Chemoprotective activity of aqueous leaf extract of Acalypha wilkesiana against cyclophosphamide-induced toxicity in rats
2019-10-17ChineduAnokwuruGodswillAnyasorOlutayoShokunbiBabajideSopekanOyindamolaOsinugaOlusolaAfolabiOlamideArojojoyeChibunduEzekielIsaiahDIRamaite
Chinedu P. Anokwuru, God'swill N. Anyasor, Olutayo S. Shokunbi, Babajide Sopekan,Oyindamola K. Osinuga, Olusola E. Afolabi, Olamide B. Arojojoye, Chibundu N. Ezekiel,Isaiah DI. Ramaite
1Department of Basic Sciences, School of Science and Technology, Babcock University, Ilishan Remo, Ogun State, Nigeria
2Department of Chemistry, School of Mathematical and Natural Sciences, University of Venda, Thohoyandou South Africa
3Department of Biochemistry, Benjamin S. Carson (Snr) School of Medicine, College of Health and Medical Sciences, Babcock University, Ilishan Remo, Nigeria
4Department of Microbiology, School of Science and Technology, Babcock University, Ilishan Remo, Nigeria
ABSTRACTObjective: To investigate the protective effect of aqueous leaf extract of Acalypha (A.)wilkesiana Muell. Arg (Euphorbiaceae) against cyclophosphamide-induced toxicity in albino rats.Methods: Twenty male albino rats were randomly divided into fi ve groups of four animals each. The control group (Ⅰ) was fed with pellets and distilled water, while groupⅡwas orally administered with only 20 mg/kg cyclophosphamide. Groups Ⅲ, Ⅳ andⅤwere coadministered with 20 mg/kg body weight cyclophosphamide and 110, 220 and 440 mg/ kg body weight A. wilkesiana leaf extract, respectively, for 7 d. After treatment, liver and kidney function biomarkers, haematological parameters, liver antioxidants, and mitochondrial membrane permeability transition pore opening were investigated.Results: A. wilkesiana leaf extract signif i cantly reduced (P<0.05) cyclophosphamide-induced increase in plasma aspartate aminotransferase, alanine aminotransferase, creatinine, uric acid and urea. It increased superoxide dismutase, catalase, glutathione-S-transferase activities and reduced glutathione levels. It also increased packed cell volume count, hemoglobin concentration and white blood cell count while inhibiting the induction of mitochondrial swelling.Conclusions: This study demonstrates that aqueous extract of A. wilkesiana leaf protected tissues against cyclophosphamide-induced oxidative damage.
Keywords:Medicinal plants Oxidative stress Chemoprotective activity Cyclophosphamide Acalypha wilkesiana
1. Introduction
Cyclophosphamide [N,N-bis(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorin-2-amine 2-oxide] is a cytotoxic alkylating drug used for the treatment of cancer. It is also used to suppress the immune system during bone marrow transplant[1,2].Cyclophosphamide is a prodrug that is metabolized into phosphramide mustard and acrolein in the liver. Phosphramide mustard is responsible for the therapeutic effect of cyclophosphamide while acrolein is toxic and responsible for the side effect[1,3]. These metabolites generate reactive species during metabolism resulting in oxidative stress[4]. These reactive species also induce mitochondrial membrane permeability transition in normal rat cells resulting in apoptosis[5].
Medicinal plants are known to possess chemoprotective activity against toxic substances. For example, aqueous ethanol extract ofCapparis spinoseleaf displayed protective effect against cyclophosphamide-induced nephrotoxicity in mice[6] whilst ethanol extract ofEquisetum arvenseameliorated cyclophosphamide induced genotoxicity in mice[7].
Acalypha (A.) wilkesianaMuell. Arg. (Euphorbiaceae), commonly called “copper plant”, is commonly used for the treatment of cold,headache, malaria, dermatological and gastrointestinal disorders,breast tumors and inf l ammation[8]. A few chemical compounds have being isolated or identif i ed. Onyeike et al.[9] reported the presence of three fl avonoids, fi ve carotenoids and 12 simple terpenes in A.wilkesianawhilst gallic acid, shikimic acid, corilagin, geraniin and quadrangularic acid M have been isolated from A.wilkesianaleaf[10,11] and corillagin has been identif i ed as the main antioxidant agent. There are reports on some protective effect of medicinal plants against cyclophosphamide induced toxicity in tissues[12,13], however,there is no known report available on the protective effect of A.wilkesianaleaf extract against cyclophosphamide-induced toxicity.Ikewuchiet al.[14] in a related study reported the hepatoprotective effect of aqueous leaf extract of A.wilkesianaagainst carbon tetrachloride induced liver damage in rats. This study therefore was designed to evaluate the potentials of aqueous leaf extract ofA. wilkesianato protect against cyclophosphamide-induced tissue damage.
2. Materials and methods
2.1. Chemicals, reagents and instrumentation
Cyclophosphamide (Topway Pharamceuticals, Nigeria), Hepes(May and Baker Lab; USA), KOH (Sigma Aldrich; USA), Mannitol(BDH Chemicals Ltd; England), EGTA (Sigma Chemical Co; USA),Bovine Serum Albumin (Sigma Aldrich, USA), Na2CO3(BDH Chemicals Ltd; England), NaOH (BDH Chemicals Ltd; England),Na-k C4O6.4H2O (Hopkins and Williams England, CuSO4.5H2O(Sigma Chemical Co; USA), Folin’s reagent (Sigma Chemical Co;USA), Rotenone (ICN Biomedicals Ltd), succinate (BDH Chemicals Ltd; England), CaCl2(May and Baker Lab; Products), spermine(Sigma-Aldrich, USA Product), UV-visible spectrophotometer(6405 Jenway), rotary evaporator (Buchi Rotavapor RE-3; Buchi Labortechnik AG, Flawil, Switzerland).
2.2. Plant material and extraction
A. wilkesianaleaves were collected from Babcock University horticulture garden and identified by Prof. E.B Esan at the Department of Basic Sciences Babcock University (Nigeria).Voucher specimen (BUH002) was deposited at the University Herbarium. Aqueous extraction was adopted based on the traditional preparation method. Dried sample (1 kg) ofA. wilkesianaleaves were soaked in 20 L distilled water for 24 h. The extraction was repeated for the same duration twice, making a total of 72 h. Each fi ltrate was refrigerated at 4 ℃ to avoid microbial contamination.The aqueous suspension was fi ltered using Whatman fi lter paper No. 1. The combined filtrates were concentrated in a rotary evaporator (BuchiRotavapor RE-3; Buchi Labortechnik AG, Flawil,Switzerland) at 60 ℃. Further evaporation of water from the extract to achieve complete dryness was achieved by evaporating in a hot-air oven at 50 ℃ for 72 h prior to storage at 4°℃ until further analysis.The extract yield was 160 g (16% yield).
2.3. Animals
Twenty (20) male albino rats weighing 120-200 g were obtained from Nigerian Institute of Medical Research, Yaba, Lagos, Nigeria.The animals in cages were acclimatized for two weeks in the animal house, then maintained and cared for according to the National Institute of Health guidelines for the Care and Use of Laboratory Animals[15]. Tap water and commercial pelleted feed were provided under room temperature [(28±2) ℃] and 12 h light/dark cycle. The study was approved by the Babcock University Health Research and Ethics Committee under approval No. BUHREC373/16.
2.4. Experimental design
The rats were randomly divided into fi ve groups (Ⅰ-Ⅴ) of four rats each. GroupⅠrats were fed for 7 d with normal commercial pelleted feed alone while animals of groupⅡwere administered orally with 20 mg/kg body weight (b.w.) cyclophosphamide daily for 7 d.Groups Ⅲ-Ⅴrats were co-administered orally with 20 mg/ kg b.w.cyclophosphamide and 110, 220 and 440 mg/kg b.w. A.wilkesianaleaf extract respectively daily for 7 d. The administered extract doses were derived from the median lethal dose (LD50) of 2 197.72 mg/ kg reported by Udobang et al[8]. At the end of the treatment period,the animals were starved overnight and sacrificed. Whole blood sample was collected by cardiac puncture using plastic hypodermic syringes and kept in heparinized bottles. Immediately, the whole blood was centrifuged at 3 000 rpm for 15 min to obtain plasma for biochemical assays. Protein concentration was estimated by the method of Lowry[16].
2.5. Hepatotoxic biomarkers
Plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were determined with the procedures described in Randox kits by Randox laboratories, UK.
2.6. Nephrotoxic biomarkers
Plasma levels of creatinine, uric acid and urea were determined using procedures described in Randox kits by Randox laboratories,UK.
2.7. Antioxidant assays
2.7.1. Determination of superoxide dismutase activity
Superoxide dismutase (SOD) activity in plasma was determined by the method of Misra and Fridovich[17]. Tissue homogenates were diluted with distilled water in ratio 1:9. An aliquot of 0.2 mL of the diluted sample was added to the 2.5 mL of 0.05 M carbonate buffer, pH 10.2 to equilibrate in the spectrophotometer cuvette and the reaction started by adding 0.3 mL freshly prepared 0.03 mM epinephrine to the mixture which was quickly mixed by inversion.The reference cuvette contained 2.5 mL of carbonate buffer, 0.3 mL of substrate (adrenaline) and 0.2 mL of distilled water. The increase in absorbance at 480 nm was monitored every 30 sec for 150 sec.
Increase in absorbance per minute =
A0= absorbance after 30 sec
A5= absorbance after 150 sec
1 unit of SOD activity was given as the amount of SOD necessary to cause 50% inhibition of the oxidation of adrenaline to adrenochrome in 1 min.
2.7.2. Determination of catalase activity
Catalase (CAT) activity was determined according to the method of Sinha[18]. Tissue sample (0.1 mL) homogenate was mixed with 1.0 mL 0.01 M phosphate buffer (pH 7.4), and incubated with 0.4 mL 0.2 M H2O2at 37 ℃ accurately for 1.0 min and reaction was stopped with 2.0 mL of 5% potassium dichromate (1:3 with glacial acetic acid). Further the samples were incubated in boiling water bath for 15 min and centrifuged at 5 000 rpm for 15 min and supernatant was used to quantify the amount of H2O2to calculate catalase activity at 570 nm. One unit represents 1.0 µmole of H2O2consumed/min/mg protein.
2.7.3. Determination of glutathione S-transferase (GST)activity
The effect of A. wilkesiana aqueous extract on GST activity was determined by the method of Habig et al[19]. The reaction medium was prepared as 0.03 mL glutathione (GSH), 0.15 mL 1-chloro-2,4-dinitrobenzene (CDNB), phosphate buffer (pH 6.5)and homogenate and the reaction was allowed to run for 60 s each before the absorbance was read against the blank at 340 nm. The temperature was maintained at approximately 31 ℃. The absorbance was measured using a spectrophotomer at 340 nm. GST activity was calculated as:
GST activity (µmol/min/mg protein)=

Extinction coefficient of CDNB = 9.6 mmol/cm
The principle is based on the fact that all known GST demonstrate a relatively high activity with as second substrate. Consequently,the conventional assay for GST activity utilizes 1-chloro-2,4-dinitobenzene as substrate. When this substance is conjugated with reduced GSH, its absorption maximum shifts to a longer wavelength.The absorption increase at the new wavelength of 340 nm provides a direct measurement of the enzymatic reaction.
2.7.4. Determination of reduced GSH
The effect of A. wilkesiana aqueous extract on reduced GSH was determined using the method described by Jollow et al[20]. Two millilitres of precipitating reagent (10% Trichloro acetic acid)was added to the obtained homogenate and then allowed to stand for 10 min. The mixture was centrifuged at 3 000 g for 5 min and 0.5 mL of the supernatant added to 4 mL of phosphate buffer and finally 0.5 mL of Ellman’s reagent (5’,5’-dithiobis (-2-nitrobenzoic acid) (DTNB)) was added. The optical densities were read within the 30 min of colour development at 412 nm using a T80 UV/Visible spectrophotometer (Leicestershire, United Kingdom).
2.8. Haematological assays
Packed cell volume (PCV), haemoglobin count (Hb) and white blood cell count (WBC) were determined in accordance to the method reported by Oyewole et al[21].
2.9. Liver and kidney mitochondrial assessment
2.9.1. Mitochondrial isolation
Mitochondrial fractions from liver and kidneys of rat were isolated using conventional differential centrifugation technique as method described by Hogeboom et al[22]. Rat liver and kidney mitochondrial fractions were isolated in a buffer solution containing 210 mM mannitol, 70 mM sucrose, 5 mM 2-(4-[2-hydroxyethyl] piperazin-1-yl) ethane sulfonic acid (HEPES) at pH 7.4 and 1 mM ethylene glycol tetra acetic acid (EGTA). Mitochondrial protein content was determined by Folin-Ciocateau method using bovine serum albumin as standard protein[16].
2.9.2. Assessment of mitochondrial membrane permeability transition
Mitochondrial membrane permeability transition was assessed according to the method of Lapidus and Sokolove[23]. Change in absorbance of mitochondria was monitored at 540 nm in a double beam UV-visible spectrophotometer (6406 Jenway, England).Mitochondrial fraction (0.4 mg/ mL) were suspended in a medium containing 210 mM mannitol, 70 mM sucrose, 5 mM HEPES-potassium hydroxide (pH 7.4), 0.8 µM rotenone and 5 mM sodium succinate. Membrane permeability transition (MPT) is induced in the presence of Ca2+(triggering agent, TG) which serves as a standard MPT trigger but not in the absence of Ca2+(Non triggering agent, NTG). The induced MPT (due to the presence of CP) in groups treated with CP alone and CP + A. wilkesiana extracts were compared to those of the control group. Reduction in the amplitude of the treatment groups (compared to NTG of the control) indicates increase in inhibition of mitochondrial membrane permeability transition (MMPT). To calculate the percentage inhibition of MMPT,NTG (control) was taken as 100% inhibition while TG (control) was taken as 0% inhibition.
2.10. Statistical analysis
Data were expressed as mean ±standard deviation (SD) of triplicate readings after analysis using SPSS version 23.0. One way ANOVA test was performed to analyze the difference between different groups.
3. Results
3.1. Hepatotoxic biomarkers
In this study, the hepatoprotective effect of A. wilkesiana leaf extract was examined using hepatic function biomarkers. Results showed that A. wilkesiana leaf extract significantly reduced (P<0.05) the cyclophosphamide induced elevated levels of plasma AST and ALT in a dose dependent manner (Table 1).

Table 1. Effects of Acalypha wilkesiana leaf extract on the liver and kidney function of rats treated with cyclophosphamide.
3.2. Nephrotoxic biomarkers
The co-administration of A. wilkesiana leaf extract and cyclophosphamide signif i cantly reduced (P<0.05) the elevated levels of plasma creatinine, urea and uric acid as compared to the group treated with cyclophosphamide alone (Table 1).
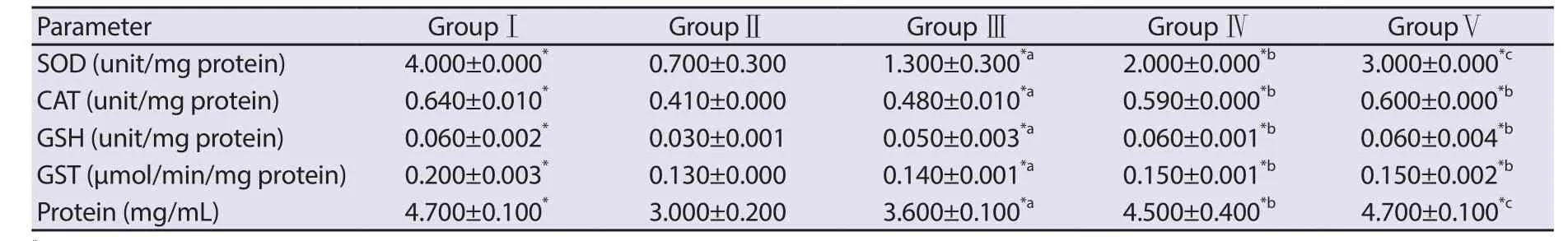
Table 2. Effect of CP and Acalypha wilkesiana leaf extract on plasma enzyme and non-enzyme antioxidant system of rats.
3.3. Antioxidant assays
The result of the protective effect of A. wilkesiana leaf extract in the antioxidant assays is presented in Table 2. Cyclophosphamide significantly reduced (P<0.05) the activities of plasma SOD, CAT,GST and the level of GSH which constitute a mutually supportive endogenous antioxidant defense team against the insurgence of reactive species generated by the cyclophosphamide metabolites.However, the co-administration of A. wilkesiana leaf extract and cyclophosphamide significantly increased the levels of these antioxidants which were dose dependent.
3.4. Haematological assays
Our result indicated that A. wilkesiana leaf extract was able to elevate significantly (P<0.05) the reduced levels of PCV and Hb and stimulate the suppression of WBC caused by cyclophosphamide(Table 3).
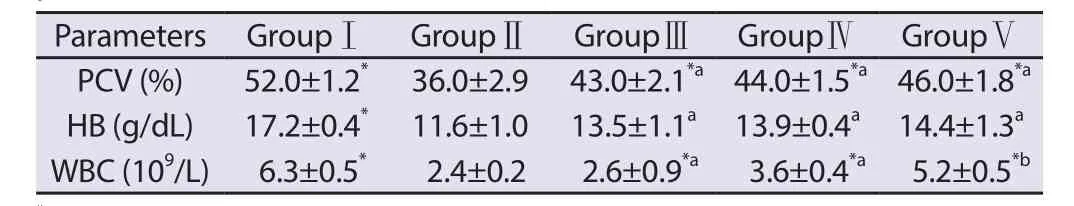
Table 3. Effect of CP and Acalypha wilkesiana leaf extract on haematological parameters in rats.
3.5. Mitochondrial membrane permeability transition pore opening
Data in Figure 1 showed a minimal (NTG) and maximal (TG)amplitude of MMPT for the control group in a succinate energized sucrose phosphate buffer. All the treatment groups also displayed minimal amplitude compared to the control NTG. The group treated with 440 mg/kg A. wilkesiana displayed the lowest amplitude compared to the control NTG while the group treated with CP 20 mg/kg displayed the highest amplitude. The presence of Ca2+induced a higher amplitude of MMPT in all treatment groups compared to NTG of the control but lower than the TG of the control (Figure 2). In a similar pattern as in Figure 1, the group treated with 440 and 220 mg/kg A. wilkesiana displayed the lowest amplitude while the group treated with 20 mg/kg CP displayed the highest amplitude. The data illustrated in Figures 1 and 2 indicated a reduction in the induction of MMPT by A. wilkesiana compared to the CP treated group (20 mg/kg CP). Animals orally treated with 20 mg/kg b.w. of cyclophosphamide displayed reduced MMPT inhibition by (19.60±0.08)% and (43.10±0.68)% in the absence and presence of Ca2+respectively. However, the doses of 110, 220 and 440 mg/ kg b.w. A. wilkesiana leaf extract inhibited cyclophosphamide-induced MMPT in a dose dependent manner both in the absence of Ca2+[(82.80±0.44)%, (85.20±0.04)%, and(93.80±0.08)%], respectively] and presence of Ca2+[(70.80±0.04)%,(86.10±0.20)%, and (87.70±0.08)%, respectively] (Table 4).

Table 4. Percentage inhibition of mitochondrial membrane permeability transition pore opening in the absence and presence of calcium ion by different doses of Acalypha wilkesiana leaf extract.
4. Discussion
The data from this present study showed that animals induced with toxicity using cyclophosphamide had an elevated plasma AST and ALT activities. Previous studies have demonstrated that rise in the plasma AST and ALT activities indicate hepatic damage[24].However, experimental groups treated with aqueous extract of A. wilkesiana leaf reduced plasma AST and ALT activities when compared with cyclophosphamide alone treated group. This suggests that A. wilkesiana leaf extract might possess bioactive compound(s)with hepatoprotective properties. The observed protective effect of A. wilkesiana leaf extract on the liver was further strengthened by the significant elevation in plasma protein concentrations in the animals co-administered with A. wilkesiana leaf extract and cyclophosphamide in a dose dependent manner when compared with cyclophosphamide treated animals. This also indicates that the leaf extract of A. wilkesiana could stimulate protein biosynthesis and perhaps ameliorate the toxic effect of cyclophosphamide.
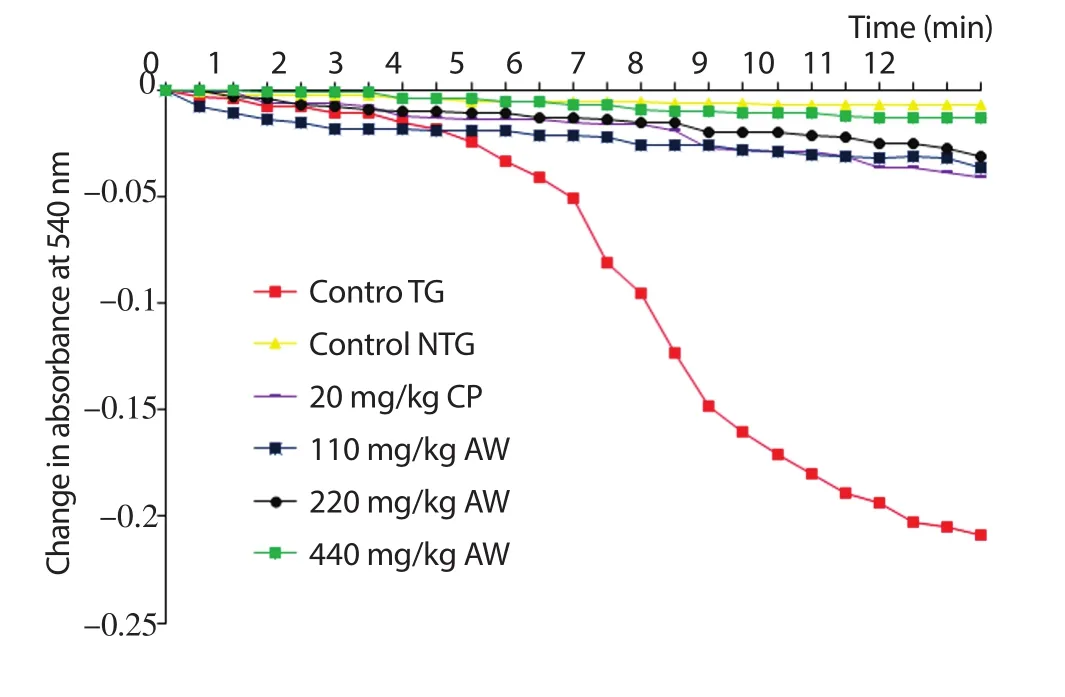
Figure 1. Change in absorbance (540 nm) for 12 min by 20 mg/kg cyclophosphamide (CP) and varying dose of aqueous leaf extract of Acalypha wilkesiana (ALEAW) on mitochondria membrane permeability transition pore opening in the absence of calcium ion energized by sodium succinate,TG: Triggering agent (presence of Ca2+), NTG: Non-triggering agent(absence of Ca2+).

Figure 2. Change in absorbance (540 nm) for 12 min by 20 mg/kg cyclophosphamide (CP) and varying dose of aqueous leaf extract of Acalypha wilkesiana (ALEAW) on mitochondria membrane permeability transition pore opening in the presence of calcium ion energized by sodium succinate,TG: Triggering agent (presence of Ca2+), NTG: Non-triggering agent(absence of Ca2+).
The signif i cant elevated creatinine in the groupⅡrats could be as a result of poor glomeruli fi ltration. The elevated urea level of the rats treated with cyclophosphamide could have resulted from increased protein catabolism evidenced by decrease in protein level or due to impairment of renal function resulting in the excessive reabsorption of urea into the blood. Therefore, elevated creatinine, urea and uric acid due to cyclophosphamide toxicity suggest that the kidneys had poor clearance of creatinine, uric acid and urea, which is a warning of possible kidney malfunction. However, the treatment of the experimental groups with different concentrations of A. wilkesiana leaf extract displayed signif i cant reduction in the plasma levels of creatinine, urea and uric acid, suggesting that the extract was able to improve the kidney clearance of these metabolites. Creatinine is a byproduct of creatine while urea is a byproduct of protein. The accumulation of serum creatinine, urea and uric acid is indicative of kidney dysfunction[25].
Superoxide anion radicals generated from tissue metabolism are converted to hydrogen peroxide by SOD. The hydrogen peroxide is fi nally neutralized to water and oxygen by catalase. Accumulation of superoxide radicals will deactivate CAT while increase in the hydrogen peroxide will inactivate SOD[26]. The increase in the activity of SOD and CAT could be as a result of the potent antioxidant activity of A. wilkesiana leaf extract as reported by Anokwuru et al[27]. Reduced GSH, a non-enzymatic antioxidant,detoxif i es toxic endogenous and exogenous substances while GST binds to lipophilic compounds and act as an enzyme for GSH conjugation reaction[28,29]. The depletion of GSH and GST by cyclophosphamide was reversed by A. wilkesiana leaf extract in a dose dependent manner as shown in Table 2. This elevation of GSH and GST activity may be due to the presence of antioxidant phytochemicals present in the A. wilkesiana leaf extract.
Cyclophosphamide is a known immunosuppressive drug and acts on the cells by suppressing the activity of the bone marrow and innate immune responses[30]. Our result showed that cyclophosphamide may have increased bone marrow suppressive effect causing aplastic anemia as shown in the signif i cant reduction of the haematocrit level of the rats. The immunosuppressive effect of cyclophosphamide is shown in the signif i cant reduction of the WBC level as compared to the control group. Treatment with the extract reversed the anemic condition induced by CP significantly and stimulated the nonspecif i c immune response. This study has shown that A. wilkesiana leaf extract could stimulate the haemopoetic system and may restore the production of immune cells.
The data obtained from the MMPT study showed that aqueous extract of A. wilkesiana leaf exhibited a mitochondrial protective effect against cyclophosphamide-induced liver damage at the subcellular level by inhibiting MMPT. This further strengthens the observed hepatoprotective property of A. wilkesiana leaf extract against cyclophosphamide induced hepatic tissue damage. The prevention of cyclophosphamide-induced mitochondrial damage by aqueous extract of A. wilkesiana leaf could be attributed to the presence of antioxidant phytochemicals. Previous studies had shown that the induction of mitochondrial membrane transition by proapoptotic or reactive oxygen molecules is one of the earliest events preceding caspase cascade during apoptotic event. However, plant materials with antioxidant property could inhibit the propagation and damaging effects of reactive species thus maintain the integrity of the mitochondria membrane and circumventing apoptosis[31].
In this present study, the observed chemoprotective activity of the aqueous extract of A. wilkesiana leaf could be due to the presence of corilagin and geraniin, which have been previously isolated.Corilagin and geraniin have been reported to possess antioxidant activity and chemoprotective potential[32,33].
This study has demonstrated that aqueous extract of A. wilkesiana leaf, which is rich in antioxidants, exhibits its chemoprotective effect against cyclophosphamide toxicity by lowering signif i cantly the elevated liver and kidney biomarkers, elevating depleted plasma antioxidant enzymes and non-enzymes, stimulating the immune system through the increase in the white blood cells, and protecting mitochondrial membrane against oxidative damage by reactive oxygen species. The fi ndings indicate that A. wilkesiana leaf extract could serve as a potential source of alternative medicine against cyclophosphamide induced tissue toxicity.
Conflict of interest statement
We declare that we have no conf l ict of interest.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Inhibitory effects of methanolic Olea europaea and acetonic Acacia laeta on growth of Babesia and Theileria
- Curcuma angustifolia ameliorates carbon tetrachloride-induced hepatotoxicity in HepG2 cells and Swiss albino rats
- Association between measles antibodies in vaccinated and naturally infected mothers with protective antibodies and the occurrence of measles in their children: A cross-sectional study in the Bavi district of Hanoi
- Long-term safety follow-up of children from a randomized-controlled phaseⅡb proof-of-concept efficacy study of the live, attenuated,tetravalent dengue vaccine (CYD-TDV) in Thailand
- Ebola virus disease: Recent advances in diagnostics and therapeutics
