Sol-gel preparation of La1-xCexCoO3 for CO oxidation: effect of Ce doping
2019-10-11ZHUJunjiangWANGShanXUXuelianZHAOYanxiYANGHaijian
ZHU Junjiang ,WANG Shan ,XU Xuelian ,ZHAO Yanxi ,YANG Haijian
(1 Institute of Catalysis for Energy and Environment,College of Chemistry and Chemical Engineering,Shenyang Normal University,Shenyang 110034,China; 2 Hubei Key Laboratory of Biomass Fibers and Eco-dyeing & Finishing,College of Chemistry and Chemical Engineering,Wuhan Textile University,Wuhan 430200,China; 3 Key Laboratory of Catalysis and Materials Science of the State Ethnic Affairs & Commission Ministry of Education,College of Chemistry and Materials Science,South-Central University for Nationalities,Wuhan 430074,China )
Abstract La1-xCexCoO3 perovskite catalysts were prepared by sol-gel method and characterized by XRD,N2 physisorption and H2-TPR measurements.The effects of Ce doping on the physicochemical properties and catalytic performances of La1-xCexCoO3 for CO oxidation were investigated.The results indicated that the doping of Ce into the perovskite framework not only increased the BET surface area,but also enhanced the redox performance and improved the CO oxidation activity.However,when the Ce doping percentage was more than 10%,CeO2 phase appeared in the sample,which covered the active site and resulted in the destruction of perovskite structure,decreasing the CO oxidation activity.
Keywords perovskite oxides; LaCoO3; Ce doping; CO oxidation
Perovskite oxides with ABO3structure are a type of ideal model catalyst for investigation in heterogeneous catalysis,in that the metal cations at both A- and B-site can be substituted by foreign metal cations without destroying the matrix structure,as long as the tolerance factor is in the range of 0.7~1.1[1].Therefore,the physicochemical properties of perovskite oxides can be tailored as desired,and its relation to the structure properties can be feasibly correlated.
The application of perovskite oxides in catalysis came into view since the report of Voorhoeve et al.in 1972[2],who found that these materials were active for hydrocarbons oxidation.Thereafter,a large number of works regarding the catalytic applications of perovskite oxides have been explored[3]and great achievements have been obtained.It is generally believed that oxygen vacancy and redox ability are two important properties of the material[1,4].The former is used to absorb and activate the oxidant,e.g.,molecular oxygen,and the latter is to catalyze the electron-transfer reaction between the reactant and the catalyst.Whereas,the relation between them is hard to separate,as the generation of oxygen vacancy would change inevitably the oxidation states of the B-site cations,and thus alters the redox ability.
The regeneration of oxygen vacancy is crucial to be considered when preparing perovskite catalysts for oxidation reactions,as the oxygen vacancy would be repaired when oxygen is present,leading to deactivation of the catalyst.To regenerate the oxygen vacancy and enable the reaction to be proceeded smoothly,two strategies can be adopted: by raising the reaction temperature or adding a material that can promote the mobility of atomic oxygen to the structure.
From the viewpoint of energy savings,it is preferable to improve the ability of catalyst for oxygen mobility.In this respect,cerium is a promising material and receives special interest both in academic studies and in practical use,because of its unique ability for oxygen storage and release.Zhu et al[5]reported that the doping of Ce to La1-xCexCoO3increased significantly the activity for aerobic oxidation of benzyl alcohol to benzaldehyde due to the stronger affinity of Ce to O atoms (relative to that of La),and the optimum Ce content [atomic ratio of Ce/(La+Ce)] was 10%.Gao et al[6]found that the Ce doping could increase the specific surface area and reduce the crystallite size of La1-xCexCoO3,thus improve the activity for plasma-catalytic oxidation of low concentration ethyl acetate (100).Xiang et al[7]reported that the presence of Ce4+in La1-xCexFeO3could strengthen the interactions with adsorbed O2and facilitate the activation of O—O bond,and consequently accelerate the reaction rate of catalytic methane combustion,with the optimum Ce content of 30%.All these results suggested that the Ce doping was effective to improve the catalytic performances of perovskite oxides,by increasing the surface area,strengthening the interaction with adsorbed oxygen,improving the reducibility,and so on.
In this work,a series of Ce-doped La1-xCexCoO3perovskites were prepared,and the effect of Ce doping on the physicochemical properties and catalytic performances of LaCoO3were investigated.XRD,N2-physisorption,O2-TPD and H2-TPR measurements were used to demonstrate the change of properties caused by Ce doping.Catalytic CO oxidation,which is an efficient route to eliminate CO from the exhausts and is a widely used model reaction[8],was selected to evaluate the catalytic performances of catalysts.First, the activities of perovskite oxides with different transition metals for CO oxidation were screened to optimize the transition metal.Thereafter,LaCoO3was selected and the effect of Ce doping on CO oxidation activity was investigated,to optimize the Ce content.The results indicated that the optimum Ce content was 10%,i.e.,La0.9Ce0.1CoO3,at which most of the Ce3+ions entered the perovskite framework,leading to enhanced surface area and redox ability,and enhance the activity for CO oxidation,with on-set temperature of 75 ℃.
1 Experimental
1.1 Preparation
The procedure is similar as reported elsewhere[5].Stoichiometric amount of La(NO3)3,Ce(NO3)3and Co(NO3)2were dissolved in distilled water,to which citric acid,in 1.2 times of the total metal ions (i.e.La3+,Ce3+and Co2+),was added.The mixture was stirred for 2 h,and then dried at 100 ℃ overnight.The resulting solid was grinded and calcined in a static air oven at 600 ℃ for 5 h,with heating rate of 1 ℃·min-1.Depending on the amount of Ce added,the sample was defined as La1-xCexCoO3,wherexranges from 0 to 0.9.
1.2 Characterizations
X-ray diffraction (XRD)patterns were recorded on a powder X-ray diffractometer (Ultima IV,Rigaku)using Cu-Kα radiation with a Ni-ltered operating.The samples were scanned in 2θrange 20-80° with a scanning speed of 5°·min-1.
N2physisorption isotherms were obtained from a Micromeritics TriStar II 3020 analyzer.The air were previously evacuated from the samples,which were then treated at 300 ℃ for 3 h in helium atmosphere.
Temperature-programmed desorption of oxygen (O2-TPD)was performed on a TP-5076 adsorption instrument (Tianjin,China),with the same procedure as described in previous work[9].
Temperature programmed reduction of hydrogen (H2-TPR)was performed on the same apparatus.The 100-mg sample was put in the reactor and treated by N2at 400 ℃ for 1 h.After cooling to room temperature,the gas was switched to H2/N2(volume ratio of 1∶10)at a flow rate of 50 mL·min-1,and after a stable baseline was reached,the temperature was raised to 800 ℃ at a heating rate of 10 ℃·min-1.The consumption of hydrogen was recorded by TCD.
1.3 Catalytic tests
CO oxidation reaction was performed in a fixed-bed tubular micro-reactor,with an internal diameter of 6 mm,a wall thickness of 1 mm and a length of 400 mm.The reaction mixture (volume ratio of Ar/CO/O2=93/0.5/6.5)with flow rate of 50 mL·min-1was passed through 0.1 g catalyst placed in the middle of the reactor,and the temperature was raised from 50 to 150 ℃.At each stage,the temperature was kept for 30 min before the activity tests to ensure that the reaction reaches equilibrium.The gas compositions,before and after the reaction,were analyzed by an on-line gas chromatograph (Agilent 7890B).The activity was evaluated by the equation: CO conversion (%)=[c(COin)-c(COout)]/c(COin)×100%,wherec(COin)andc(COout)represented the inlet and outlet CO concentrations.
2 Results and discussion
In the previous work[10],we have shown that LaCoO3with perovskite structure can be yielded when prepared by sol-gel method using citric acid as complexant and calcined at the temperature of 600 ℃,while the formation of perovskite structure failed when Mn,Cu and Zn,instead of Co,was used as the B-site metal,due to their half-filled (3d54s2for Mn)or full-filled electrons (3d104s1-2for Cu and Zn)in the 3d orbit.Hence,LaCoO3was chosen for investigation in this work and the calcination temperature was set at 600 ℃.
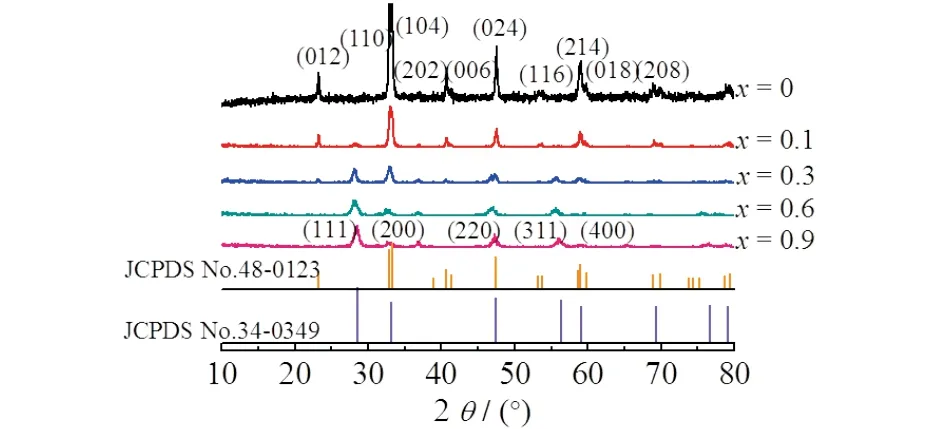
Fig.1 XRD patterns of the La1-xCexCoO3 (0.1≤x ≤1)
Fig.1 depicted the XRD patterns of La1-xCexCoO3(0≤x≤0.9).As expected,LaCoO3,without Ce addition,possessed the perovskite structure.However,the peak at 2θ=33.3° that assigned to the characteristic peak of LaCoO3weakened,and a new peak at 2θ=28° that assigned to CeO2increased with the Ce content,suggesting that the LaCoO3perovskite structure was destroyed and CeO2was separated.The CeO2phase became the majority and the perovskite structure almost disappeared atx≥0.6.This indicated that the incorporation of Ce into the perovskite framework was not feasible at large amount.Previous researches have shown that the maximum Ce content incorporated into the ABO3-type perovskite oxides was less than 10%[11],supporting our results.
N2physisorption measurements showed that the BET surface area of LaCoO3was significantly increased after Ce doping,especially at Ce content of 10 % (Fig.2a).The surface area and pore volume increased from 9.3 m2·g-1and 0.06 cm3·g-1for LaCoO3to 20.3 m2·g-1and 0.11 cm3·g-1for La0.9Ce0.1CoO3,indicating that the incorporation of Ce into the perovskite framework facilitated the improvement of surface area or the creation of pores.Indeed,a significant increase in the pore volume was observed fromx=0 to 0.1,and La0.9Ce0.1CoO3that accomodated the most amount of Ce in the framework showed the largest pore volume.However,with the further increase of Ce content,the surface area and pore volume decreasd due to the separation of CeO2,which blocked the pores created in the structure,and more seriously,destroyed the perovskite structure,as observed in the XRD patterns.These results indicated that only the Ce that incorporated into the perovskite structure could improve the surface area.Fig.2b showed the pore size distribution of the samples.An exception was observed atx=0.6,which showed pore size centered at 33 nm and was possibly due to the holes made between the particles.
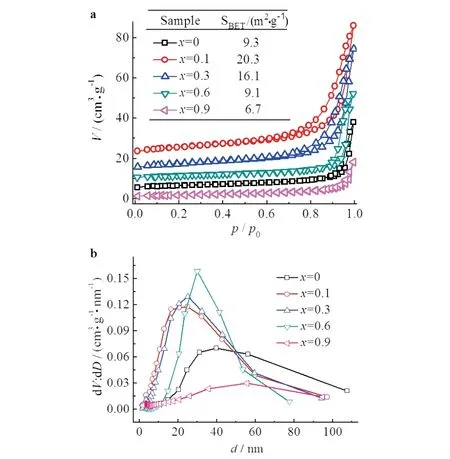
Fig.2 N2 physisorption isotherms(a)and the corresponding pore size distribution(b)of La1-x CexCoO3 (0.1≤x≤1)
Fig.3 presented the O2-TPD graph obtained from the LaxCe1-xCoO3samples.Normally,two desorption peaks appeared in the O2-TPD graph of perovskite oxides,with the first one,locating at temperature below 600 ℃,attributed to the oxygen chemically adsorbed on the oxygen vacancy,and the second one,locating at temperature above 600 ℃,attributed to the lattice oxygen[9,12].
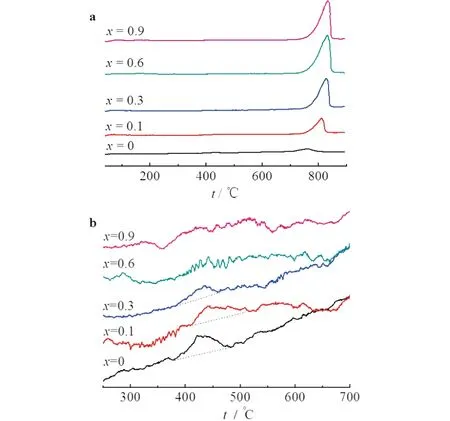
a)the graph of overview; b)the graph with magnified view at temperature between 250 and 700 ℃
Fig.3a displays that only one obvious peak,at temperature above 700 ℃,appeared in the graph of overview,which could be that the first desorption peak was too weak to observe.Indeed,when magnifying the graph at temperature of 300-700 ℃ (see Fig.3b),a desorption peak at about 500 ℃ appeared.
For the first desorption peak,the peak area decreased and the peak temperature increased with the increase of Ce content.This could be that for samples fromx=0 to 0.1,Ce3+ions were incorporated into the perovskite framework and some of them were transformed into Ce4+ions.The presence of Ce4+ions would decrease the amount of oxygen vacancy according to the principle of electroneutrality,and strengthen the interaction with oxygen atoms (e.g.,Ce4+―O vs.La3+—O)because of its strong affinity,thus leading to smaller peak area and higher peak temperature.For samplesx≥ 0.3,because the separation of CeO2phase and the destruction of perovskite structure,the amount of oxygen vacancy decreased and no peak in the graph appeared atx≥0.6 where the perovskite stucture was fully destroyed.
For the second desorption peak,the peak area increased all alone with the Ce contents,in accordance to what had been reported in the previous work[13].The increase fromx=0 to 0.1 was that the lattice oxygen was drew and loosed by Ce3+/4+,facilitating the release of lattice oxygen.The further increase atx≥ 0.3 was mostly attributed to the oxygen released from Co3O4,which was yielded accompanying the CeO2separation,as discussed by Alamdari et al[14].
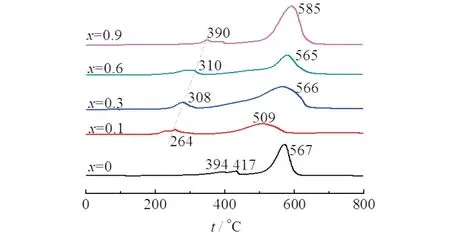
Fig.4 H2-TPR curve of the LaxCe1-xCoO3 (0≤x ≤0.9)
Redox abilities of La1-xCexCoO3evaluated by H2-TPR measurements were shown in Fig.4.In general,two reduction peaks appeared for the samples,with the first attributed to the reduction of Co3+to Co2+,and the second to the reduction of Co2+to Co0[11,15].The first reduction temperature decreased greatly after Ce addition,from 417 ℃ for LaCoO3to 264 ℃ for La0.9Ce0.1CoO3,indicating that the presence of Ce promoted the reduction of Co3+to Co2+.This could be attributed to the strong affinity of Ce4+cation for oxygen,which caused the rupture of Co—O bond by the way of Co3+—O + Ce3+=Co2++ Ce4+—O.Thereafter,the oxygen of Ce4+—O was rapidly removed by H2(e.g.,Ce4+—O + H2=Ce3++ H2O—e-)due to its capability for oxygen releasing.That is,the Ce3+cations acted as a bridge of the reaction between H2and Co3+—O,facilitating the reduction of Co3+to Co2+.
As the Ce content increased,the temperature of the first reduction peak moved toward the high temperature region.At this time,the oxygen of Co3+—O couldn′t be abstracted efficiently as that of La0.9Ce0.1CoO3due to the separation of CeO2and the destruction of perovskite structure (Fig.1).Moreover,the CeO2covered on the surface would restrain the reduction of Co3+.As a result,the reduction temperature was shifted to high regions.
As for the second reduction peak,a significant decrease in the peak temperature is observed fromx=0 to 0.1 due to the Ce incorporation in the perovskite structure.The co-existence of Ce3+ions promoted the rupture of Co―O bond as discussed above,thus the reaction between H2and Co—O could be easily occurred,showing reduction temperature of 509 ℃.Thereafter,CeO2was separated and the perovskite structure was destroyed,resulting in the formation of Co2O3and/or Co3O4.The reduction has less relation to the Ce3+ions,but could relate to the Ce doping,as the perovskite phase was degraded and more Co2O3and/or Co3O4would be produced with the increase of Ce doping.
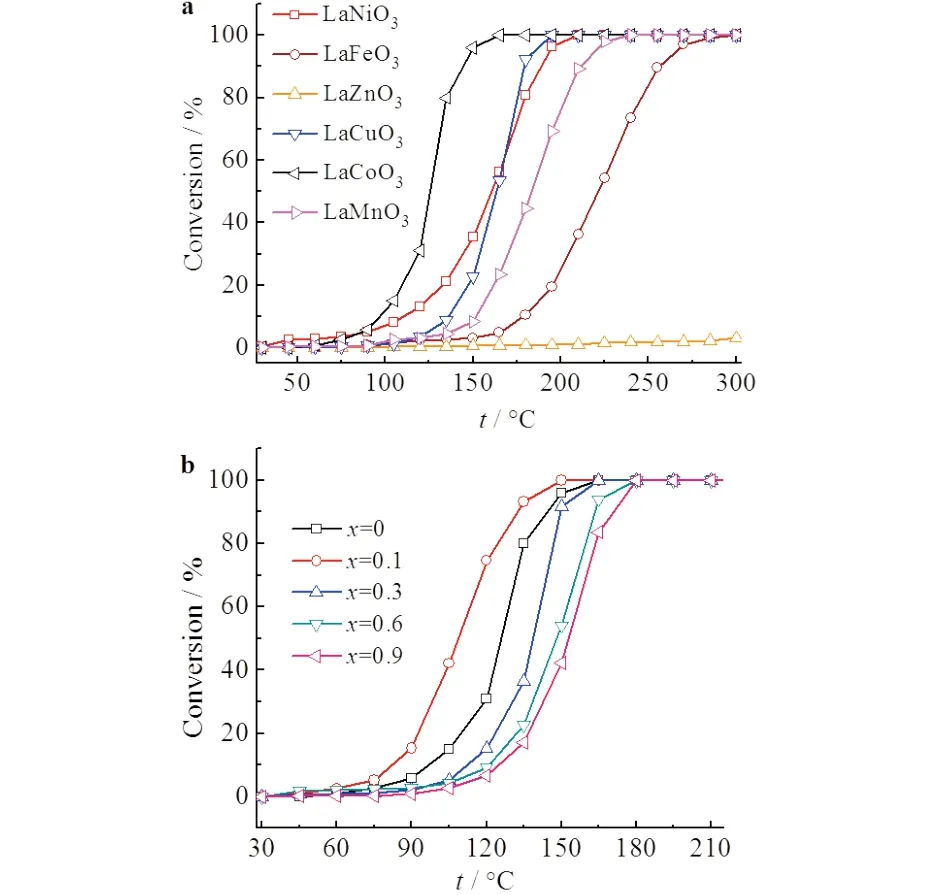
a)LaBO3 (B=Mn,Fe,Co,Ni,Cu,Zn);b)LaxCe1-xCoO3 (0≤x≤0.9)
Fig.5 showed the CO oxidation acitivity measured from LaBO3and LaxCe1-xCoO3.For LaBO3with different transition metals,Co was the best B-site metal for CO oxidation,and the activity was in sequence of LaCoO3> LaCuO3≈ LaNiO3> LaMnO3> LaFeO3> LaZnO3(Fig.5a),in accordance to what had been reported in the previous work[16].The lowest activity of LaZnO3could be that the perovskite structure was not formed,and both La3+and Zn2+cations had stable oxidation states and were not suitable to catalyze electron-transfer reaction including CO oxidation.
Fig.5b presented the effect of Ce doping on the activity of LaxCe1-xCoO3for CO oxidation.With the increase of Ce doping,the activity first increased and then decreased,with the best obtained atx=0.1,which showed an on-set temperature of 75 ℃,and was significantly lower than that of LaCoO3(90 ℃).Together with the BET surface area and the redox ability discussed in Fig.2 and Fig.3,it was inferred that the best CO oxidation activity of La0.9Ce0.1CoO3was attributed to the improved surface area and redox ability caused by Ce doping.However,the Ce doping should be controlled,otherwise it would deteriorate the reaction by separating CeO2phase and destroying the perovskite structure.
3 Conclusion
Effect of Ce doping on the catalytic performances of LaxCe1-xCoO3for CO oxidation was investigated.XRD patterns indicated that the content of Ce doping to the perovskite framework was less than 10 %,otherwise CeO2phase would be separated and the perovskite structure would be destroyed.Therefore,the sample with 10% Ce doping showed the largest BET surface area,the best redox ability and the highest CO oxidation activity.It could be explained that at high Ce doping,the pores were blocked,the structure was destroyed,and the active sites were covered by the separated CeO2phase,thus leading to decreased activity.
