Downregulation of iASPP Expression Suppresses Proliferation,Invasion and Increases Chemosensitivity to Paclitaxel of Head and Neck Squamous Cell Carcinoma In Vitro
2019-10-15ZhengzhengLiuWeiluKuangWenjingZengJianyunXiaoYongquanTian
Zhengzheng Liu, Weilu Kuang, Wenjing Zeng,Jianyun Xiao, Yongquan Tian*
1Department of Oncology, 2Department of Pharmacy, 3Department of Otolaryngology Head and Neck Surgery, Xiangya Hospital, Central South University, Changsha 410008, China
Key words: human head and neck squamous cell carcinoma; iASPP; chemosensitivity; paclitaxel
Objective Our previous study has revealed that iASPP is elevated in human head and neck squamous cell carcinoma(HNSCC) and iASPP overexpression signifcantly correlates with tumor malignant progression and poor survival of HNSCC. This study investigated the function of iASPP playing in proliferation and invasion of HNSCC in vitro.Methods HNSCC cell line Tu686 transfected with Lentiviral vector-mediated iASPP-specific shRNA and control shRNA were named the shRNA-iASPP group and shRNA-NC group, respectively. The non-infected Tu686 cells were named the CON group. CCK-8 assay, flow cytometry, transwell invasion assay were performed to detect the effects of iASPP inhibition in vitro.Results Our results demonstrated that the proliferation of shRNA-iASPP cells at the time of 72 h (F=32.459, P=0.000),96 h (F=51.407, P=0.000), 120 h (F=35.125, P=0.000) post-transfection, was significantly lower than that of shRNANC cells and CON cells. The apoptosis ratio of shRNA-iASPP cells was 9.42% ± 0.39% (F=299.490, P=0.000), which was significantly higher than that of CON cells (2.80% ± 0.42%) and shRNA-NC cells (3.18% ± 0.28%). The percentage of shRNA-iASPP cells in G0/G1 phase was 74.65% ± 1.09% (F=388.901, P=0.000), which was strikingly increased,compared with that of CON cells (55.19% ± 1.02%) and shRNA-NC cells (54.62% ± 0.88%). The number of invading cells was 56 ± 4 in the shRNA-iASPP group (F=84.965, P=0.000), which decreased significantly, compared with the CON group (111 ± 3) and the shRNA-NC group (105 ± 8). The survival rate of shRNA-iASPP cells administrated with paclitaxel was highly decreased, compared with CON cells and shRNA-NC cells (F=634.841, P=0.000).Conclusion These results suggest iASPP may play an important role in progression and aggressive behavior of HNSCC and may be an efficient chemotherapeutic target for the treatment of HNSCC.
HEAD and neck squamous cell carcinoma(HNSCC) is the sixth leading cancer worldwide,[1]causing a significant morbidity with an incidence of approximately 550 000 cases worldwide annually.[2]Despite advances in diagnosis and treatment, HNSCC is still a serious threat to human life with a 5-year survival rate of only 50% to 60%.[3]Similar to all solid tumors, a series of genetic alterations are common in the initiation and progression of HNSCC. Therefore, identifying novel therapeutic targets and biomarkers, and furthermore elucidating their underlying mechanism are efficient ways for prediction, diagnosis and further successful prevention and therapy for HNSCC patients.[4,5]
The apoptosis stimulating protein of p53 (ASPP)family, including 3 members, ASPP1, ASPP2, and iASPP,was identified as specific regulators of p53-, p63-,and p73-mediated apoptosis.[6]iASPP, encoded by PPP1R13L gene, is the evolutionally conserved inhibitory member of the ASPP family. It can specifically inhibit p53-mediated cell apoptosis, and its overexpression confers resistance to ultraviolet radiation and cisplatin-induced apoptosis in cultured cells.[7]Furthermore,the oncogenic role of iASPP has been shown in many kinds of human cancers, such as breast carcinomas,[7]acute leukemia,[8]hepatocellular carcinoma,[9]ovarian cancer,[10]gastric cancer,[11]colorectal cancer,[12]nonsmall-cell lung cancer,[13]prostate cancer,[14]et al. In our previous study, we have demonstrated that iASPP is elevated in human HNSCC and iASPP overexpression significantly correlates with tumor malignant progression and poor survival in HNSCC.[15]
However, the role of iASPP playing in tumorigenesis and progression of HNSCC is not clear. In this study, firstly we used a lentiviral RNAi system to inhibit the expression of iASPP in HNSCC cell line—Tu686 cells. Then, we investigated the effects of downregulation of iASPP expression on HNSCC cell proliferation,apoptosis, cell cycle, invasion and paclitaxel chemosensitivityin vitro.
MATERIALS AND METHODS
Cell culture
HNSCC cell line Tu686 was established from a primary tumor located in the base of tongue. The Tu686 cell line kindly provided by Dr. Zhuo (Georgia) Chen(Emory University Winship Cancer Institute, Atlanta,Georgia) was cultured in a Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium (1:1) supplemented with 10% fetal bovine serum (FBS) and antibiotics in a humidified atmosphere with 5% CO2at 37°C. Exponentially growing cells were used in the subsequent experiments.
Lentiviral vectors mediated iASPP-specific shRNA transfection and grouping
iASPP-specific shRNA lentiviral particles (sc-72100-V/200 µl, Santa Cruz) are a pool of concentrated, transduction-ready viral particles containing 3 target-specific constructs that encode 19-25 nt (plus hairpin) shRNA designed to knock down iASPP gene expression in human Tu686 cells.
Tu686 cells (2×104) were seeded into 24-well plates, and divided into three groups: LV-shRNA-iASPP,LV-shRNA-NC, and non-infected groups (CON). Then the medium containing iASPP shRNA or control shRNA lentiviral particles was added to Tu686 cells of the LVshRNA-iASPP or LV-shRNA-NC group and incubated for 12 h according to the manufacturer’s protocol. The silencing efficiency was analyzed by RT-PCR and western blotting 72 h post-infection.
Quantitative real-time RT-PCR analysis
Quantitative real-time RT-PCR (qPCR) analysis was performed as described in our previous study.[15]Total RNA was extracted using Trizol reagent (Invitrogen)according to manufacturer’s protocol. First-strand cDNA was synthesized with a Revert Aid First Strand cDNA Synthesis Kit (FERMENTAS, USA). qPCR was performed on a MiniOpticon Real-Time PCR System using SYBR green Master Mixture. The primers for iASPP and GADPH amplification were as follows: iASPP (180 bp),5’-GAAAGCCTGGAACGAGTCTG-3’ (forward) and 5’-GCGCTAGTGAGGTTGTCCTT-3’ (reverse); GADPH (179 bp), 5’-CGACCACTTTGTCAAGCTCA-3’ (forward) and 5’-ACTGAGTGTGGCAGGGACTC-3’ (reverse). The mRNA expression levels of iASPP were normalized against GADPH and determined by 2-ΔΔCTmethod.[16]
Western blotting
Western blotting was performed as previously described.[17]The same amount of proteins (50 μg) was separated by 10% SDS-PAGE, and the dilution of the antibodies was as follows: anti-iASPP rabbit polyclonal antibody (ab-34898, 1:1500, Abcam), anti-bax rabbit polyclonal antibody (1:1000, Cell Signaling), and anti-bcl-2 rabbit polyclonal antibody (1:1000, Proteintech Group).
Cell proliferation assay
Cell Counting Kit-8 (CCK8, Beyotime, China) assay was carried out to draw cell growth curves according to manufacturer’s protocol. Briefly, cells were seeded into a 96-well plate at a density of 3.0×103/well, cultured with medium containing 10% FBS in a humidified atmosphere with 5% CO2at 37°C and exposed to fresh media every other day. The absorbance at 450 nm of the cells in each group was measured at 24, 48, 72,96 and 120 h post-transfection, and then cell growth curve was generated.
Flow cytometry
After incubated with serum-free medium for 24 h, the cells were washed twice with PBS at 4°C and resuspended to a concentration of 1×106/ml. Subsequently, the cells were incubated with Annexin V-FITC and propidium iodide for 30 min. Fluorescence-activated cell sorting analysis was performed with a CytomicsTM FC500 instrument (Beckman Coulter, USA). Cell apoptosis and cell cycle distribution were measured.
Transwell invasion assay
Transwell invasion assay was performed as previously described.[18]A 24-well transfected chamber (Costar,Cambridge, MA, USA) was used to evaluate the invasiveness of the transfected cells according to manufacturer’s instructions. In brief, 2×104cells in 100 μl serum-free medium were seeded into the upper chamber of the transwell that had been pre-coated with matrigel overnight. The lower chamber was filled with 600 μl DMEM/F12 containing 10% FBS to induce chemotaxis.After 48 h of incubation, the cells were fixed by methanol and stained with crystal violet. The invading cells that were on the lower surface of the filter membrane,were counted under a microscope at ×200 magnification from at least five random fields. Each experiment was repeated three times.
Analysis of sensitivity of Tu686 cells to chemotherapeutics paclitaxel
Cells seeded in a 96-well plate were cultured overnight, and incubated with different concentrations of paclitaxel (0.01, 0.1, 1, 10, 102, 103, 104nmol/L) for 48 h in a CO2incubator. The cell viability was assessed by CCK8 assay. The cell survival rate was calculated according to the measured OD450 value: cell survival rate = (experimental group OD450― blank OD450)/(control group OD450―blank OD450) × 100%. And the half maximal (50 percent) inhibitory concentration(IC50 value) of paclitaxel was calculated. Apoptosis and cell cycle distribution were assayed with flow cytometry.
Statistical analysis
All statistical analyses were performed with SPSS 19.0 software. Quantitative data in this study were presented as mean ± SD. Two-tailed Student’st-test and one wayANOVAfollowed by Student-Newman-Keuls’s post hoc test were used to compare statistical differences between groups.P<0.05 was considered statistically significant.
RESULTS
iASPP shRNA lentiviral particles efficiently silence the expression of iASPP in HNSCC cell line Tu686
qPCR analysis and Western blotting showed lentivirus-delivered iASPP shRNA effectively infected Tu686 cells, and inhibited iASPP mRNA expression more than 80% (Figure 1A) and iASPP protein expression over 70% (Figure 1B, 1C).
iASPP knockdown inhibits the cellular proliferation in vitro
To investigate the function of iASPP on cellular proliferation of HNSCC cellsin vitro, a CCK-8 assay was used.The results showed the proliferation of Tu686 cells was markedly inhibited after knockdown of iASPP gene expression. The OD450 value of shRNA-iASPP cells at the time of 72 h (F=32.459,P=0.000), 96 h (F=51.407,P=0.000), 120 h (F=35.125,P=0.000) post infection,was significantly lower than that of shRNA-NC cells and CON cells. (Figure 2).
iASPP knockdown induces apoptosis and cell cycle arrest at G0/G1 phase
To clarify the possible mechanisms involved in iASPP-mediated cell proliferation, flow cytometry was applied to detect apoptosis and cell cycle. The results showed that the apoptosis ratio of shRNA-iASPP cells was 9.42% ± 0.39%, which was significantly higher than that of CON cells (2.80% ± 0.42%) and shRNA-NC cells (3.18% ± 0.28%,F=299.490,P=0.000;Figure 3).
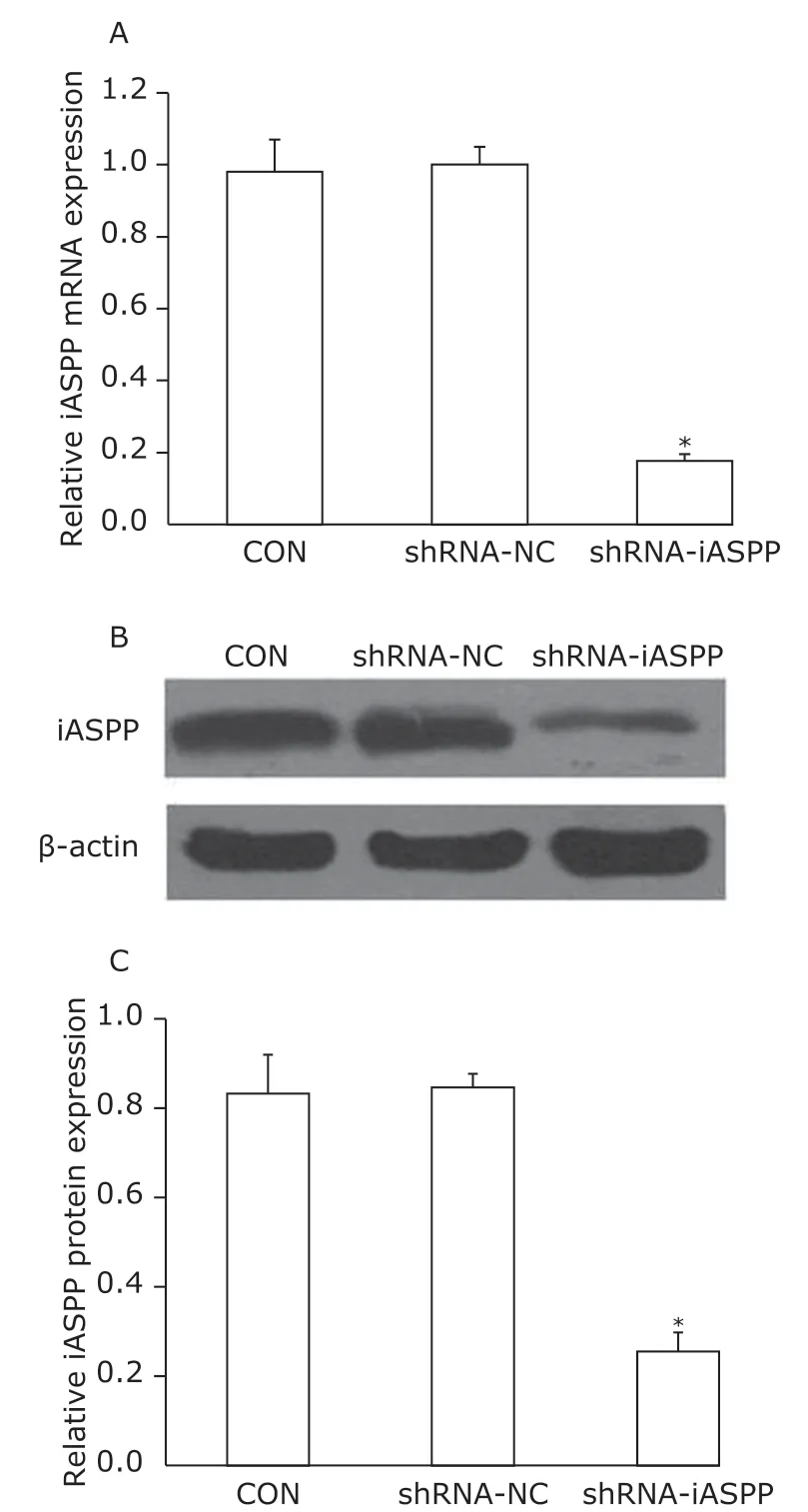
Figure 1. Knockdown of iASPP expression by lentiviral vector-mediated shRNA interference in Tu686 cell lines.CON: non-infected cells; shRNA-NC: cells transfected with control shRNA lentiviral vector; shRNA-iASPP: cells transfected with iASPP lentiviral vector.
The percentage of shRNA-iASPP cells at G0/G1 phase was 74.65% ± 1.09% (F=388.901,P=0.000),which was strikingly increased, compared with that of CON cells (55.19% ± 1.02%) and shRNA-NC cells(54.62% ± 0.88%). Whereas the percentage of shRNA-iASPP cells at S phase was 17.54% ± 1.21%(F=176.759,P=0.000), which decreased significantly,compared with CON cells (32.56% ± 0.98%) and shRNA-NC cells (32.50% ± 1.17%). The percentage of shRNA-iASPP cells at G2/M phase was 7.81% ± 0.76%(F=59.999,P=0.000), which reduced prominently,compared with CON cells (12.24% ± 0.68%) and shRNA-NC cells (12.87% ± 0.31%, Figure 4).
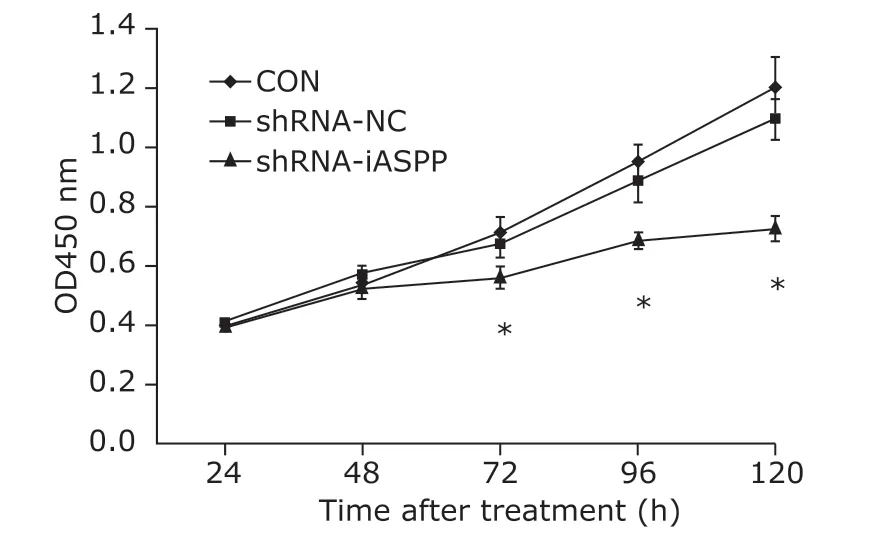
Figure 2. The effect of iASPP knockdown on the proliferation of Tu686 cells. Cell proliferation was measured by the CCK-8 assay every 24 hour for 5 days. Results were expressed as means of three independent experiments ± SD(*P < 0.01, compared with shRNA-NC cells and CON cells).
The potential effects of downregulation of iASPP expression on some apoptosis-related proteins such as bax, bcl-2 were also evaluated by western blotting.The results demonstrated that downregulation of iASPP expression obviously inhibited the expression of anti-apoptotic protein bcl-2, but significantly increased the expression of pro-apoptotic protein bax (Figure 5).
Silencing iASPP expression inhibits cell invasion in vitro
To elucidate the effect of iASPP on HNSCC cell invasion, transwell invasion assay was carried out. The result demonstrated invasiveness of the Tu686 cells decreased significantly in shRNA-iASPP cells, compared with CON cells and shRNA-NC cells (Figure 6). The number of invading cells was 56 ± 4 in the shRNA-iASPP group (F=84.965,P=0.000), which decreased significantly, compared with the CON group(111 ± 3 ) and the shRNA-NC group (105 ± 8). These results suggests that iASPP plays an important role in mediating cell invasion of HNSCC, which is a crucial influence of progression and aggressive behavior of HNSCC.
Down-regulated iASPP expression sensitizes Tu686 cells to paclitaxel in vitro
To evaluate the effect of knockdown of iASPP expression on chemosensitivity of HNSCC Tu686 cells to paclitaxel, different concentrations of paclitaxel were employed to treat CON cells, shRNA-NC cells and shRNA-iASPP cells for 48 h.
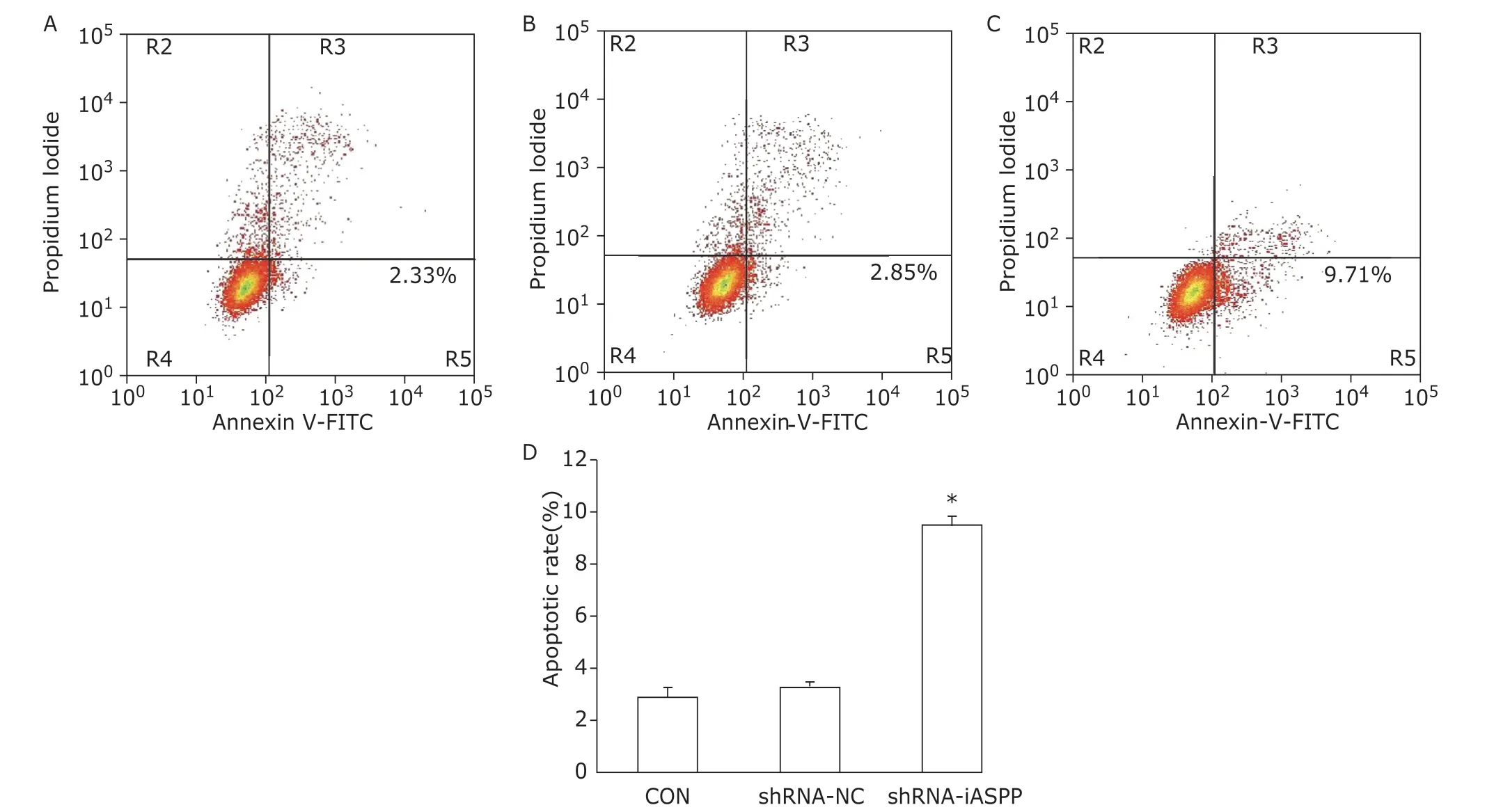
Figure 3. The effect of knockdown of iASPP on cell apoptosis of Tu686 cells was analyzed by flow cytometry.
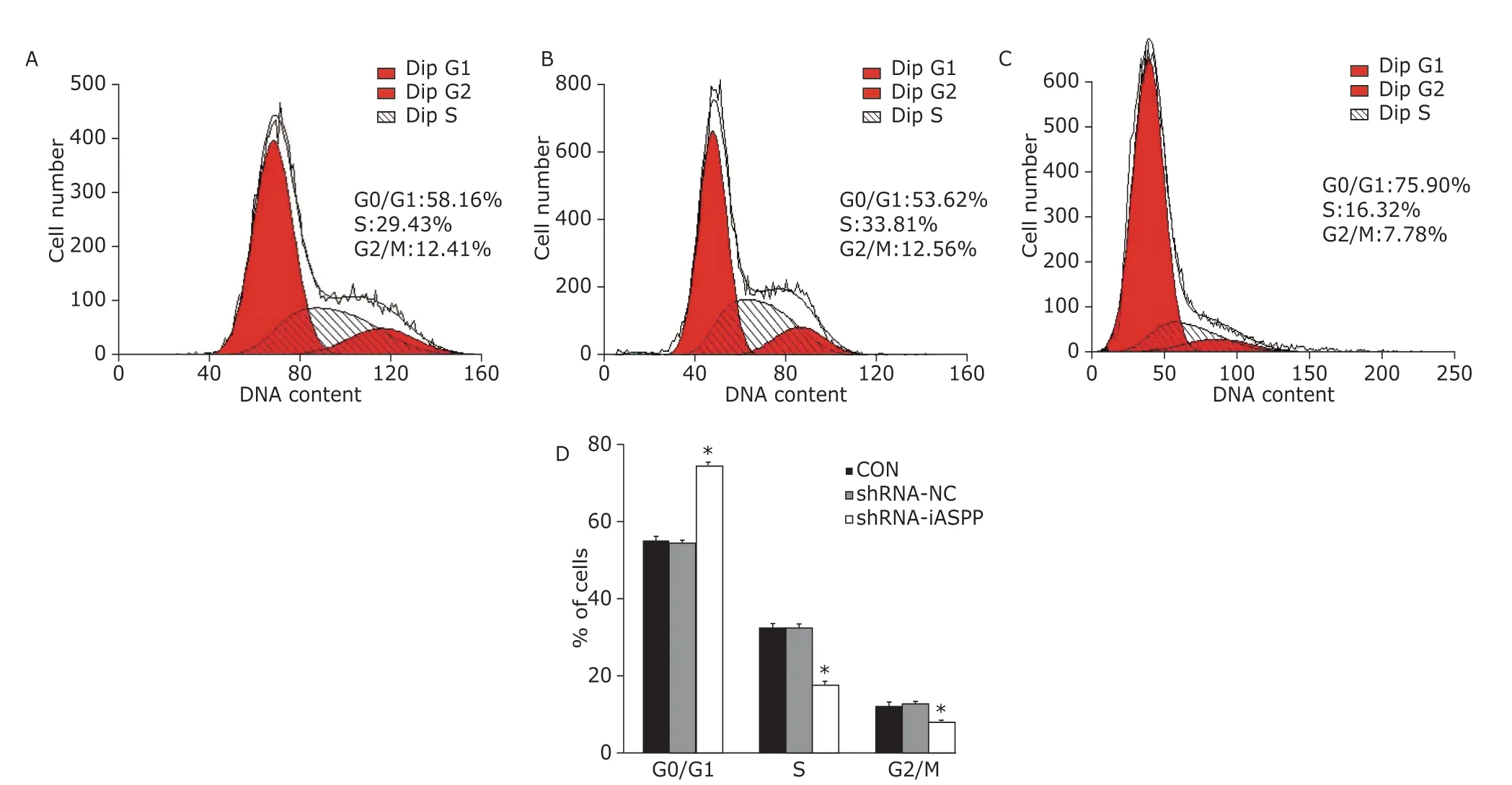
Figure 4. The effect of knockdown of iASPP on cell cycle distribution of Tu686 cells was analyzed by flow cytometry.
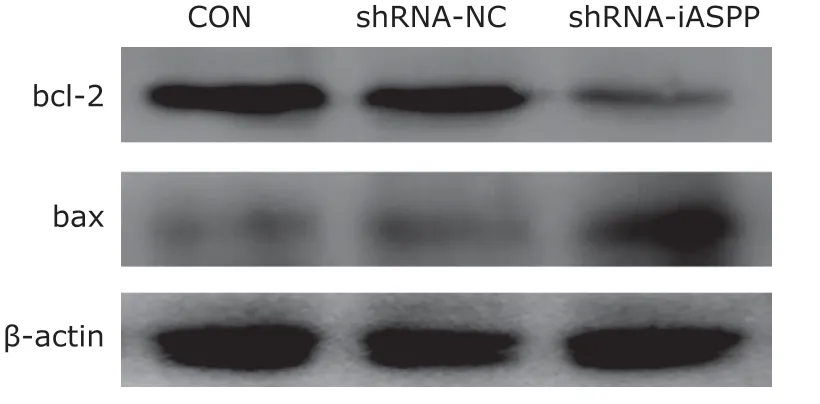
Figure 5. The effect of knockdown of iASPP expression on the expression of apoptosis-related proteins in Tu686 cells was analyzed by western blotting.
The survival rate of shRNA-iASPP cells exposed to paclitaxel was highly decreased, compared with CON cells and shRNA-NC cells (F=634.841,P=0.000; Figure 7). Calculated by SPSS 19.0, IC50 value of paclitaxel for shRNA-iASPP cells, CON cells and shRNA-NC cells was 3.84±0.28, 47.46±2.12 and 50.93±2.27 nmol/L, respectively. These results demonstrated the chemosensitivity of shRNA-iASPP cells to paclitaxel was highly increased, compared with CON cells and shRNA-NC cells.
After treatment with IC30 of paclitaxel, flow cytometry showed the apoptosis ratio of shRNA-iASPP cells was strikingly increased, compared with CON cells and shRNA-NC cells (18.27%±1.13%vs. 7.31%±0.33%, 8.20%±0.34%,F=220.453,P=0.000; Figure 8). Flow cytometry also demonstrated the percentage of shRNA-iASPP cells at G2/M phase was obviously increased, compared with CON cells and shRNA-NC cells(28.68% ± 0.76%vs. 19.21% ± 0.89%, 17.99% ±0.13%;F=219.075,P=0.000), whereas the percentage of cells at S phase decreased significantly, compared with CON cells and shRNA-NC cells (15.11% ± 1.09%vs. 26.38% ± 2.04%, 26.58% ± 0.67%,F=66.760,P=0.000). For G0/G1 phase, no obvious difference was found among the three groups (56.21% ± 1.82%vs. 54.41% ± 1.15%, 55.42% ± 0.56%,F=1.479,P=0.300; Figure 9). These data demonstrated inhibiting iASPP expression can significantly promote cell apoptosis and induce cell cycle arrest, which can strengthen chemosensitivity of HNSCC cell line to paclitaxel. It suggests that iASPP may be a novel and efficient chemotherapeutic target for HNSCC treatment.
DISCUSSION
We investigated the function of iASPP associated with tumorigenesis, invasiveness and chemosensitivity of HNSCCin vitro. The results have clearly demonstrated that silencing iASPP gene expression could promote apoptosis, arrest cell cycle at G0/G1 phase, inhibit cell proliferation and invasion ofin vitroHNSCC cell line,and strengthen chemosensitivity of HNSCC cell line to paclitaxel. All these findings suggest that iASPP could play an important role in progressive and aggressive behavior of HNSCC and might be a novel and efficient chemotherapeutic target for HNSCC treatment.
Several studies have revealed that iASPP knockdown inhibits cellular proliferation and induces cell apoptosis in several cancers such as colorectal cancer,[19]spinal chordoma,[20]leukemia,[21]cervical cancer,[22]glioblastoma,[23]gastric cancer,[24]et al.,which were in consistent with the results of our study.Our result showed downregulation of iASPP expression increased bcl-2 expression and decreased bax expression, which were similar to those found in spinal chordoma.[20]The above results indicated that iASPP can inhibit cell apoptosis by regulating apoptosisrelated proteins.
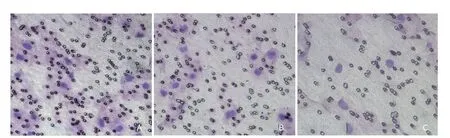
Figure 6. Downregulation of the expression of iASPP inhibited the invasion of Tu686 in vitro. Matrigel invasiveness assay in transwell culture chamber showed invasiveness decreased significantly in the shRNA-iASPP group (C), compared with the CON group (A) and shRNA-NC group (B).
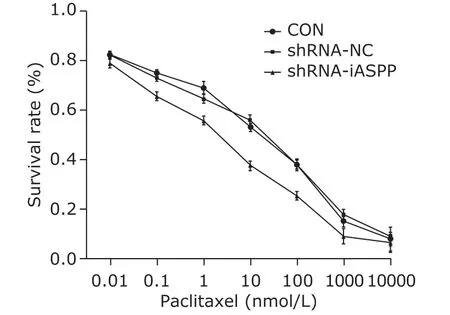
Figure 7. Knockdown of iASPP expression can strengthen chemosensitivity of HNSCC Tu686 cell line to paclitaxel.CON, shRNA-NC and shRNA-iASPP cells were treated with increasing concentrations of paclitaxel for 48 h, and the cell survival rate was assessed by CCK-8 assays. Results represent the mean ± SD of three independent experiments.
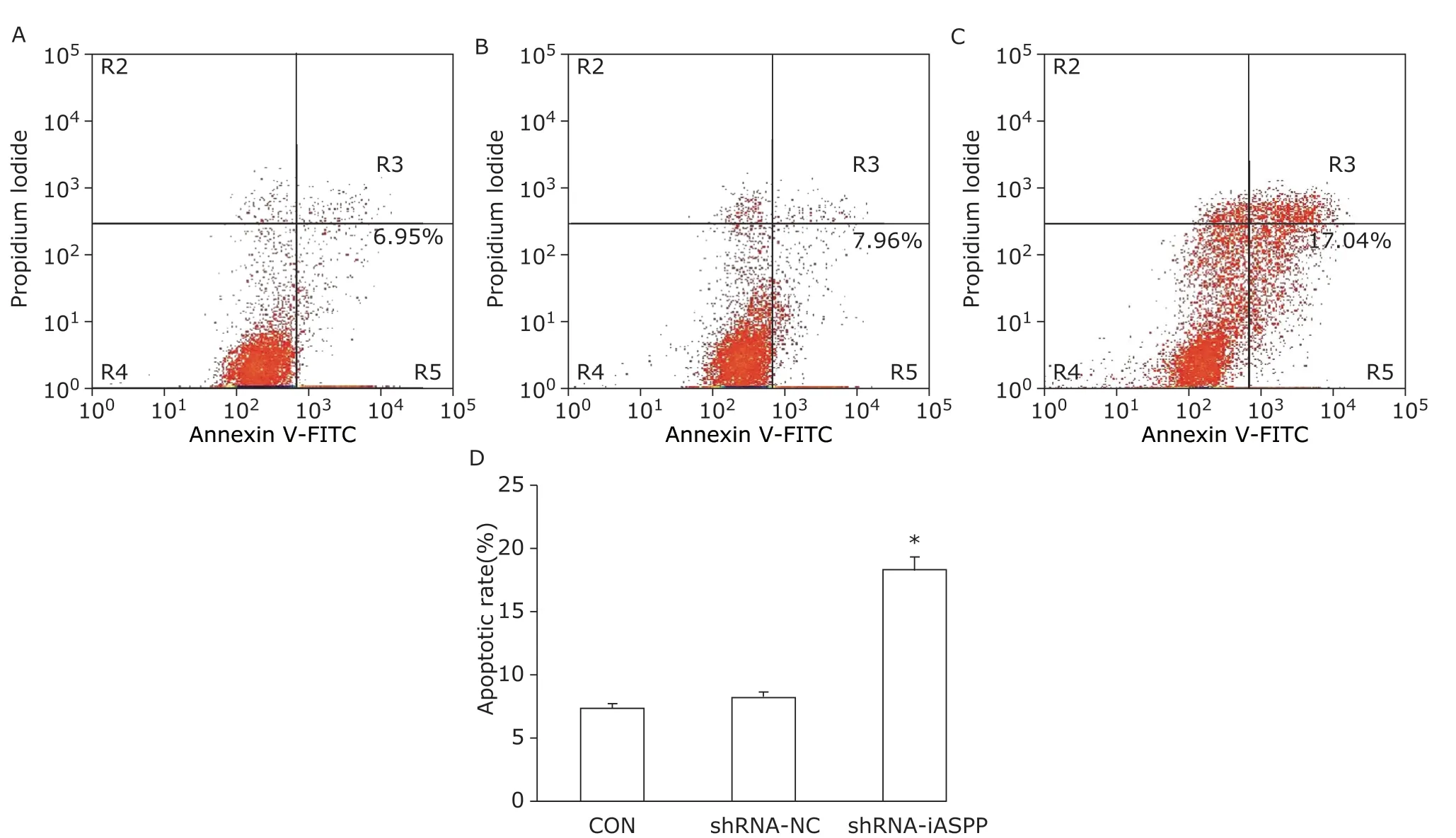
Figure 8. The effect of knockdown of iASPP expression on cell apoptosis of Tu686 cells treated with paciltaxel for 24 hours was analyzed by flow cytometry. Representative histograms showing apoptosis of CON (A), shRNA-NC (B) and shRNAiASPP cells (C). The apoptosis ratio of shRNA-iASPP cells was significantly higher than that of CON cells and shRNA-NC cells (D) (*P=0.000). Results of graphic D represent the mean ± SD of three independent experiments.
In our previous study, we have found overexpression of iASPP was significantly correlated with lymph node metastasis.[15]This study has verified silencing iASPP expression can inhibit invasion of the Tu686 cellsin vitro. These results revealed iASPP might associate with lymph node metastasis of HNSCC, which is crucial for invasion and metastasis of HNSCC. Consistently,Morriset al.[25]reported that the increased iASPP was associated with tumor invasive growth, metastatic disease, and cancer-related mortality. They revealed that iASPP was enriched in highly metastatic prostate cancer cells compared to primary cells, and iASPPoverexpressing cells distributed at the invasive leading edge mostly. Maet al.[20]regarded that the elevated iASPP expression had significant association with tumor invasion. It has also demonstrated that downregulated iASPP expression could remarkably weaken the proliferative and invasive properties of the pancreatic adenocarcinoma cells and the central nervous system lymphoma cells.[26,27]Previous studies showed some microRNAs could regulate iASPP expression and mediate malignant progression including cellular invasion of many tumor cells, such as glioblastoma,prostate cancer and colorectal cancer.[28-30]Zhaoet al.[31]revealed the interaction of lncRNA-microRNA-iASPP and identified the H19/miR-140/iASPP axis may participate in regulating proliferation and invasion of glioma cells. Xionget al.[32]verified that iASPP can stimulate epithelial-mesenchymal transition by regulating the downstream signaling pathway of miR-20a-FBXL5/BTG3 in cervical adenocarcinoma cells. Lianget al.[27]suggested that miR-184 targeting iASPP could suppress invasion of human central nervous system lymphoma cells through regulating PI3K/AKT signaling pathway.iASPP has been shown to promote oncogenic featuresviainteracting with p53 and inhibiting its ability to regulate transcription of target genes and repressing the expression of p53-responsive miR-34a.[22]All these evidences indicate that the regulatory mechanisms of iASPP on invasion are complicated and diverse. Therefore, further researches are necessary to explore the definite mechanism in future.
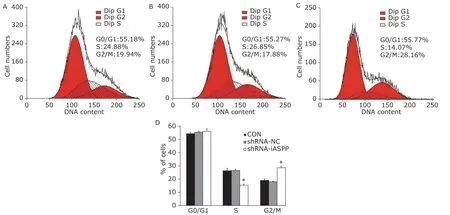
Figure 9. The effect of knockdown of iASPP expression on cell cycle distribution of Tu686 cells treated with paciltaxel for 24 hours was analyzed by flow cytometry. Representative histograms showing cell cycle distribution of CON (A), shRNA-NC(B) and shRNA-iASPP cells (C). The percentage of shRNA-iASPP cells in G2/M phase was strikingly increased (*P=0.000),and the percentage of cells in S phase decreased significantly (*P=0.000). Results of graphic D represent the mean ± SD of three independent experiments.
Chemotherapy is part of the systemic treatment for HNSCC. Paclitaxel, a tubulin-stabilizing anti-cancer agent, is now widely used to treat HNSCC. Many trials have indicated that a three-drug regimen consisting of taxel (including docetaxel), cisplatin and 5-fluorouracil(TPF) improves outcomes of patients with HNSCC.[33-35]However, drug-resistance seriously interferes with the effectiveness of chemotherapy. In fact, increased iASPP expression in human malignant cells is mainly associated with their chemoresistance.[7]Yuet al.[36]reported the expression of iASPP in gastric carcinoma(GC) patients with cisplatin resistance was significant higher than that in the healthy control group. In addition, higher expression of iASPP was also detected in cisplatin-resistant cancer cell lines. They concluded that iASPP induced cisplatin resistance in GC patients and iASPP might be a novel therapeutic strategy for the treatment of cisplatin-resistant GC. Jianget al.[10]reported that iASPP overexpression in ovarian cancer cells conferred resistance to paclitaxel by reducing mitotic catastrophe in a p53-independent mannerviaactivating separase, whereas silencing iASPP expression enhanced paclitaxel-mediated mitotic catastrophe through inactivating separase. Xionget al.[32]found that knockdown of iASPP suppressed cervical cancer cell proliferation and sensitized cervical cancer cells to cisplatinin vivo. Jiaet al.[37]observed that in response to cell damage stimuli, iASPP cells can protect hematopoietic cells against apoptosis; in the meantime these apoptosis-resistant cells would have more mutation accumulation. Caoet al.[38]found that iASPP was highly elevated in human cervical cancer, and overexpression of nuclear iASPP was correlated with poor prognosis and chemoresistance/radioresistance,suggesting that iASPP might serve as a novel potential prognostic marker and therapeutic target for cervical cancer. Furthermore, knockdown of endogenous iASPP caused a significant increase of p53-mediated apoptosis induced by chemotherapeutics in human malignances.[7,39]In this study, we confirmed that suppression of iASPP expression can significantly promote cell apoptosis and induce cell cycle arrest, which can strengthen chemosensitivity of HNSCC cell line to paclitaxel. All these evidences suggest that iASPP may be a novel and efficient chemotherapeutic target for HNSCC treatment.
In conclusion, knockdown of iASPP expression inhibited proliferation and invasion, and strengthen chemosensitivity of HNSCC to paclitaxelin vitro. It suggests iASPP might play an important role in progression and aggression of HNSCC and serve as a potential therapeutic target for HNSCC. Furthermore,the potential mechanisms involved iASPP in the tumorigenesis and progression of HNSCC should be studied in the future.
Conflicts of interest statement
The authors declare no conflicts of interest.
杂志排行
Chinese Medical Sciences Journal的其它文章
- Research on the Antitumor Compounds from Cephalotaxus Hainanensis
- Artificial Musk R&D and Manufacturing
- Management of an Adult with Goodpasture’s Syndrome Following Brain Trauma with Extracorporeal Membrane Oxygenation: A Case Report
- Transvaginal Reduction of a Heterotopic Cornual Pregnancy with Conservation of Intrauterine Pregnancy
- Research Progress on Diagnosis and Treatment of Chronic Osteomyelitis
- Association between GSTT1 Homozygous Deletion and Risk of Pancreatic Cancer: A Meta Analysis
