Comparison of efficacy of continuous and intermittent walking exercise on blood pressure and renal function in elderly hypertensive subjects: study protocol for a randomized controlled trial
2019-10-12PiyapongPrasertsriOrachornBoonlaJatupornPhoemsapthaweeKanoknuchNaravorathamPetcharatTrongtosak
Piyapong Prasertsri, Orachorn Boonla Jatuporn Phoemsapthawee, Kanoknuch Naravoratham Petcharat Trongtosak
1 Faculty of Allied Health Sciences, Burapha University, Chonburi, Thailand
2 Faculty of Sports Science, Kasetsart University, Kamphaeng Saen Campus, Nakhon Pathom, Thailand
3 Exercise and Nutrition Sciences and Innovation Research Group, Burapha University, Chonburi, Thailand
Abstract
Key words:blood pressure; cardiovascular disease; cardiovascular risk factors; clinical registration; exercise; glomerular filtration rate; heart rate variability; oxidative stress; randomized controlled trial
INTRODUCTION
Research background
Hypertension (HTN) or high blood pressure (BP) is defined as abnormally high arterial BP. According to the Joint National Committee 7 (JNC7), HTN is defined as systolic BP level of ≥ 140 mmHg and/or diastolic BP level ≥ 90 mmHg.1The overall prevalence of HTN is similar between genders, but differs with age. For those 65 years old or older, HTN affects women more than men.2,3HTN is a major global health problem. HTN profoundly increases risk of chronic heart disease, stroke, and coronary heart disease, heart failure, peripheral vascular disease, and renal impairment.1,4,5Around 7.5 million deaths or 12.8% of the total of all annual deaths worldwide from HTN.Exercise is a useful adjunctive therapy in treating HTN. It has been shown that low-intensity exercise is effective for reducing BP in patients with HTN and cardiac rehabilitation,6-9as well as enhancing renal function in patients with chronic renal failure.10-12Most of the studies reported have applied continuous exercise, and there have been few studies of the efficacy of intermittent exercise. Additionally, there has been reported that elderly individuals are declined in physical fitness and exercise tolerance.13-15
Main objective
The purpose of this study is to evaluate and compare theefficacy of continuous and intermittent walking exercise on BP, renal function, and BP-related variables including cardiac autonomic function, cardiovascular risks, and oxidative stress in elderly individuals with HTN. This randomized controlled trial will reveal the difference in efficacy of continuous and intermittent walking exercise in the reduction of BP and the enhancement of renal function in elderly individuals with HTN. The objective data will show the potential efficacy of the two walking patterns as a non-pharmacological treatment of HTN.
METHODS/DESIGN
Study design
This is a prospective, single-center, randomized controlled trial at Faculty of Allied Health Sciences, Burapha University, Chonburi Province, Thailand. Fifty elderly subjects with HTN will be recruited from Mueang District, Chonburi Province, Thailand. The subjects will be randomized into two groups. The continuous walking exercise group (n= 25) will perform walking exercise continuously for 30 minutes/day, 3 days/week for 12 weeks. The intermittent walking exercise group (n= 25) will perform walking exercise intermittently for 10 minutes/session, 3 consecutive sessions/day with 1-minute interval between sessions, 3 days/week for 12 weeks. BP, renal function, cardiac autonomic function, cardiovascular risks, and oxidative stress will be evaluated before and after 12 weeks. Figure 1 shows the flow chart of the trial protocol.

Figure 1: Flow chart of the trial protocol.
Study setting
Faculty of Allied Health Sciences, Burapha University, Chonburi Province, Thailand.
Study population
Recruitment
We recruited 50 elderly subjects with HTN from Sub-district Health Promoting Hospital and Aging Society in Mueang District, Chonburi Province, Thailand from May to June 2018. Leaflets that contain the details of the study will be sent to staffs of each instution. Subjects who are interested in participating in the study will directly call the study leader and study assistant. After screening, based on the inclusion and exclusion criteria and provision of informed consent, the subjects will be enrolled in our study.
Eligibility criteria
Fifty elderly subjects, aged 60-80 years, who have been diagnosed with HTN and lived in Mueang District, Chonburi Province, Thailand will be enrolled in this study.
Inclusion criteria
Subjects who meet all of the following criteria will be considered for study inclusion:
· Aged 60-80 years
· Subjects who are diagnosed as having HTN or who have systolic BP 140-159 mmHg or diastolic BP 90-99 mmHg
· Provision of signed informed consent
Exclusion criteria
Subjects who meet one or more of the following conditions will be excluded from the study:
· Subjects who have obesity, cardiovascular disease, diabetes mellitus, renal disease, or thyroid disease
· Subjects who have musculoskeletal disorders which are obstacle to practice walking i.e., knee osteoarthritis
· Subjects who exercise regularly (more than 3 times/week)
· Unwilling to participate exercise program
Withdrawal criteria
Subjects who meet one or more of the following criteria during the trial will be withdrawn from the study:
· Unable to comply with the exercise program (practice less than 80% of the program)
· Unable to attend post-test visit
· Willing to quit the study
In a screening of the subjects, a physical examination including systolic BP, diastolic BP, and heart rate (HR) will perform along with the distribution of questionnaire forms. Classification of HTN in this study will be defined using 2013 European Saiety of Hypertension/European Society of Cardiology (ESH/ESC) Guidelines. According to the guidelines, HTN is classified as resting systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg. In addition to BP level, subjects with HTN will be classified as having a history, diagnosis, and/or treatment of HTN. Questionnaires which will be used in the screening of subjects include a health questionnaire form that will be usedto examine the subjects' underlying diseases, prior medication treatments, and history of illness. A Thai general health questionnaire will be used to examine the subjects' mental health. Subjects who have diabetes mellitus, thyroid disease, cardiovascular disease and/or renal disease will be excluded from the study. Subjects who regularly smoked, consumed alcohol or exercised will also be excluded.
Grouping, randomization and blinding
A random number table will be generated using IBM SPSS Statistics 21.0 (IBM SPSS, Chicago, IL, USA). Subjects will be numbered according to the sequence of enrollment. Eligible subjects with odd-number will be assigned into the continuous walking exercise group (n= 25), while those with even-number will be assigned into the intermittent walking exercise group (n= 25). This study is an open trial; all subjects will be aware of grouping and assessment.
Interventions
Continuous walking exercise group
Subjects in the continuous walking exercise group (n= 25) will perform walking exercise continuously for 30 minutes/day, 3 days/week, for 12 weeks. In a performing of continuous walking exercise, subjects will be instructed to walk rhythmically with the rate of 60 paces/minute or 1 pace/second.
Intermittent walking exercise group
Subjects in the intermittent walking exercise group (n= 25) will perform walking exercise intermittently for 10 minutes/session, three consecutive sessions/day with 1-minute interval between sessions, 3 days/week, for 12 weeks. Subjects in the intermittent walking exercise group will practice walking as same rhythm as those in the continuous walking exercise group.
Outcome measures
Primary outcome measures
Before and after 12 weeks of walking exercise intervention, systolic BP and diastolic BP levels will be measured using a digital automatic BP monitor (Microlife BP 3AQ1, Widnan, Switzerland). The BP cuff will thoroughly wrap on a subject's arm. The lower edge of the cuff will about 1 inch above the bend of elbow. systolic BP, diastolic BP, and HR will be measured for 3 times and 5 minutes apart. The mean among the three readings will be reported as the systolic BP, diastolic BP, and HR for each subject. Serum creatinine and uric acid concentrations will be evaluated as for renal function. In addition, estimated glomerular filtration rate and estimated creatinine clearance will also be calculated.
Secondary outcome measures
Before and after 12-week intervention cardiac autonomic function will be evaluated by HR variability (HRV) analysis. The HRV measurement will be attained by a Lead II electrocardiography for 10 minutes using PowerLab 4/30 (ADInstruments, Australia). Analysis of HRV parameters will include time domain and frequency domain. The time domain comprises the standard deviation of normal beat-to-beat (R-R) intervals (SDNN) and the root-mean-square of successive R-R (RMSSD). The frequency domain consists of the values of total power (TP), very low, low, and high frequency powers (VLF: 0 to 0.04 Hz, LF: 0.04 to 0.15 Hz, and HF: 0.15 to 0.4 Hz), and LF/HF ratio. Cardiovascular risks and oxidative stress will also be evaluated. After 12-hour overnight fasting, subject's blood will be drawn in the morning by venepuncture technique and will be collected in glucose, clot activator, and EDTA tubes. Blood samples for 2 mL in a glucose tube will be analyzed for plasma glucose concentration. Blood samples for 3 mL in a clot activator tube will be analyzed for concentrations of serum lipid profile (high-dendity lipoprotein cholesterol (HDLC), low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), and total cholesterol (TC)). Atherosclerogenic index will be calculated using the values of serum TC and HDL-C concentrations. Blood samples for 3 mL in an EDTA tube will be analyzed for oxidative stress including plasma malondialdehyde and blood glutathione and glutathione disulfide.
The schedule of outcome measurement assessments is shown in Table 1.
Safety assessment
During a 12-week intervention, subjects will be closely followedviatelephone communication by a study assistant. Adverse events including injury associated with the study intervention will be recorded. If severe adverse events occur, the details of the adverse events will be reported to the principle investigator and the Human Ethics Committee within 24 hours.
Sample size estimation
A statistical formula for comparison of means of two groups was used in this study. A study of Hernández-Torres et al.16was applied to the formula. They reported that mean difference in HDL-C concentration after continuous and intermittent exercise was 6 mg/dL, with SD 12.7. The decision was made to require 80% power with aP-value less than 0.05. Hence, the anticipated sample size will be 20 subjects per group and the total will be 50 subjects, including 25% drop out rate estimation.
Statistical analysis
IBM SPSS Statistics 21.0 (IBM Company, Chicago, IL, USA) will be conducted to analyze data. Shapiro-Wilk test will be used to evaluate the normality of distribution and data will be expressed as the mean ± SD. Pairedt-test will be used for intragroup comparison of outcomes before and after 12-week intervention. Two-samplet-test will be used to compare outcomes between the two groups.P-value less than 0.05 will be considered statistically significant.
Data collection and management
Missing data processing
In the case of missing data, the data obtained from any subject who will not properly complete the intervention will be recorded, yet not included for analysis.
Baseline data collection
The baseline information that will be collected includes demographic data, general disease history, and clinical data (Table 2).
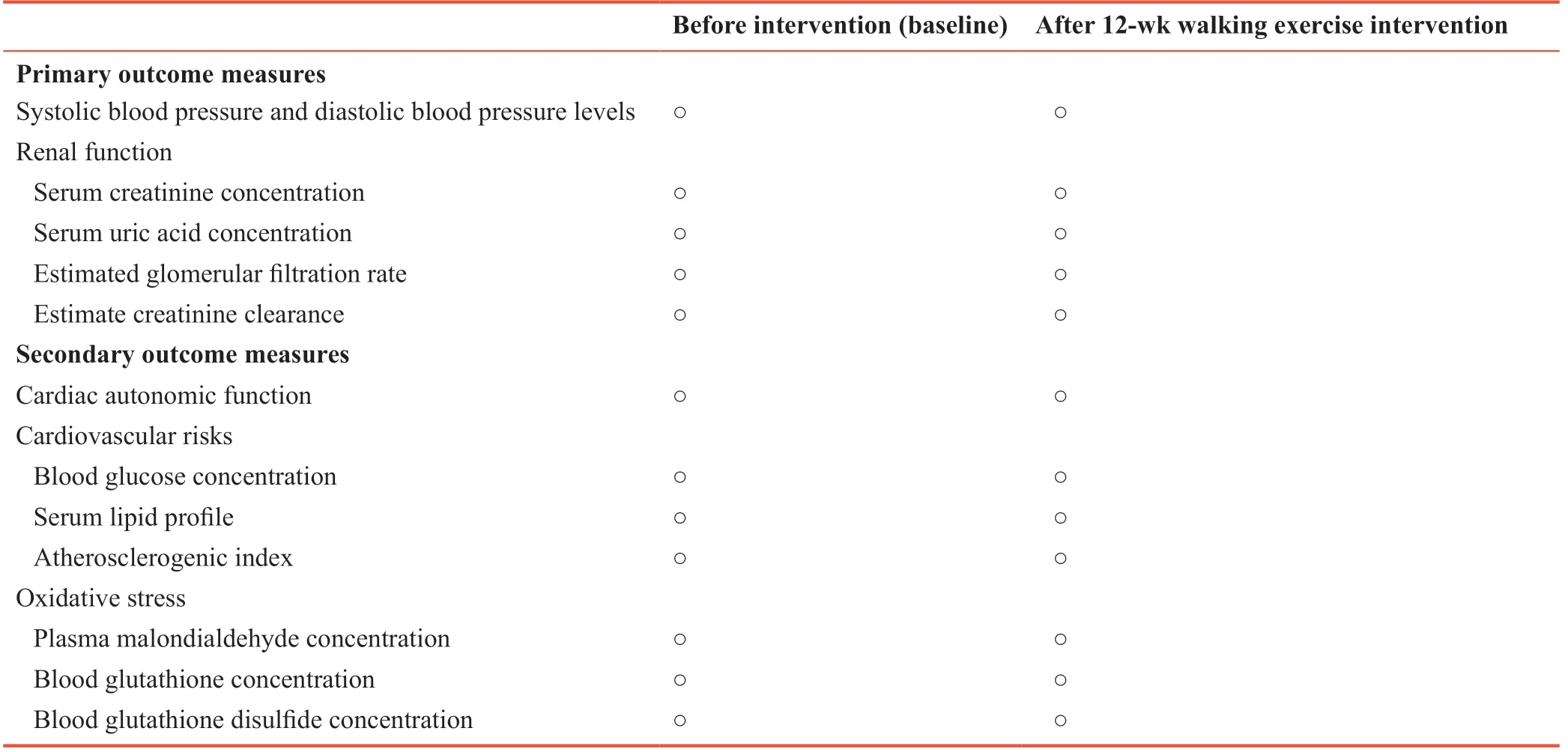
Table 1: Schedule of outcome measures

Table 2: Baseline data and general disease history of the enrolled subjects
Data management
The entire trial process will adhere to the quality management standards for clinical trials. Data of each subject will be recorded completely, accurately, clearly, and objectively. All data will be input into the computer, locked, and stored. To ensure the accuracy of the data, two data entry personnel will perform double entry and proofreading, separately. After checking, the database will be locked by the principle investigator, and will not be altered.
Monitoring
Audits
Initial stage: The study protocols have been approved by the Human Ethics Committee of the Burapha University. The trial agreement has been signed.
During the trial: During recruitment of subjects, regular monitoring ensures that the enrolled subjects will comply with the inclusion criteria and be excluded if they meet the exclusion criteria. All procedures will be corrected in accordance with the scheme to certify data reliability.
Final stage: After the subject completes all examinations, a final visit will be required to confirm the data precision.
Quality control of the clinical trial
During the trial, sponsor inspectors will conduct regular periodic visits to the research center to ensure all adherences to the research project. The original data will be checked to ensure that the data are precise and complete.
Ethics and dissemination
Ethical considerations and informed consent
The study protocol will be conducted in accordance with theDeclaration of Helsinki. The data will be reported in accordance with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) (Additional file 1). All subjects will be informed of their role in the study by a researcher prior to sign a consent form. The consent form will provide information regarding collection, usage, and storage of subject data. This study was approved by the Human Ethics Committee of the Burapha University on January 5, 2018 (approval No. 218/2560).
Dissemination
We plan to present results in open access scientific publications and at scientific conferences and meetings.
DISCUSSION
Achievements and existing problems
Low-intensity exercise has been shown to enhance efficacy of medical treatment of HTN. Elderly individuals are limited in exercise tolerance due to their decline in physical fitness. Evaluation of the efficacy of continuous and intermittent low-intensity exercise will provide a novel information as a therapeutic treatment in elderly individuals with HTN.
Limitations of this study
The intervention of this study is a home-based exercise training program. Although during a 12-week intervention subjects willbe closely followed by a study assistant and will be asked to record their exercise schedule, we cannot completely control physical activities as well as food consumption behavior of subjects. In addition, alteration in medication administered of subjects may influence the objectivity of the outcomes of this study.
Strengths of this study
Comparison of the efficacy of continuous and intermittent walking exercise on BP, renal function, and BP-related variables including cardiac autonomic function, cardiovascular risks, and oxidative stress in elderly individuals with HTN has not previously been reported.
Significance of this study
This is a prospective, randomized controlled trial aiming to evaluate the efficacy of continuous and intermittent walking exercise on reducing BP and enhancing renal function in elderly individuals with HTN. In addition, BP-related variables including cardiac autonomic function, cardiovascular risks, and oxidative stress will be investigated.
In this study, we attempt to evaluate the efficacy of intermittent walking exercise that is expected to reduce BP, improve renal function, improve cardiac autonomic function, improve cardiovascular risks, and improve oxidative stress in elderly individuals with HTN as same as continuous walking exercise. The intermittent walking exercise can be applied as an effectively therapeutic treatment of HTN in elderly individuals.
TRIAL STATUS
Recruitment of subjects began on May 2, 2018. The completion of trial will be on October 27, 2018.
Additional file
Additional file 1: SPIRIT checklist.
Author contributions
Study design, manuscript writing, and subject recruitment: PP. Data collection and analysis: PP, OB, JP, KN and PT. All authors approved the final version of the manuscript.
Conflicts of interest
None declared.
Financial support
This work was supported by the National Research Council of Thailand under Grant 230/2561 (to PP).
Institutional review board statement
The study protocol has been conducted in accordance with theDeclaration of Helsink. This trial was approved by the Human Ethics Committee of the Burapha University (approval No. 218/2560). Written informed consent is obtained from all participants. This trial was registered in the Thai Clinical Trials Registry (TCTR) (identification No. TCTR20180226003) on February 23, 2018.
Declaration of patient consent
The authors certify that they will obtain all appropriate patient consent forms. In the forms the patients will give their consent for patients' images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement
This study follows the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidance for protocol reporting.
Biostatistics statement
The statistical methods of this study are reviewed by the biostatistician of Burapha University.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
Individual participant data will be in particular shared after deidentification. Anonymized individual data will be available through presentations at scientific meetings and/or by publication in a peer-reviewed journal without end date. The data will also be available immediately upon request from those who wish to access the data. If requested, study protocols and outputs of statistical analysis will be available.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
Clinical Trials in Degenerative Diseases的其它文章
- A study protocol on effectiveness of theory-based intervention on self-care and glycated hemoglobin among type 2 diabetes patients in National Center for Diabetes in Yemen
- Effects of electroanalgesia on knee osteoarthritis: study protocol for a randomized, triple-blind, placebo-controlled trial
- Changes in insulin resistance and inflammatory factors in cataract patients with glaucoma after phacoemulsification and trabeculectomy: a self-controlled trial
