A study protocol on effectiveness of theory-based intervention on self-care and glycated hemoglobin among type 2 diabetes patients in National Center for Diabetes in Yemen
2019-10-12AbeerYahyaAhmedAlWashaliHejarAbdulRahmanHayatiKadriShaharSurianiIsmailYahyaElezzy
Abeer Yahya Ahmed Al-Washali, Hejar Abdul Rahman Hayati Kadri Shahar Suriani Ismail Yahya A. Elezzy
1 Department of Community Health, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia 43000 UPM Serdang, Selangor, Malaysia
2 Faculty of Medicine, Sana'a University, Head of Medical Department, Al Thawra General Teaching Hospital, Sana'a, Yemen
Abstract
Key words:diabetes; diet; foot care; HbA1c; Information-Motivation-Behavioral Skills; physical activity; self-care; Yemen
INTRODUCTION
Diabetes is one of the non-communicable diseases, which becomes a health problem worldwide due to a continuous increase in its prevalence.1The World Health Organization estimates that 422 million adults have diabetes mellitus worldwide.2Type 2 diabetes accounts for 85-95% of all diabetes in high-income countries and may account for an even higher percentage in low and middle-income countries.3Globally in past 30 years, type 2 diabetes prevalence has been rapidly increased.4According to the World Health Organization, about 9% of adults over 18 years have diabetes.5
Diabetes is a complex, chronic illness that needs unceasing medical care with multifactorial risk reduction strategies. Diabetes management aims to prevent diabetes-related morbidity, mortality, and decrease diabetes economic costs. To achieve these aims, adequate glycemic control has been recommended.6Many studies indicate that self-care behaviors influence glycemic control.7,8Self-care represents a key factor for diabetes patients to maintain the quality of life and to prevent serious disease complications.9Adequate self-care has been shown to improve blood glucose levels, glycated haemoglobin (HbA1c), and dietary habits.10
Despite the importance of glycemic control, data from several studies have shown that the diabetic patients in Yemen have poor glycemic control with the mean HbA1C of 9%.11-13Poor glycemic control among the majority of patients is an alarming sign of low-quality diabetes care in this country. Little is reported to assess the self-care behaviors among diabetic patients in Yemen. However, studies from the neighboring countries showed that there is a poor adherence to the diabetes self-care behavior among patients with type 2 diabetes.14-16In Oman, D'Souza et al.15found that there were inadequateself-care behaviors among the majority of adults with type 2 diabetes with poor glycemic control. In Saudi Arabia, Sabbah and Al-Shehri17reported that there was a poor practice on the management plan of diabetic care particularly the nonpharmacological component of the plan.
Inadequate diabetic self-care remains a significant problem facing health care providers and populations in all settings. Inadequate self-care increases patient's morbidity and mortality, costs of medication and laboratory tests and cost in time and effort of the care providers.18The purpose of this study was to develop, implement and evaluate the effect of Information-Motivation-Behavioral Skills (IMB) model intervention on diabetes self-care behaviors (diet, physical activity and foot care) and HbA1c among patients with type 2 diabetes in the National Center for Diabetes in Yemen.
METHODS/DESIGN
Design
An open-label parallel, randomized controlled trial will be carried out in the National Center for Diabetes in Yemen (Figure shows the CONSORT flow chart of the study procedure). This trial was registered in the Australian New Zealand Clinical Trials Registry (ANZCTR) on July 17, 2017 (registration number: ACTRN12617001037392; http://www.anzctr.org.au/ACTRN12617001037392.aspx.). The screening and enrollment of the participants will be carried out for 1 month. After providing informed consent, the eligible participants will be randomized either to an intervention group or a control group (usual care). The assessments will be conducted at baseline and 6 months post-intervention. Table 1 summarizes the timing of the trial.
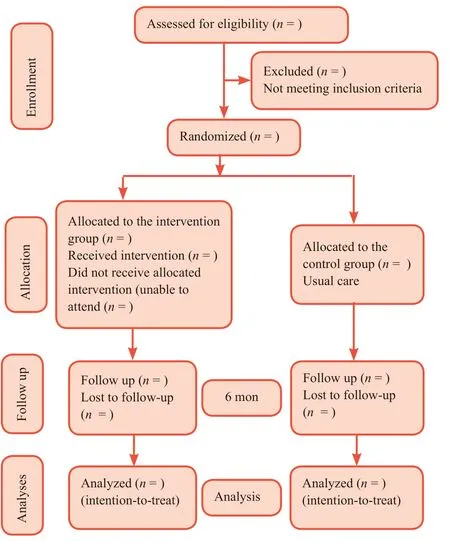
Figure 1: CONSORT flow chart.

Table 1: Study assessment schedules
Setting
The study will be conducted in National Center for Diabetes, in Sana'a, Yemen. The center was inaugurated in March 2007 and is hosted by the main referral hospital in Yemen, Al Thawra Modern General and Teaching Hospital. The hospital is located in Sana'a city, the capital of Yemen, and it is a tertiary hospital.
Eligibility criteria
The eligible participants will be male and female Yemeni patients, aged 18 years or older, and have a clinical diagnosis of type 2 diabetes mellitus (according to the diagnostic criteria formulated by the World Health Organization in 199919) for at least 1 year and their HbA1c will be between 7% and 12%. Patients will be excluded from the study if they meet one of following criteria: severe diabetes complications, cognitive impairment, pregnant woman, and not providing informed consent.
Sample size
The formula by Rosner (2011)20will be used for sample size estimationn= 2σ2 (Z1-β + Z1-α/2)2 / (μ1μ2)2. The primary outcome measure used for sample size determination will be the change in diet behavior score. Results from previous diabetes randomized controlled trial21showed that the difference in the mean diet behavior score between the intervention and control groups was 0.77, and the standard deviation (σ2) was 3.6485. Taking into account an additional 20% for attrition rate, the final sample size will be 242 participants.
Randomization
The participants will be randomized to the intervention group or the control group using block randomization to ensure equal group sizes. An independent person will create the randomization sequence using computer software program (Random Allocation Software Version 1.0)22that generates the random sequences with a 1:1 allocation using random block sizes. The block sizes will not be disclosed, to ensure concealment. Sequentially numbered, opaque, sealed envelopes will be used to conceal the randomization sequence. A nurse, who knows nothing about the research, will allocate the participants. The name of the patients will be written on the envelope before it is opened.
Intervention methods
Intervention group
The intervention will be designed based on the IMB model23to address patients' diet, physical activity, foot care information, motivation, and behavioral skill deficits. Intervention foci will be derived from an extensive review of the literature and lengthy conversations with experts in the field.
The researcher will use power point presentation, discussion and role-play to deliver the intervention. All participants in the intervention group will be allocated into small subgroups consisting of 20 participants for more effective interaction within the group. The intervention will consist of one session lasting 3 hours and will include all IMB model elements.
The questions to be discussed will be used to motivate the patients. The questions to be discussed for diet and physical activity were developed in a previous study.24Osborn24used the Information Motivation Behavioral Skill model of health behavior change to promote self-management behaviors in Puerto Ricans with diabetes. Only two questions for foot care will be developed for this study. The first one will be about the barriers of foot care, and the second one will be about their goal and plan for foot care.
Information:Information related to the diet, physical activity, and foot care will be presented. The researcher will present a brief introduction about the food contents and which food content raises blood glucose level and the ways to control the blood glucose level through monitoring carbohydrate intake. To enhance physical activity information, the researcher will explain the benefits of physical activity, the risk of inactivity and the ways to increasing physical activity during the day. The foot care information will include diabetes-related foot problems, the importance of foot care and how to take care of the feet.
Motivation:The researcher will use the discussion to enhance motivation to perform diabetes self-care behaviors (diet, physical activity, and foot care). The discussion will include asking open-ended questions, affirming desirable behaviors and negotiating goals that are realistic and attainable. The researcher also will serve as a source of social normative support for diabetes self-care behavior change, and help patients identify other sources of such support.
Behavioral skills:Patients will be given behavioral skills training on how to monitor carbohydrates portion sizes, integrate physical activity into their lifestyle by doing activities at home that do not require costly equipment and how to wash, dry and moisten their feet. The patients will also be given training on how to inspect the bottom of the feet, test water temperature as well as check shoes before wearing.
Control group
The control group will receive the usual care, which will include a combination of physician monitoring, medical treatment and an optional education session about the healthy diet. The control group will be in the waiting list to get the intervention, which will be at the end of the study after posttest data collection
Baseline assessment
The socio-demographic information will be measured with five items. Participants will report their gender, age, the highest level of completed education, employment status and marital status. The medical history information will be measured with three items. Participants will report their diabetes duration and type of medication they used for diabetes. Patient Health Questionnaire (PHQ-9) will be used25to assess the depression. The PHQ has nine items and is used to make a diagnosis of depressive disorder.
Outcome measures
Primary outcome measures
The primary outcomes of this study will be diabetes self-care behaviors (diet, physical activity, foot care) and hemoglobin A1c (HbA1c). These outcomes will be assessed at baseline and after a 6-month follow-up. Self-care behavior will be assessed with the 11-item Summary of Diabetes Self-Care Activities (SDSCA) scale.26The SDSCA scale measures frequency of self-care activity in the last 7 days for five aspects of the diabetes regimen: general diet (following the healthy diet), specific diet (eating fruits/low-fat diet), foot care, blood-glucose testing, exercise, and cigarette smoking. For this general analysis, diet, foot care and exercise will be used.
HbA1c will be measured with the Tina-quant turbidimetric immunoassay in the laboratory of the National Center for Diabetes. Three mL of the venous blood will be collected from the participants to measure HbA1c.
Secondary outcome measure
The secondary outcome will be the change in the IMB of diabetes self-care behaviors (diet, physical activity, foot care). The IMB measures for diet and physical activity were developed in a previous study by Osborn et al.20The same measurement will be used in this study to measure IMB for diet and physical activity. The IMB measures for foot care will be developed for this study.
A pilot study will be conducted to validate the questionnaire and the intervention module and to check if one month is enough to achieve adequate participant enrolment to reach target sample size.
Data management
Double data entry will be used to enter the data, so we can check for mismatches or out-of-range values. Access to the study data will be restricted. Every effort will be made to ensure the confidentiality of any identifying information that is obtained in connection with this study. To keep it private, a number (not the participant's name) will be assigned to the information participants give. All participants' information will be kept in locked cabinets and will be destroyed when the study is done. All the information of participants will only be used for research, and will only be seen by the researcher.
Statistical analysis
All analyses will be performed using SPSS version 23 (IBM, Armonk, NY, USA),P-value less than 0.05 will be considered as significant, and intention-to-treat analysis will be used to analyze the data. The following strategies will be conducted to minimize and handle the missing data:
a) The investigator tries to limit the burden to the participant by reducing required visits (only two visits) and amount of data collected (not all self-care behaviors will be included in this study).
b) The investigator will encourage the participants to complete the study. Intermittent phone calls will be used to keep the study participants engaged in the study. The HbA1c test will be offered free to all the participants to encourage the participants to complete the study.
c) The investigator will follow up all randomized subjects, even if they withdraw from an allocated intervention.
d) During the analytical stage, the missing data will be analyzed to determine the amount and patterns of missing data and variables associated with missing data.
e) The primary analysis will be performed using maximum likelihood estimation.
f) Sensitivity analysis will be performed to assess the assumptions made in the primary analysis.
Baseline group equivalence will be assessed using Pearson's chi-square tests for categorical variables and Student'st-test for continuous variables. Analysis of covariance and multivariate analysis of covariance models will be used to test the intervention effect on a diet, physical activity and foot care behavior, HbA1c and diet, physical activity and foot care information, motivation, and behavioral skills.
Data monitoring
The data monitoring committee has not been formed for this study because of the short duration between recruitment and follow-up and low-risk nature of this study. The research team will keep track of the progress of this study. The research team will not carry out an interim analysis because this study does not involve any drugs or medical procedures.
Ethics and dissemination
Study protocol: 1.0. The protocol was approved by the Ethics Committee for Research Involving Human Subjects Universiti Putra Malaysia on February 7, 2017 (reference No. FPSK(FR16)015) and Al Thawra Hospital Ethics Committee (reference No. 2/2018). This trial will be conducted in accordance with theDeclaration of Helsinki. All the patients will provide written informed consent. The findings of this study will be submitted to an open-access peer-reviewed journal. A summary of the findings will be sent to the National Center of Diabetes in Yemen, so the participants and the healthcare professionals can know the findings of this study. A full study report will be sent to the National Information Center in Yemen and will be available to the public.
DISCUSSION
This is a 6-month theory-based intervention for patients with type 2 diabetes with the aim to improve self-care behaviors and decrease HbA1c. The IMB model guides intervention development. This intervention will provide the participants with information related to the diabetes self-care behaviors, motivate them to do these behaviors, and finally provide them with skills to perform the self-care behaviors.
We expect that the self-care behaviors will improve and decrease HbA1c in the intervention group compared with the control group. The results of this study will increase our understanding of how a theory-based intervention can guide the development of the intervention to improve diabetes selfcare behaviors.
TRIAL STATUS
Patient recruitment began in February 2018. Analysis of primary outcome measure will be completed in July 2019, and the study will be finished in September 2019. We are currently analyzing the study outcomes.
Additional file
Additional file 1: Ethical Approval Documentation.
Additional file 2: Model consent form.
Additional file 3: SPIRIT checklist.
Acknowledgments
We would like to thank the personnel from the health care centers that are important in the recruitment of study participants. We would also like to thank the Bio-pharmaceutical Industry in Yemen for their support.
Author contributions
Study concept and design: AYAW, ARH, KSH, IS, YAE; principle investigator: AYAW; manuscript drifting: AYAW, KSH. All authors gave final approval for publication.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Financial support
None.
Institutional review board statement
The protocol was approved by the Ethics Committee for Research Involving Human Subjects from Universiti Putra Malaysia on February 7, 2017 (reference No. FPSK(FR16)015) and Al Thawra Hospital Ethics Committee (reference No. 2/2018). All the patients will provide written informed consent.
Declaration of patient consent
The authors certify that they will obtain all appropriate patient consent forms. In the forms the patients will give their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement
This study followed the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidance for protocol reporting.
Biostatistics statement
The statistical methods of this study were reviewed by the biostatistician of University Putra Malaysia.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
Anonymized individual data will be available immediately after study publication upon request from those who wish to access the data for 3 years. Personal results will also be available to participants upon request. Results will be disseminated through presentations at scientific meetings and/or by publication in a peer-reviewed journal.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, andbuild upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
Clinical Trials in Degenerative Diseases的其它文章
- Effects of electroanalgesia on knee osteoarthritis: study protocol for a randomized, triple-blind, placebo-controlled trial
- Comparison of efficacy of continuous and intermittent walking exercise on blood pressure and renal function in elderly hypertensive subjects: study protocol for a randomized controlled trial
- Changes in insulin resistance and inflammatory factors in cataract patients with glaucoma after phacoemulsification and trabeculectomy: a self-controlled trial
