Association between GSTT1 Homozygous Deletion and Risk of Pancreatic Cancer: A Meta Analysis
2019-10-15JunpengCuiMeiyiLinZhenghaoLiuBaolinLiu
Junpeng Cui, Meiyi Lin, Zhenghao Liu, Baolin Liu*
1Sixth Department of General Surgery, Shengjing Hospital of China Medical University, Shenyang 110004, China
2Department of Rheumatology, First Affiliated Hospital of China Medical University,Shenyang 110001, China
3China Medical University, Shenyang 110013, China
Key words: pancreatic cancer; gene; GSTT1; susceptibility; meta analysis
Objective To clarify the possible association of GSTT1 homozygous deletion with the susceptibility to pancreatic cancer.Methods We searched PubMed database, Chinese Journal Full Text Database (CNKI), and EMBASE to find the eligible studies published up to April 18, 2018 for evaluating the relationship between GSTT1 homozygous deletion and pancreatic cancer. The frequency of null genotype for GSTT1 between the pancreatic cancer group and the healthy control group was compared with Chi-square test, and odds ratios(ORs) value and 95% confidence interval (95% CI) were calculated.Results A total of 9 studies met the inclusion criteria, and 5952 cases consisting of 2387 pancreatic cancer patients and 3565 healthy controls were included in the meta analysis. Compared with the control group,frequency of null genotype for GSTT1 in the pancreatic cancer group was higher (33.4% vs. 38.7%, OR = 1.26,95%CI = 1.01-1.58, P = 0.04).Conclusion GSTT1 homozygous deletion individuals may have higher susceptibility to pancreatic cancer.
PANCREATIC cancer, one of the most common malignant tumors worldwide, has a high mortality rate with a 5-year survival rate of no more than 10%.[1,2]So far, although certain risk factors such as smoking, obesity, genetics, diabetes, diet, inactivity, have been identified,[3-5]the underlying molecular mechanisms of the cancer remains elusive.[6,7]Several case-control studies published previously have revealed that genetic susceptibility might relate to the risk of pancreatic cancer in different individuals.[8,9]
Glutathione S-transferases (GSTs), known as a family of phase II detoxification isozyme, play an important role in protecting cells from harmful chemical substances by catalyzing the conjugation of glutathione with a variety of electrophilic compounds, such as tobacco carcinogens and anticancer agents, etc.[10,11]Reduced glutathione S-transferase T1 (GSTT1) is the most common member of the soluble GSTs family. It has been proved thatGSTT1 gene polymorphism had a correlation with clinical prognosis of hepatocellular carcinoma and gastric cancer.[12-14]Individuals with homozygous deletion (null genotype) ofGSTT1 gene showed loss or deficiency of catalytic enzyme activity and increased susceptibility to the DNA damage induced by carcinogens.[11]A large amount of attention has been paid to the possible correlation betweenGSTT1 null genotypes and risk of pancreatic cancer, however, results from different laboratories do not coincide with each other. Therefore, we performed the meta analysis to clarify the underlying relationship betweenGSTT1 null genotype and pancreatic cancer risk.
MATERIALS AND METHODS
Literature search
We searched PubMed database, Chinese Journal Full Text Database (CNKI), and EMBASE using the search terms: “Pancreatic” OR “pancreas”; “cancer” OR “carcinoma” OR “tumor” OR “tumour” OR “neoplasm” OR“cancers”; “Polymorphism” OR “variant” OR “mutation”.The search was limited to the articles published up to April 18, 2018.
Inclusion and exclusion criteria
Case-control studies that investigated frequency of null genotype forGSTT1 of pancreatic cancer were included in the study. Studies having the incomplete sample data and improper statistical methods were excluded.
Study selection and data extraction
Data extracted from the eligible studies by two independent reviewers included: study characteristics (design,country, ethnics, publication year), genotyping methods,number of cases of pancreatic cancer and normal controls who carried null genotype ofGSTT1. Differences in study inclusion and data interpretation were resolved by consensus of the two reviewers.
Quality evaluation
Quality of studies was evaluated by using Newcastle-Ottawa scale according to the quality of selection, comparability and exposure. The references achieving a total score of 6 or higher would be finally enrolled in the study.
Statistical analysis
Statistical analysis was carried out with RevMan5.3 and Stata12.0 (StataCorp, College Station, Texas).Chi-squaretest was used to compare frequency of null genotype forGSTT1 between the pancreatic cancer group and the control group, and odds ratios (ORs) and 95% confidence intervals (CIs) were estimated. The inconsistency index(I2) statistic was performed to determine the heterogeneity of the included studies. If there was a significant heterogeneity (P< 0.1,I2> 50%), a random effect model was used for data analysis, or else a fixed effect model was used. Furthermore, sensitivity analysis was carried out by omitting each study involved in the Meta-analysis one by one to determine whether the data are stable and credible.The publication bias was assessed by Egger’s test and funnel plot drawing with RevMan5.3 software.Pvalues less than 0.05 were considered statistically significant.
RESULTS
Search results
There were 1089 articles were identified. After screened according to inclusion and exclusion criteria, 9 articles containing 8 written in English and 1 in Chinese were finally included. Duellet al.'s report was divided into three studies based on ethnicity.[15]The flow chart of literature screening is shown in Figure 1.
Characteristics of the included studies
A total of 9 case-control studies, including 2387 pancreatic cancer cases and 3565 healthy controls, met the inclusion criteria. The subjects were Caucasians,Asians and Africans, respectively. The basic characteristics of the included articles are illustrated in Table 1.Quality assessment results of the included studies are shown in Table 2.
Association of GSTT1 null genotype with pancreatic cancer
Heterogeneity analysis showedI2was 67% (P= 0.04),which was over the cut-off value (50%), therefore random effect model was used. As shown in Figure 2,the difference in frequency of null genotype forGSTT1 between the pancreatic cancer group and the control group was statistically significant (38.7%vs. 33.4%,OR= 1.26, 95%CI= 1.01-1.58,P= 0.04), suggesting thatGSTT1 gene deficiency would be associated with susceptibility of pancreatic cancer.
Sensitivity
Sensitivity analysis suggested that our results were relatively stable (Figure 3).
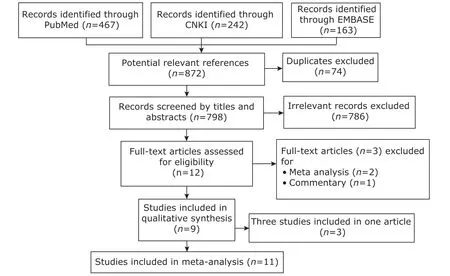
Figure 1. The flow chart of literature screening.
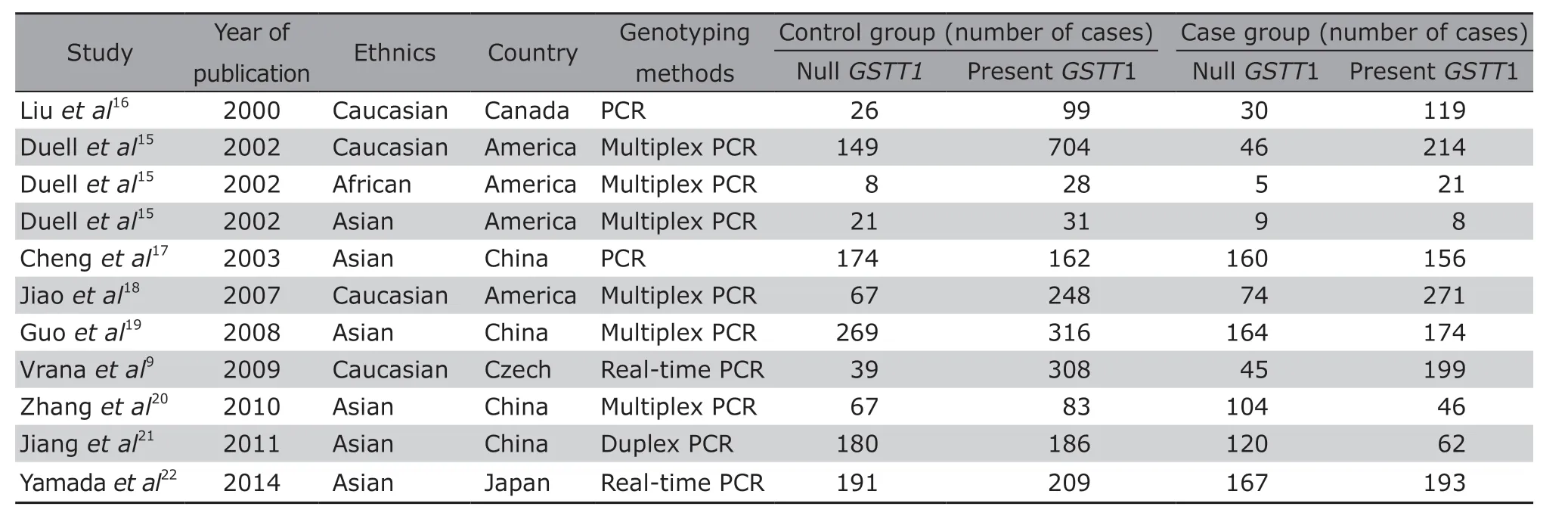
Table 1. Baseline characteristics of the studies included in the literature
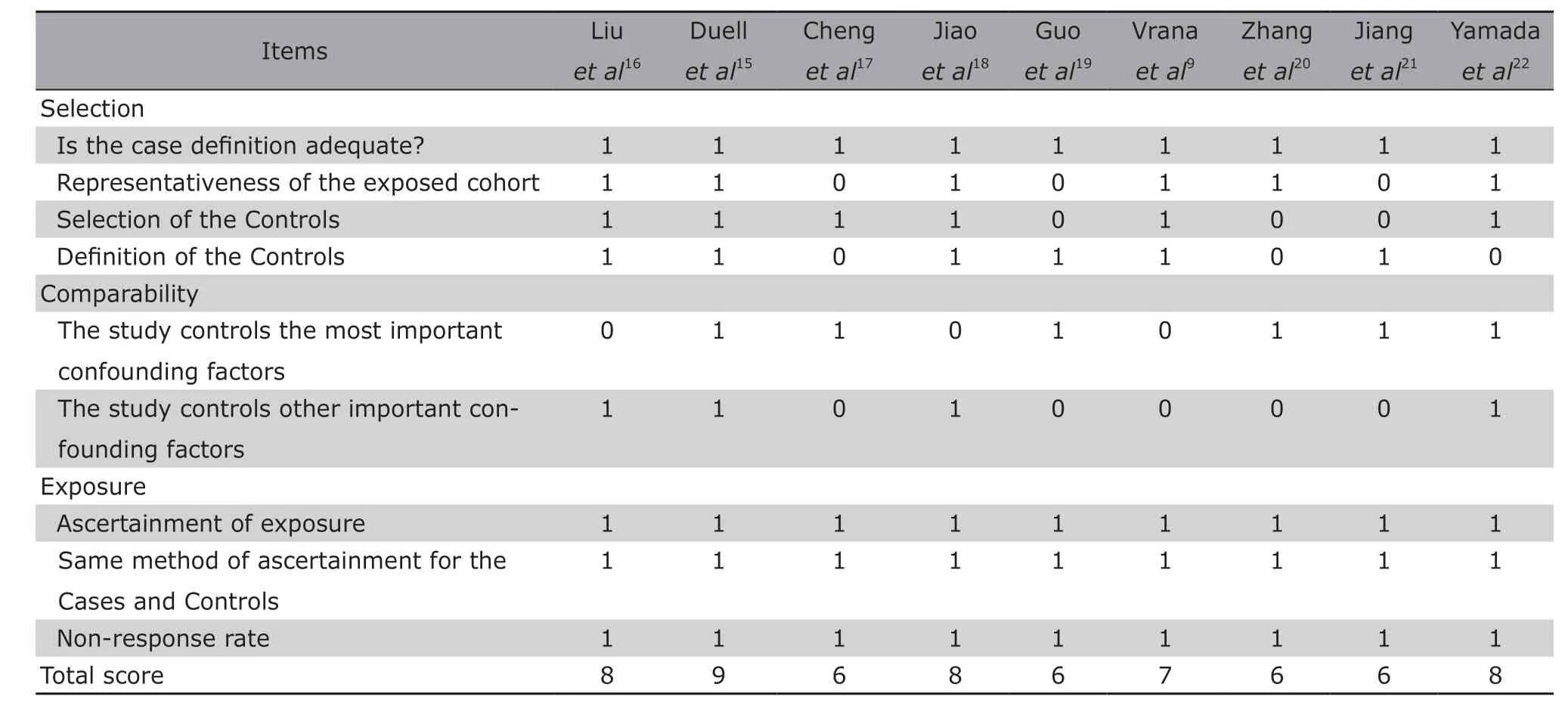
Table 2. Quality assessment of the included studies using Newcastle-Ottawa scale

Figure 2. Forest plot of the relationship between GSTT1 null genotype and risk of pancreatic cancer.
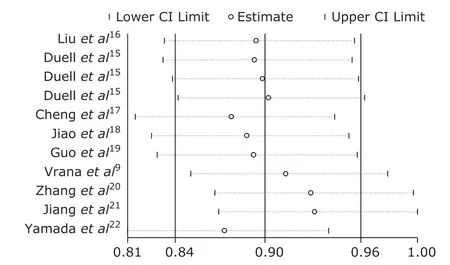
Figure 3. Sensitivity test of original studies.
Publication bias
The shape of the Funnel plot was basically symmetrical(Figure 4) and Egger’s test confirmed that there was no publication bias too (P= 0.294).
DISCUSSION
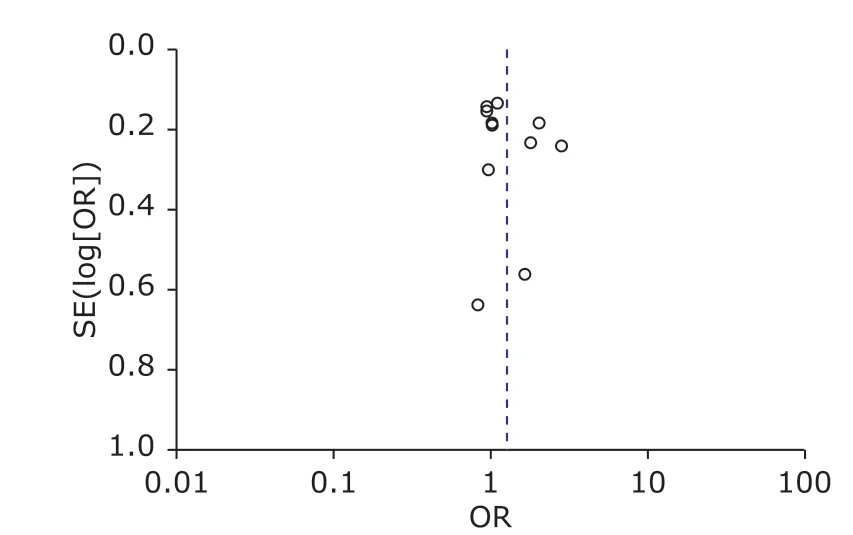
Figure 4. Funnel plot for publication bias test of the meta-analysis.
At present, the pathogenesis of pancreatic cancer has not been fully elucidated, but it is generally acknowledged that genetic factors play an important role in the occurrence of pancreatic cancer. GSTs,mainly including μ (GSTM), θ (GSTT), π (GSTP) and α (GSTA) members, belong to the second stage isoenzyme super family, which is able to detoxify endogenous oxidative stress products and exogenous carcinogens.[23]Homozygous deletion ofGSTT1 gene(null genotype) has been considered as a potential risk factor for various cancers, such as gastric cancer and renal cell carcinoma, etc.[24-26]Loss of enzyme activity induced by deficiency ofGSTT1, which decreases the ability of body to detoxify endogenous and exogenous carcinogens,[26,27]brings the individuals to be susceptible to pancreatic cancer.
In this paper, we enrolled 9 eligible casecontrol studies that researched the relationship between homozygous deletion ofGSTT1 gene and pancreatic cancer, to find the role ofGSTT1 deletion in susceptibility to pancreatic cancer. Meta analysis showed the frequency ofGSTT1 homozygous deletion in the pancreatic cancer group was higher than that in the healthy control group (P=0.04). Therefore,we concluded that individuals with homozygous deletion ofGSTT1 may have a higher susceptibility to pancreatic cancer. However, more large sample randomized controlled studies are needed to make a more accurate estimation.
Conflicts of interest statement
The authors have no conflict of interest to disclose.
杂志排行
Chinese Medical Sciences Journal的其它文章
- Research on the Antitumor Compounds from Cephalotaxus Hainanensis
- Artificial Musk R&D and Manufacturing
- Management of an Adult with Goodpasture’s Syndrome Following Brain Trauma with Extracorporeal Membrane Oxygenation: A Case Report
- Transvaginal Reduction of a Heterotopic Cornual Pregnancy with Conservation of Intrauterine Pregnancy
- Research Progress on Diagnosis and Treatment of Chronic Osteomyelitis
- IL-36β Promotes Inflammatory Activity and Inhibits Differentiation of Keratinocytes In Vitro
