Influence of oxygen plasma treatment on the surface properties of indium-tin oxide thin films
2019-10-11GUJinhuaLUZhouKANGHuai
GU Jinhua, LU Zhou, KANG Huai
(1 Experimental Teaching and Laboratory Management Center, South-Central University for Nationalities, Wuhan 430074, China;2 College of Electronic Information Engineering, South-Central University for Nationalities, Wuhan 430074, China)
Abstract The surfaces of indium-tin oxide (ITO)thin films were modified by oxygen plasma discharge.The effect of oxygen plasma treatment on the surface properties of ITO films and the changes in surface properties of treated ITO films with the ageing time were investigated through the measurements of chemical composition, contact angle, surface energy and polarity.Experimental results show that oxygen plasma treatment improves the stoichiometry of ITO surface, increases the surface energy and polarity, and enhances surface wettability of ITO, so as to improve the surface properties of ITO owing to the effective removal of the contaminants of the ITO surface.In addition, with the increment of ageing time after oxygen plasma treatment, the improved stoichiometry, surface energy and polarity of the ITO surface tend to decay, and the optimized ITO surface properties become worse and worse.
Keywords indium-tin oxide, oxygen plasma treatment, surface properties
Indium-tin oxide (ITO)is a commonly used substrate for the construction of organic optoelectronic devices because ofits unique characteristics for instance low electric resistivity, high visible transmittance, excellent adhesion to substrates, chemical stability and easy patterning ability[1-3].It is well known that the organic light-emitting device (OLED)usually consists of a sandwich structure with the organic thin film deposited onto the ITO transparent anode and covered by patterned top metal cathode contacts[4-8].Since the organic thin film is in direct contact with the ITO anode, the surface characteristics of the ITO anode plays an important role in improving the OLED performance.Many studies have been reported on modifying the surface properties of the ITO.They include annealing process[9-11], oxygen or argon plasma[12-14],oxygen glow discharge[15], ultraviolet-ozone clearing[16,17], electro-chemical treatment[18], acid and base adsorption[19], SF6plasma[20], low energy ion beam treatment[21], and coating treatment with self-assembled monolayers[22,23].Among them, oxygen plasma was considered as a promising and efficient treatment because it results in the highest work function, the lowest sheet resistance, and the smoothest surface[13,14].In this work, oxygen plasma treatment was carried out on the ITO substrates.The influence of oxygen plasma treatment on the surface properties of ITO films and the changes in surface properties of treated ITO samples with the ageing time were studied by X-ray photoelectron spectroscopy (XPS), contact angle and surface energy measurements.
1 Theoretical
Surface energy is a direct manifestation of intermolecular forces.The surface of a solid, like that of a liquid, possesses additional free energy, but owing to the reduced molecular mobility this free energy is not directly observable, and it must be measured by indirect methods.
When a liquid drop is in contact with an ideally smooth, homogeneous, planar, and nondeformable surface, it exhibits an equilibrium contact angle that can be expressed by Young’s equation[24]:
γL·cosθ=γS-γSL,
(1)
where γLis the surface tension of the liquid, γSthe surface energy of the solid,γSLthe interfacial tension between the solid and the liquid, andθthe equilibrium contact angle formed by the liquid on the solid.
It was proposed that thesurface energy (γ)is composed of two components[24]: the polar component (γp)including three different intermolecular forces due to permanent and induced dipoles, and hydrogen-bonding, whereas the dispersion component (γd)is due to instantaneous dipole moments.Their relationship is given by the following equation:
γ=γp+γd,
(2)
The work of adhesion between the solid and the liquid (the negative of the Gibbs energy of adhesion),Wa, can be expressed by the Dupré equation:
Wa=γS+γL-γSL,
(3)
The Young-Dupré equation, therefore, is achieved by combining Eqs.(1)and (3):
Wa=γL·(1+cosθ),
(4)
This equation implies that the solid-liquid work of adhesion can be simply calculated from liquid surface tension and contact angle measurements.
According to the theory of fractional polarity[24], the work of adhesion between the solid and the liquid can also be expressed by the following equation:
(5)
Combination of the above with Eq.(4)yields
(6)
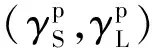
(7)
(8)

(9)
(10)
2 Experimental
2.1 Sample preparation
Commercial ITO-coated glasses with film thickness and sheet resistance of 150 nm and 30Ω/sq respectively were used and cut into 3.0 cm×3.0 cm plates in this experiment.Prior to their use, the ITO samples were routinely cleaned by rubbing in a detergent, rinsing in deionized water, successive ultrasonification with acetone and isopropanol each for 15 min, and finally dried in a flow of nitrogen.Then, the ITO samples were modified using oxygen plasma discharge (oxygen purity was 99.999%)at room temperature.The reaction chamber was evacuated to 10 Pa pressure and the working pressure during the plasma discharge was about 15 Pa.The gas flow rate, the discharge power and the treatment duration were 20 sccm, 35 W and 6 min, respectively.
2.2 Surface analysis
The XPS measurements were carried out by a XSAM-800 X-ray photoelectron spectrometer, using a monochromatic Al Kα(hν=1486.60 eV)as X-ray source.Typically the operating pressure in the analysis chamber was about 210-7Pa.All binding energies were referenced to the binding energy of the carbon C 1s peak at 285.0 eV.For the calculation of the atomic concentrations, a linear background correction was done, and the peak areas were corrected with empirical sensitivity factors, the instruments transmission function and the specific mean free path lengths[25].
The contact angles were measured by the sessile drop technique[24]using a JY-82 type contact angle analyzer at 20°C in an environmental chamber.The reported values of contact angles are the mean of five measurements.Distilled water (H2O)and methylene iodide (CH2I2, from Beijing Chemical Reagents Company)were used as the probe liquids[23], and their surface tension and surface tension components are listed in Table 1.
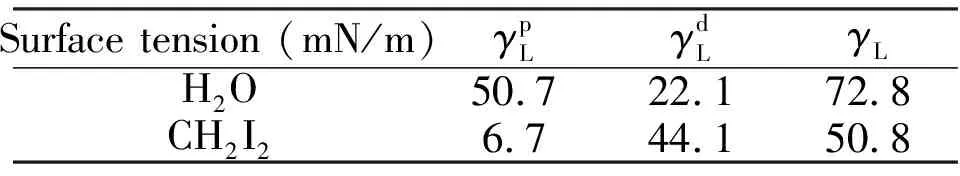
Tab.1 The surface tension components of probe liquids
3 Results and discussion
3.1 XPS analysis
Fig.1 shows the high-resolution O 1s XPS spectra for the ITO samples.The O 1s XPS spectrum exhibits an asymmetric peak that was fitted to two components: the main peak O(1)centred at about 530.4 eV can be assigned to O2-ions in the tetrahedral interstices of the face-centred-cubic In3+ion array, while the shoulder peak O(2)centred at about 532.0 eV is due to residual surface contaminants[24].
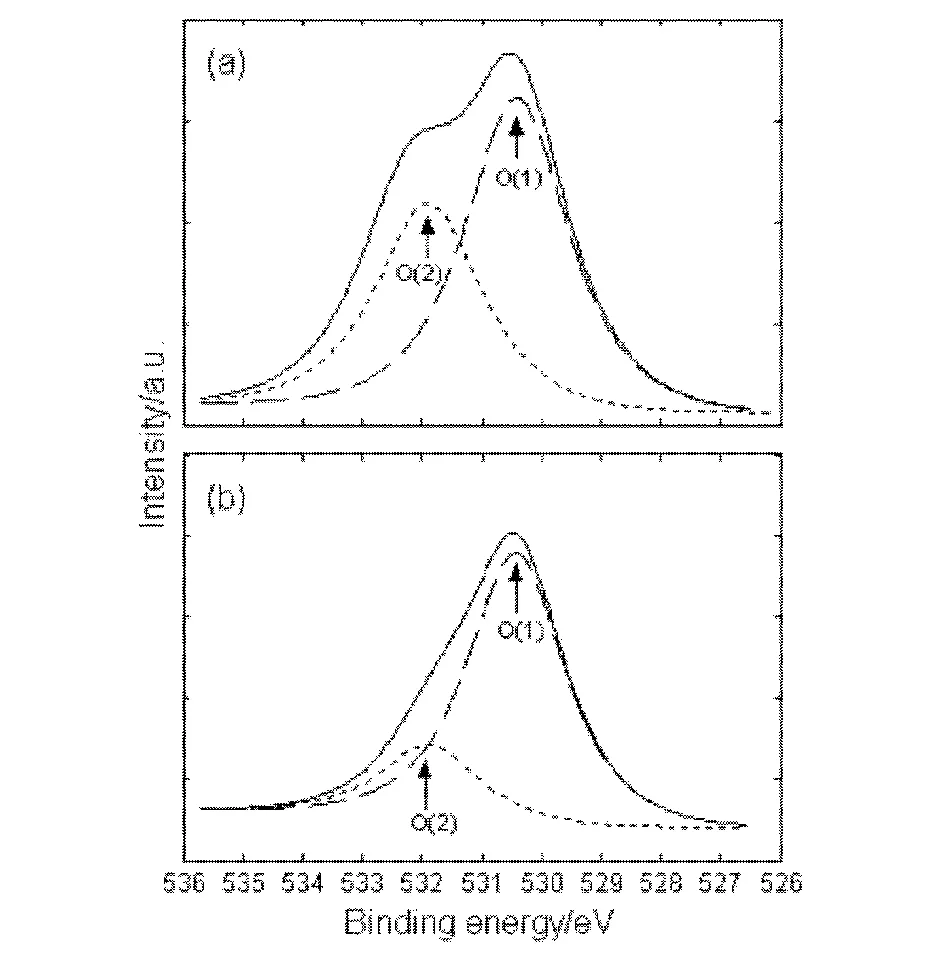
Fig.1 O 1s high-resolution XPS spectra for (a)untreated and (b)treated ITO samples
The intensity of O(2)for the treated ITO is observed to largely decrease compared to that of the non-treated ITO, indicating the oxygen plasma treatment effectively removes the contaminants from the ITO surface.The XPS analysis results of the ITO samples are summarized in Table 2.Note that the ratios of O/In and C/In respectively change from 1.132 and 0.342 on the non-treated ITO to 1.929 and 0.129 on the treated ITO, while the Sn/In ratio is hardly changed by the plasma discharge, remaining near 0.160.This means the oxygen plasma treatment used here does not etch the ITO surfaces.Note also that besides containing the elements of In, Sn and O, the carbon element also exists on the ITO samples, suggesting that carbon is the only major contaminant.Similar results have also been reported in the previously literatures[12,13,26].The concentrations of In, Sn, O and C on ITO surface are observed to change from 38.0, 6.0, 43.0, 13.0 at.% to 31.0, 5.2, 59.8, 4.0 at.% respectively after the plasma discharge.The result demonstrates that the oxygen plasma treatment largely increases the oxygen concentration and decreases the carbon concentration, and improves the surface stoichiometry, and thereby effectively enhances the work function of the ITO, since the ITO work function is determined by the oxygen composition or by the carbon concentration[27-29].
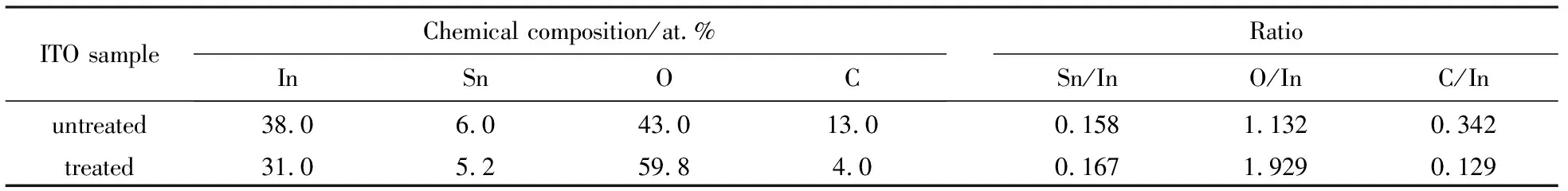
Tab.2 XPS analysis results of the ITO surfaces
Fig.2 gives the evolution of chemical composition of ITO surfaces as a function of the time after plasma discharge.As can be seen, the concentrations of In, O and C are affected by the elapsed time after plasma discharge, except Sn concentration is not changed.The marked changes in the concentrations of In, O and C can be observed within 2 h after the plasma discharge.After 21 h, rather little change is found and the concentrations of In, O and C exhibit to reach the plateau values of 37.5, 43.9 and 12.7 at.%, respectively.The change in chemical composition of treated ITO surface probably results from the partial release of plasma-introduced oxygen from the ITO surface and the re-contamination of the surface by hydrocarbons in air during the elapsed time after the plasma discharge.The results indicate that the improvement in the surface stoichiometry due to the plasma discharge is not stable.

Fig.2 Chemical composition as a function of the time after plasma discharge for the treated ITO samples
3.2 Surface wettability
Fig.3 shows the contact angle as a function of the time after plasma discharge for the treated ITO samples.As can be seen, the oxygen plasma discharge brings about a large decrease in contact angle.The H2O contact angleθ1is decreased from 67.2° to 20.1°, and the CH2I2contact angleθ2from 51.6° to 35.5°, indicating that the treated ITO surface appears to be highly polar.
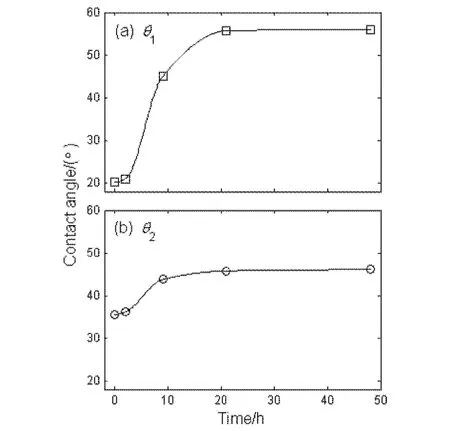
Fig.3 Contact angle as a function of the time after plasma discharge for the treated ITO samples
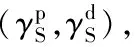

Fig.4 Surface energy as a function of the time after plasma discharge for the treated ITO samples
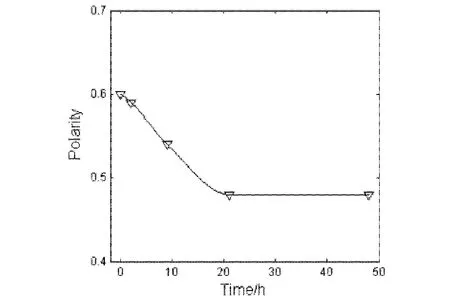
Fig.5 Polarity as a function of the time after plasma discharge for the treated ITO samples
4 Conclusion
In this study, the ITO surfaces were treated by the oxygen plasma discharge.The surface properties of ITO films were investigated through the measurements of XPS, contact angle, surface energy and polarity.Experimental results reveal that the oxygen plasma discharge improves the stoichiometry of the surface and enhances the surface wettability of ITO, so as to optimize the surface properties of ITO substrates.And the improved surface properties benefited from the oxygen plasma discharge is observed to decay with the increase of the time after plasma discharge.The difference in chemical composition, surface energy and polarity between the untreated and treated ITO surfaces appears to become smaller with the increased time after the plasma discharge.The plasma effects were re-treated 21 h after storage, indicating that re-contamination occurred on the surfaces.
