorescence assay as described previously[24]. Briefly, in a 96-well plate, five selected concentrations: 0.25×, 0.5×, 1×, 2×, and 4× the IC50of MOE and AAL extracts were combined with DA and AQ at a constant ratio (1:1)[26] and examined against the in vitro culture of B.bovis, B. bigemina, B. divergens, B. caballi and T. equi. The degree of synergism was determined as the weighted average of combination index (CI) values using a formula [(1×IC50)+(2×IC75)+(3×IC90)+(4×IC95)/10] and the results were presented using the reference combination index scale: <0.90 (synergism), 0.90-1.10 (additive),and >1.10 (antagonism). Each experiment was repeated in three separate trials.
2.11. Chemotherapeutic effects of MOE and AAL extracts on B. microti in mice
The growth inhibition of the two extracts was also evaluated against B. microti infection in mice as described previously[24]. Twentyf i ve BALB/c mice were housed under a pathogen-free environment with controlled temperature (22 ℃) and humidity and a 12 h light/dark cycle and divided equally into fi ve groups. Four of the groups were injected intraperitoneally with 1 × 107B. microti-iRBCs, while one group was left uninfected to act as the negative control. When the parasitemia in the infected mice reached 1%, the mice were injected daily with each specif i c drug for fi ve days. MOE and AAL extracts were administrated orally at a dose of 150 mg/kg to the fi rst and second groups, respectively. While, the third and fourth groups were intraperitoneally (i.p.) injected with 25 mg/kg of DA and double distilled water, respectively. To validate the efficacy of the combination of MOE with DA and AQ in vivo, thirty BALB/c mice were divided into six groups and inoculated intraperitoneally with 1 × 107B. microti-iRBCs. Another group consisting of fi ve mice was left uninfected and untreated to act as negative control. The fi rst and second groups were administered an i.p. injection of double distilled water and 25 mg/kg of DA, respectively, while the third and fourth groups received an oral administration of 20 mg/kg and 150 mg/kg of AQ and MOE, respectively. The fi fth and sixth groups were treated with a combination of MOE + DA (75 mg/kg +12.5 mg/kg) and MOE + AQ (75 mg/kg +10 mg/kg), respectively, via a route similar to that for the single drug. The levels of parasitemia in all mice were detected daily by Giemsa-stained thin blood smears prepared from venous tail blood every 48 h until 40 days post-infection (p.i.). The hematocrit (HCT) percentage, hemoglobin (HGB) and RBCs count were monitored every 96 h and measured using a Celltac α MEK-6450 automatic hematology analyzer (Celltac α MEK-6450, Nihon Kohden, Japan). Each experiment was repeated twice.
2.12. Genomic DNA extraction and PCR detection of B.microti
A nested PCR (nPCR) that targeted the B. microti small-subunit rRNA (ss-rRNA) gene was performed in accordance to the previously described protocol[24] and the genomic DNA was extracted from the blood samples collected from all mice groups on day 40 p.i. using a QIAamp DNA Blood Mini Kit (Qiagen, Tokyo, Japan). PCR cycling conditions were performed as previously described[24] and the bands were considered positive with an expected size of 154 bp.
2.13. Statistical analysis
3. Results
3.1. In vitro growth inhibition
The MOE (Figure 1A) and AAL (Figure 1B) extracts inhibited the growth of all tested species in a dose-dependent manner. The IC50sof MOE and AAL extracts on B. bovis, B. bigemina, B. divergens,B. caballi, and T. equi were (107.1±9.2), (47.7±2.3), (101.1±8.9),(105.5±11.1), (19.3±2.1) µg/mL, and (3.8±0.1), (3.3±0.7), (4.3±3.7),(11.1±2.1), and (1.5±0.1) µg/mL, respectively. The IC50sof AAL on B. bovis, B. bigemina, B. divergens, B. caballi, and T. equi were lower than those of MOE extracts (Table 1). DA and AQ inhibited the growth of all tested parasites with IC50values shown in Supplementary Table 1. The drug efficacy was not influenced by the concentration of the diluent since there was no signif i cant difference in the inhibition observed between the positive control well containing the DMSO and untreated wells. The precultivation of cattle and horse RBCs with the two extracts was performed to evaluate its direct effects on the host RBCs. No significant differences were observed between the extracts-treated cattle and horse RBCs and the non-treated RBCs (data not shown).
3.2. Viability assay and morphological changes of treated parasites
A viability assay was performed to determine the concentration of the extracts that could completely clear the parasites after the treatment of successive four days followed by the withdrawal of the drug pressure. B. bovis, B. bigemina, B. divergens, B. caballi, and T.equi treated with MOE could not regrow at concentrations of 4× IC50(428.4, 190.8, 404.4, 422.0, and 77.2 µg/mL, respectively). The AAL extract suppressed the growth of B. bovis, B. caballi, and T. equi at 4× IC50(15.2, 44.4, and 6.0 µg/mL, respectively), while B. bigemina and B. divergens could regrow even at 4× IC50(Supplementary Table 2).The morphological changes of B. bovis, B. bigemina, B. divergens,B. caballi and T. equi treated with MOE and AAL extracts were observed in Giemsa-stained blood smears. Micrographs were taken of B. bovis (Figure 2A) and B. divergens (Figure 2B) treated with MOE at 428.4 and 404.4 µg/mL, respectively and AAL extracts at 13.2, 15.2, and 34.4 µg/mL, respectively. Abnormally dividing parasites at 24 h were observed as compared to the piriform shape of normal B. bovis and B. divergens, while drug-treated cultures showed higher numbers of degenerated parasites than did the control cultures at 72 h.
3.3. Cytotoxicity of MOE and AAL extracts on MDBK,NIH/3T3 and HFF cell lines
The toxic effects of MOE and AAL extracts on the host were evaluated on MDBK, NIH/3T3, and HFF cell lines. The EC50sof MOE extract on MDBK, NIH/3T3, and HFF cell lines were (794.7±41.9),>1 500, and >1 500 µg/mL, respectively. The EC50sof AAL extracts on MDBK, NIH/3T3, and HFF cell lines were (873.9±17.5), >1 500, and >1 500 µg/mL, respectively. In a separate assay, DA and AQ at a concentration of 100 µM did not show any inhibition of MDBK, NIH/3T3, or HFF cell viability (Supplementary Table 1). The selectivity indexes of the two extracts, defined as the ratio of cell line EC50to the parasite IC50, are shown in Table 1.

Table 1. IC50 and selectivity index of methanolic Olea europaea and acetonic Acacia laeta extracts.
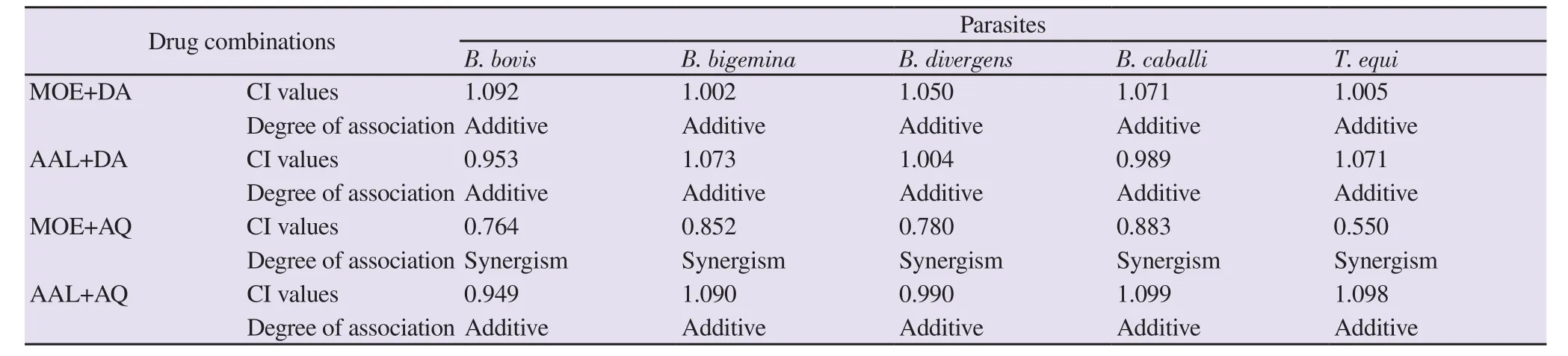
Table 2. Effect of combinations of methanolic Olea europaea and acetonic Acacia laeta with diminazene aceturate and atovaquone against Babesia and Theileria parasites in vitro.
3.4. Combination treatment in vitro
The combination effect of MOE and AAL extracts with DA and AQ against B. bovis, B. bigemina, B. divergens, B. caballi, and T. equi was determined in vitro. The MOE extract or AAL extract+DA combined treatments showed an additive relationship against tested species.The combination effects of MOE or AAL and AQ were found to be synergetic and additive on tested parasites, respectively (Table 2).
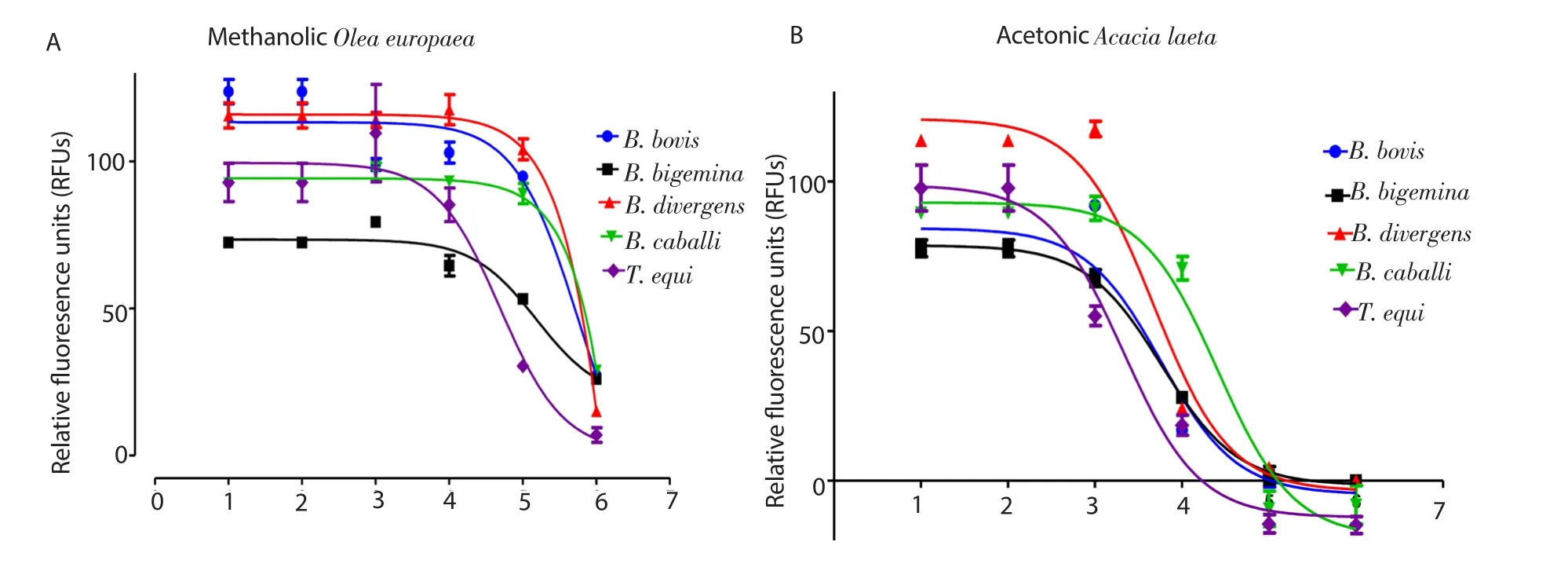
Figure 1. Correlation between relative fl uorescence units and the log concentrations of methanolic Olea europaea (nM) (A) and acetonic Acacia laeta (B) on Babesia and Theileria parasites. The results were determined by fl uorescence assay after 96 h of incubation.

Figure 2. Morphological changes in Babesia bovis (A) and Babesia divergens (B) treated with methanolic Olea europaea (MO) and acetonic Acacia laeta (AA).Light micrographs in an in vitro culture were taken after 24 (a) and 72 h (b). The arrows show abnormally dividing parasites observed at 24 h as compared to the piriform shape of normal control parasite (c), while drug-treated cultures showed higher numbers of degenerated parasites than did control cultures at 72 h.
3.5. Chemotherapeutic effect of MOE and AAL extracts on B.microti in mice

Figure 3. Effects of methanolic Olea europaea and acetonic Acacia laeta on Babesia microti in vivo. (A) Growth inhibition and (B) changes in hematocrit (HCT)percentages of MOE- and AAL on Babesia microti-treated mice. The arrow indicates fi ve consecutive days of treatment. Asterisks(*) indicate statistically signif i cant (P<0.05) differences of parasitemia between treated groups and the untreated control group based on unpaired t-test analysis.

Figure 4. Growth inhibition of MOE combinations on Babesia microti in vivo. The arrow indicates fi ve consecutive days of treatment. Asterisks(*) indicate statistically signif i cant (P<0.05) differences of parasitemia between treated groups and the untreated control group based on unpaired t-test analysis.
The inhibitory effect of MOE and AAL extracts were further evaluated against B. microti in mice. In the treated groups, the level of parasitemia increased at a signif i cantly lower percentage[ANOVA; F(1.313, 8.363)=5.252, P<0.001 for MOE-treated; and ANOVA; F(0.484, 7.652)=3.713, P<0.001 AAL-treated group] than in the control group from day 6 to 12 p.i. Peak parasitemia levels in the monotherapy-treated groups were 12.3%, 22.3%, 5.0%,and 5.4% on day 8 in 150 mg/kg MOE extract, 150 mg/kg AAL,25 mg/kg DA, and 20 mg/kg AQ, respectively, as compared with 62.6% peak parasitemia in the control group (Figure 3A). Parasitemia was undetectable by microscopic examination in mice treated with 25 mg/kg DA, 20 mg/kg AQ, 150 mg/kg MOE and 150 mg/kg AAL starting on days 14, 18, 24 and 29 p.i., respectively. A comparison of HCT percentage showed signif i cantly a higher HCT percentage in the MOE and AAL extract-treated groups [ANOVA; F(0.541,4.490)=4.283, P=0.001] than in the infected-untreated group(Figure 3B). In the combination chemotherapy-treated groups,the peak parasitemia level reached 12.8% and 17% in 75 mg/kg MOE+12.5 mg/kg DA on day 8 and 75 mg/kg MOE+10 mg/kg AQ on day 12, respectively (Figure 4). Furthermore, parasitemia was undetectable in mice on days 17 and 21 p.i. with MOE+DA and MOE+AQ, respectively, upon microscopic examination. A comparison of the HGB count [ANOVA; F(1.282, 5.684)=7.719,P<0.001], HCT percentage [ANOVA; F(2.193, 4.923)=6.86,P<0.001) and RBCs count (ANOVA; F(1.882, 6.54)=8.323,P=0.008] showed signif i cant increases in the MOE, MOE + DA, and MOE+AQ-treated groups as compared with the infected-untreated group (Figure 5A-C). The parasite DNA was not detected in 25 mg/kg DA, 75 mg/kg MOE+12.5 mg/kg DA on day 40 (Figure 6).

Figure 5. Changes in the hemoglobin (HGB) (A), hematocrit (HCT) (B) and red blood cells count (RBCs) (C) in MOE-treated mice. The arrow indicates fi ve consecutive days of treatment. Asterisks(*) indicate statistical signif i cance (P<0.05) based on unpaired t-test analysis.

Figure 6. Molecular detection of parasites DNA in the blood of treated groups. The distillate water (DDW) was used as negative control and M is for the marker. The arrow shows the expected band length of 154 bp for positive cases of Babesia microti.
4. Discussion
Current treatment options for bovine and equine piroplasmosis showing various problems, including the emergence of parasite resistance as well as unwanted side effects on treated animals[27].Additionally, Lemieux et al. documented medicinal failures in some severe human cases infected with human babesiosis after azithromycin, and AQ administration[28]. Therefore, newer and safer antibabesial compounds are urgently required.
The current study showed that MOE and AAL extract have potent growth-inhibitory effects against Babesia and Theileria parasites.Since Babesia and Plasmodium parasites belong to the same Apicomplexa phylum, our results are consistent with the fi ndings of ahin and Bilgin, who demonstrated the therapeutic efficacy of O. europaea extract for treating fever and some tropical diseases such as malaria[15]. Additionally, they revealed that the medicinal activity of O. europaea is due to the presence of unique bioactive compounds, namely phenolics, tocopherols, and carotenoids[15].In the like manner, Deshmukh et al. documented that Acacia extract has suppressive activity as well as curative and prophylactic effects against chloroquine-sensitive Plasmodium berghei NK-65-infected mice and its antiplasmodial effect has been attributed to the presence of many phytochemicals, such as alkaloids, terpenes, and fl avonoids[29]. Taken together, it is likely that the growth-inhibiting effect of the two extracts might be due to more than one bioactive ingredient acting in synergy to restrict Babesia and Theileria parasites growth. A viability assay showed that MOE and AAL extracts eliminated the regrowth of Babesia and Theileria parasites.This fi nding is compatible with an earlier report that showed the appreciable reduction and clearance of Plasmodium parasites after treatment with Acacia extract[29].
Morphological changes suggested that MOE and AAL extracttreated parasites were unable to egress and, subsequently, died within the iRBCs. The possible explanation is that Babesia and Theileria utilized opposite actions against MOE and AAL extract as the parasites continued to transform and grow leading to malformed merozoites, which eventually caused parasite damage. Moreover,further damage and death of MOE and AAL extract-treated parasites resulted from maintaining the drug pressure. This is consistent with previous studies that revealed that the organic extract of Acacia had potent antiparasitic activity against Plasmodium falciparum and Leishmania parasites[30].
Cytotoxic study showed that MOE and AAL affected only the viability of the MDBK cell line with a higher selectivity index. This suggests that MOE and AAL extracts’ bioactive ingredients were more likely to affect Babesia and Theileria than the host cells. This fi nding is compatible with Song et al. who showed that O. europaea extract reduced PGE2 production without exhibiting any cytotoxic activity on RAW264.7 and HEK293 cells[31]. Furthermore, the safety of Acacia extracts was demonstrated in the Vero cell line[32].The combination effects of MOE and AAL extract with DA and AQ in vitro showed additive and synergetic effects against all tested parasites. Together, these fi ndings suggest that these extracts could be considered as a potential candidate for chemotherapy against piroplasmosis.
The inhibitory effect of MOE and AAL was also evaluated against B. microti infection in mice as well as the combination effect of MOE with DA or AQ. The effectiveness of MOE and AAL extract at a dose of 150 mg/kg was comparable to that shown by DA and did not reveal any apparent toxic symptoms in mice. Interestingly,the combination of MOE+DA showed a higher efficiency against B.microti in mice than did the single treatment.
For analyzing the presence of B. microti DNA, PCR assay was performed on blood samples collected on day 40 p.i. Interestingly,the present study conf i rmed the absence of B. microti DNA in MOE+ DA combination-treated groups, which conf i rm the importance of the combination treatment. The chemotherapeutic effects produced by the two extracts on B. microti not only support their antibabesial action but also give credence to their medicinal prospects by identifying and characterizing the bioactive compounds that are responsible for their antibabesial effect.
The MOE extract treatment resulted in exceptional and robust hematological parameters in mice. Interestingly, Şahin and Bilgin and Alajmi et al. documented the remarkable antioxidant activity and anti-inf l ammatory properties after treatment with O. europaea extracts and A. laeta polyphenols[15,19]. Such curative properties are desirable in new antiparasitic agents because Babesia and Theileria infections are correlated with the overproduction of reactive oxygen and nitrogen species, resulting in oxidative stress[33]. In conclusion,the present study documents for the fi rst time that MOE and AAL crude extracts restrict the growth of Babesia and Theileria parasites in vitro and in vivo. Further studies are required to isolate and identify the active principle(s) responsible for the observed antiparasitic action. They may be considered lead compounds in the search for effective and novel antibabesial drugs.
Conflict of interest statement
The authors declare no conf l icts of interest.
Acknowledgments
We would like to thank Dr. Atia Mohamed, Department of plant,Faculty of science, Damanhour University, Egypt for helping in plant identif i cation. Furthermore, the authors would like to thank Dr.Makoto Igarashi and Ms. Nthatisi Innocentia Molefe for assisting with the culture of MDBK, NIH/3T3, and HFF cell lines.
Foundation project
This study was supported by the Japan Society for the Promotion of Science (JSPS) (KAKEN Grant Number: 18H02337).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Curcuma angustifolia ameliorates carbon tetrachloride-induced hepatotoxicity in HepG2 cells and Swiss albino rats
- Chemoprotective activity of aqueous leaf extract of Acalypha wilkesiana against cyclophosphamide-induced toxicity in rats
- Association between measles antibodies in vaccinated and naturally infected mothers with protective antibodies and the occurrence of measles in their children: A cross-sectional study in the Bavi district of Hanoi
- Long-term safety follow-up of children from a randomized-controlled phaseⅡb proof-of-concept efficacy study of the live, attenuated,tetravalent dengue vaccine (CYD-TDV) in Thailand
- Ebola virus disease: Recent advances in diagnostics and therapeutics
