不同水分条件下尿素与硫酸铵对碱性水稻土N2O释放途径的影响
2019-09-10邢志强席瑞泽杨永强朱俊胡红青付庆灵
邢志强 席瑞泽 杨永强 朱俊 胡红青 付庆灵
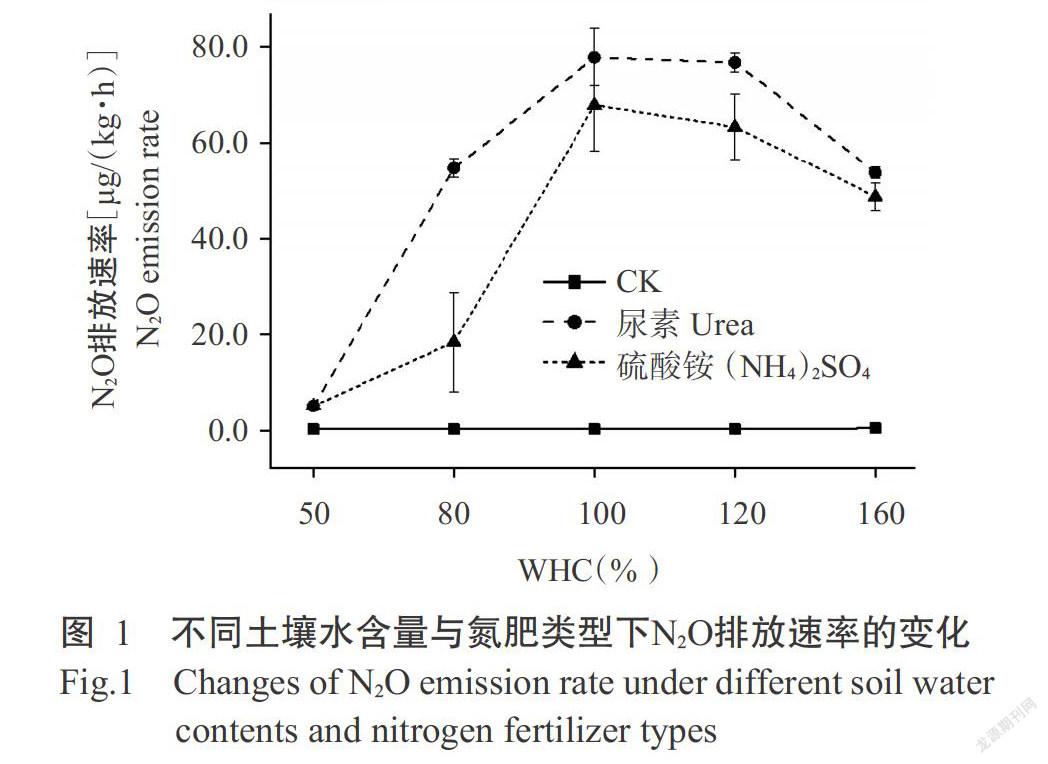
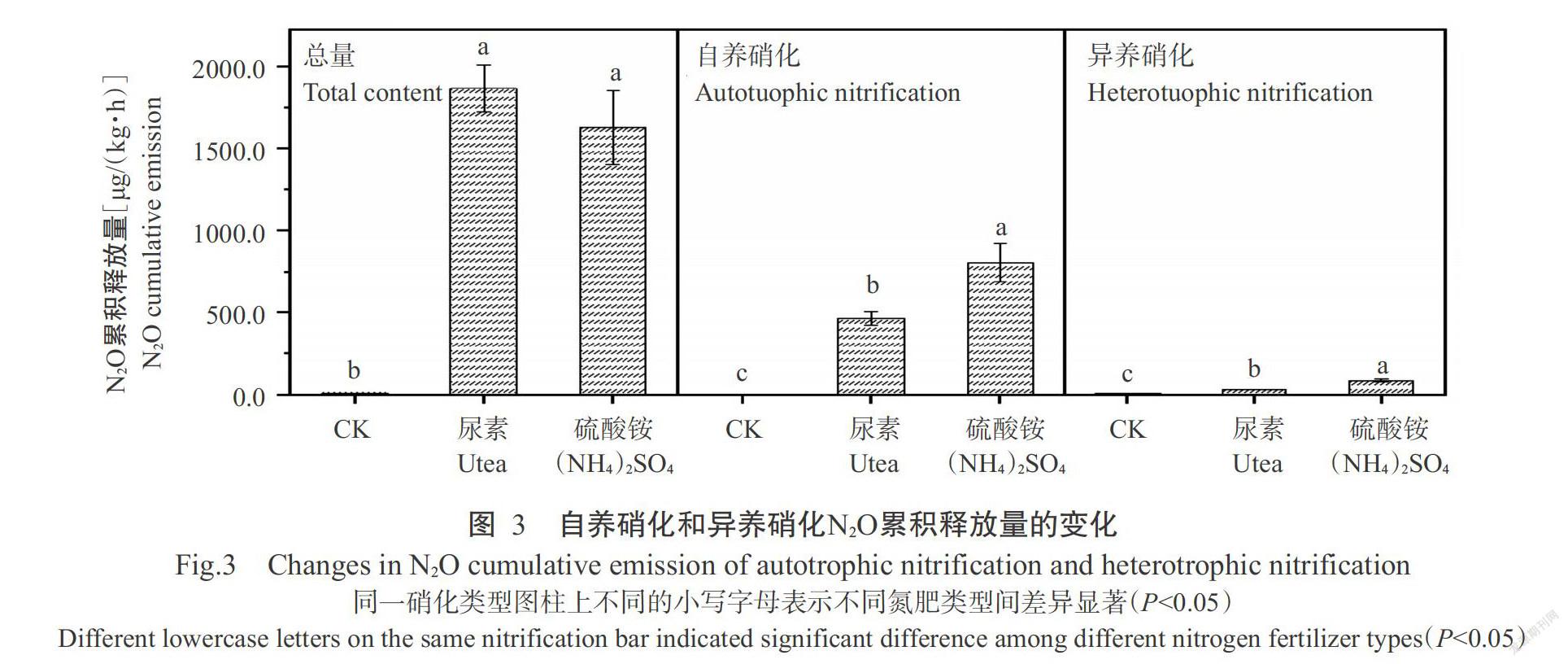
摘要:【目的】明確土壤水含量与氮肥类型对碱性水稻土N2O释放总量及释放途径的影响,为制定合理的农田氮素管理措施及减少土壤N2O排放提供理论依据。【方法】以潮土性水稻土(pH 7.9)为供试土壤,通过室内培养调整不同的土壤水含量,即调节土壤最大持水量(WHC)分别为50%、80%、100%、120%和160%,施用尿素与硫酸铵(N 100 mg/kg)两种氮肥,使用乙炔(C2H2,10 Pa)与氧气(O2,100 kPa)抑制100% WHC时不同氮肥处理的自养硝化与反硝化过程,利用气相色谱及流动分析仪测定不同处理下土壤的N2O排放量、硝态氮与铵态氮含量。【结果】对比不施肥处理(CK),尿素和硫酸铵处理的N2O排放速率与累计排放量明显提高,其中尿素对N2O排放速率的影响大于硫酸铵,且随着土壤水含量的增加,尿素和硫酸铵处理的N2O排放速率均呈先增大后减小的变化趋势。施氮后土壤自养硝化和异养硝化作用中N2O排放速率均有所提高,且施氮处理自养硝化对N2O排放的贡献大于异养硝化作用,但尿素与硫酸铵对自养和异养硝化过程N2O排放贡献的影响存在差异。硫酸铵提高了自养硝化对N2O排放的贡献,降低了异养硝化的贡献,分别由CK的31.0%提高到49.0%,以及从63.0%降低至5.3%;尿素却同时降低了自养硝化和异养硝化对N2O排放的贡献,分别由31.0%降低到25.0%及由63.0%降低到1.7%。【结论】碱性水稻土N2O释放速率随土壤水含量的增加呈先增加后减小的趋势,不同土壤水含量下尿素的N2O累积释放量均高于硫酸铵。添加氮肥降低了异样硝化对N2O释放的贡献,硫酸铵与尿素分别由自养硝化和反硝化作用起主导作用。因此,旱地土壤施用尿素、水田施用铵态氮肥有利于减少N2O释放。
关键词: 碱性水稻土;N2O;自养硝化;反硝化;异养硝化
中图分类号: S153 文献标志码: A 文章编号:2095-1191(2019)11-2429-07
Effects of urea and ammonium sulfate on N2O release pathway in an alkaline paddy soil
XING Zhi-qiang1, XI Rui-ze1,2, YANG Yong-qiang1, ZHU Jun1,
HU Hong-qing1, FU Qing-ling1*
(1College of Resources & Environment, Huazhong Agricultural University,Wuhan 430070, China; 2Soil and Fertilizer Workstation of Linfen, Linfen, Shanxi 041000, China)
Abstract:【Objective】The effects of soil water content and nitrogen fertilizer type on alkaline paddy soil N2O release and release route were clarified,which provided a scientific basis for improving nitrogen use efficiency and reducing soil N2O release. 【Method】In this experiment,fluvo-aquic soil was used as the tested soil,soil moisture contents were adjusted through indoor culture, which was adjusting the maximum water holding capacity(WHC) to be 50%,80%,100%,120% and 160%, applying two types of nitrogen fertilizers: urea and ammonium sulfate(N 100 mg/kg). Acetylene(C2H2,10 Pa)and oxygen(O2,100 kPa) were used to inhibit the autotrophic nitrification process and denitrification process of different nitrogen fertilizer types during 100% WHC. N2O release,nitrate nitrogenand ammonium nitrogen contents were determined by gas chromatography and flow analyzer. 【Result】The results showed that compared with no fertilization treatment(CK), urea and ammonium sulfate treatments significantly increased soil N2O emission rate and cumulative emissions,and the effects of urea was greater than that of ammonium sulfate,and N2O emission rate increased first and then decreased with the increase of water content. The N2O emission rate of soil autotrophic nitrification and heterotrophic nitrification increased after nitrogen application,and the contribution of autotrophic nitrification to N2O emission was greater than that of heterotrophic nitrification. However,the effects of urea and ammonium sulfate on the contribution of autotrophic nitrification and heterotrophic nitrification to N2O emissions were different. The application of ammonium sulfate increased the contribution of autotrophic nitrification to N2O emissions from 31.0%(CK) to 49.0% and reduced the contribution of heterotrophic nitrification from 63.0% to 5.3%;while urea reduced both the contribution of autotrophic nitrification and heterotrophic nitrification to N2O emissions,from 31.0% to 25.0% and 63.0% to 1.7%,respectively. 【Conclusion】With the increase of soil water content, the release rate of alkaline paddy soil N2O increases first and then decreases. The cumulative release amount of urea N2O under different water contents is higher than that of ammonium sulfate. The addition of nitrogen fertilizer reduces the contribution of heterogeneous nitrification to N2O release. Ammonium sulfate and urea are dominated by autotrophic nitrification and denitrification, respectively. Therefore, the application of urea in dry soil and the use of ammonium nitrogen fertilizer in paddy field are beneficial to reduce N2O release.
Key words: alkaline paddy soil; N2O; autotrophic nitrification; denitrification; heterotrophic nitrification
0 引言
【研究意义】氧化亚氮(N2O)是除二氧化碳(CO2)和甲烷(CH4)之外的第三大温室气体,其中约70%的N2O来源于陆地生态系统,而农业土壤中的微生物氮循环过程约贡献了45%的N2O排放量(Solomon et al.,2007)。作为世界上最主要的粮食作物,水稻在全世界的种植面积约1.55亿ha,我国是世界上最大的稻米生产国,稻米产量约占全球的26%(Zhu et al.,2011;Ge et al.,2017)。为满足日益增长的粮食需要,越来越多的氮肥被施入到土壤中,其中1%~5%氮素以N2O的形式损失(Galloway et al.,2008;Shcherbak et al.,2014)。每年约有6.8 Tg N2O-N的释放源于氮肥及有机肥的使用,占大气中N2O释放量的65%(Ravishankara et al.,2009)。因此,了解不同氮肥对水稻土N2O释放量及释放途径的影响,对于提高氮素利用效率及减缓全球变暖具有重要意义。【前人研究进展】水稻生长期间频繁的干湿交替提供了独特的生物化学环境,源于有机质与根系分泌物矿化或氮肥释放的氨在氧化层及根际土附近发生硝化反应(Ke et al.,2013;Li and Wang,2013;Li et al.,2014),而硝态氮或亚硝态氮转移到还原层发生反硝化反应逐步还原成N2O或N2(Ishii et al.,2011)。与旱地相比,水稻土由于强烈的厌氧环境使N2O可进一步被还原成N2从而降低N2O的释放,但在水稻生长过程中的排水与干湿交替期间仍可观察到N2O的集中释放,整个水稻生长期水稻土N2O-N释放量为9~35 Gg(Yao et al.,2010;Cai,2012)。土壤水含量与氮肥类型显著影响土壤N2O的释放量与释放过程,随着水含量逐渐增加,N2O释放量随之增加(Burger and Venterea,2011;Sainju,2016)。也有研究发现,土壤最大持水量(WHC)为35%~60%时N2O主要由硝化过程产生,而WHC为70%时反硝化作用是N2O释放的主要过程(Morse and Bernhardt,2013;Lan et al.,2014;Müller et al.,2014)。Bateman and Baggs(2005)研究发现,当土壤孔隙水含量(WFPS)为70%时,土壤N2O的产生全部源自反硝化作用,而WFPS在60%时硝化作用贡献了N2O产生量的81%。Liu等(2007)研究显示,在WFPS为60%时加入硫酸铵比硝酸钾可产生更多的N2O,表明硝化作用与随后的反硝化作用是低水含量时N2O释放的主要途径,而WFPS为75%时的N2O释放量显著高于WFPS为60%的N2O释放量。Lebender等(2014)研究发现,当WHC在70%时施用尿素与硫酸铵后N2O累积释放量显著提高,其中尿素处理释放量大于硫酸铵。Liu等(2017)通过使用15N分别标记氯化铵与硝酸钾,观察到温度为25 ℃,WFPS分别为50%、70%和85%时,硝化作用对N2O释放的贡献分别为87%、80%和53%。郑欠等(2017)研究发现,当WFPS为80%时,土壤N2O的产生主要源于反硝化过程,当WFPS为95%时,硝化作用为N2O排放的主要过程,且N2O排放量随着水含量的增加而升高。【本研究切入点】目前已有的研究主要集中在水含量对硝化作用和反硝化作用对N2O释放的影响及低于饱和水含量时N2O释放与水分的关系,关于硝化作用中的自养硝化与异养硝化过程及淹水条件对N2O释放的影响则鲜有关注。与土壤中自养硝化细菌仅可进行对铵态氮的氧化作用相比,异养硝化细菌虽然利用效率较低,但由于在环境中的数量及生长速率远高于自养硝化细菌,且既可利用铵态氮又能利用有机氮,因此在某些环境中,异养硝化作用对N2O释放的贡献可与自养硝化相当甚至超过自养硝化。同时,氮肥类型对N2O释放影响的研究存在相互矛盾的结果,尚缺乏明确的定论。【拟解决的关键问题】通过室内模拟水稻生长过程中的水分变化,研究不同水分条件下氮肥类型对碱性水稻土N2O释放总量及排放途径相对贡献的影响,为制定合理的农田氮素管理措施及减少土壤N2O排放供理论依据。
1 材料与方法
1. 1 土壤样品
供试土壤为潮土性水稻土,采自湖北省潜江市广华农场(东经121°69′,北纬30°41′,海拔26.4 m),土壤含17%砂粒、60%粉粒及23%黏粒。取0~20 cm耕层土壤,混匀后过5 mm筛,一部分-20 ℃保存用于后续的培养试验,另一部分风干后测量土壤基本理化性质。土壤持水能力82.2%,pH 7.9,有机质37.3 g/kg,全氮2.24 g/kg,铵态氮11.8 mg/kg,硝态氮26.6 mg/kg。
1. 2 土壤水含量与氮肥类型对N2O排放的影响
将土壤样品放入25 ℃恒温培养箱中预培养7 d,然后称取相当于20 g干土重量的新鲜土样置于300 mL培养瓶中,以水溶液的形式將尿素与硫酸铵(N 100 mg/kg)加入土壤,随后调节至不同的土壤水含量,即调节WHC分别为50%、80%、100%、120%和160%,然后使用橡胶塞密封培养瓶。于培养开始后0、12和24 h分别使用气密注射器抽取10 mL气体样品,使用气相色谱仪(Agilent GC7890A)分析其中的N2O浓度,培养结束后测定其中的硝态氮和铵态氮浓度。每处理重复3次,同时进行不施肥的空白对照(CK)试验。
1. 3 土壤N2O排放途径试验
试验设3种处理:处理Ⅰ,土壤;处理Ⅱ,土壤+100 kPa O2;处理Ⅲ,土壤+10 Pa C2H2+100 kPa O2。分别加入尿素和硫酸铵(N 100 mg/kg)并调节水含量为100% WHC。将培养瓶密封后抽气至真空,按试验处理注入不同气体后放入25 ℃恒温培养箱避光培养24 h,其余条件与1.2相同。利用差减法计算不同途径N2O排放通量:
N2O总量=N2O空气
N2O自养硝化=N2O氧气-N2O氧气+乙炔
N2O异养硝化=N2O氧气+乙炔
式中,N2O总量为培养期间N2O释放量,N2O自养硝化为培养期间自养硝化的N2O释放量,N2O异养硝化为培养期间异养硝化的N2O释放量,N2O空气为处理Ⅰ中的N2O释放量,N2O氧气为处理Ⅱ中的N2O释放量,N2O氧气+乙炔为处理Ⅲ中的N2O释放量。
1. 4 N2O排放速率计算
根据培养开始后0、12和24 h测定的N2O浓度,使用Slope函数计算N2O浓度随时间变化的曲线斜率,选择|R2|>0.9的数值,按以下公式计算N2O排放速率:
P=[dcdt]×[vMv]×[Mww]×[273T]
式中,P为N2O排放速率[μg/(kg·h)];dc/dt为培养瓶中N2O浓度随时间线性变化的曲线斜率[μL/(L·h)];v为培养瓶中气体的体积(L);Mv为标准状态下1 mol气体体积(L);Mw为N2O的摩尔质量(g);w为干土质量(g);T为温度(K)。
1. 5 统计分析
使用Origin 2018C绘图、SPSS 25.0进行方差分析,采用最小显著性差异法(LSD)比较不同处理间N2O排放速率与排放通量的差异。
2 结果与分析
2. 1 土壤水含量与氮肥类型对N2O排放的影响
由图1可知,在不同水含量条件下,施氮处理与CK间的N2O排放速率差异明显,且除WHC为50%外,尿素处理N2O排放速率均高于硫酸铵处理。随土壤水含量的增加,尿素和硫酸铵处理的N2O排放速率呈先增大后减小的变化趋势,在WHC为100%时达最大值,分别为77.7和67.7[μg/(kg·h)],当WHC上升到160%时,二者的N2O排放速率与最大值相比分别下降30%和29%。
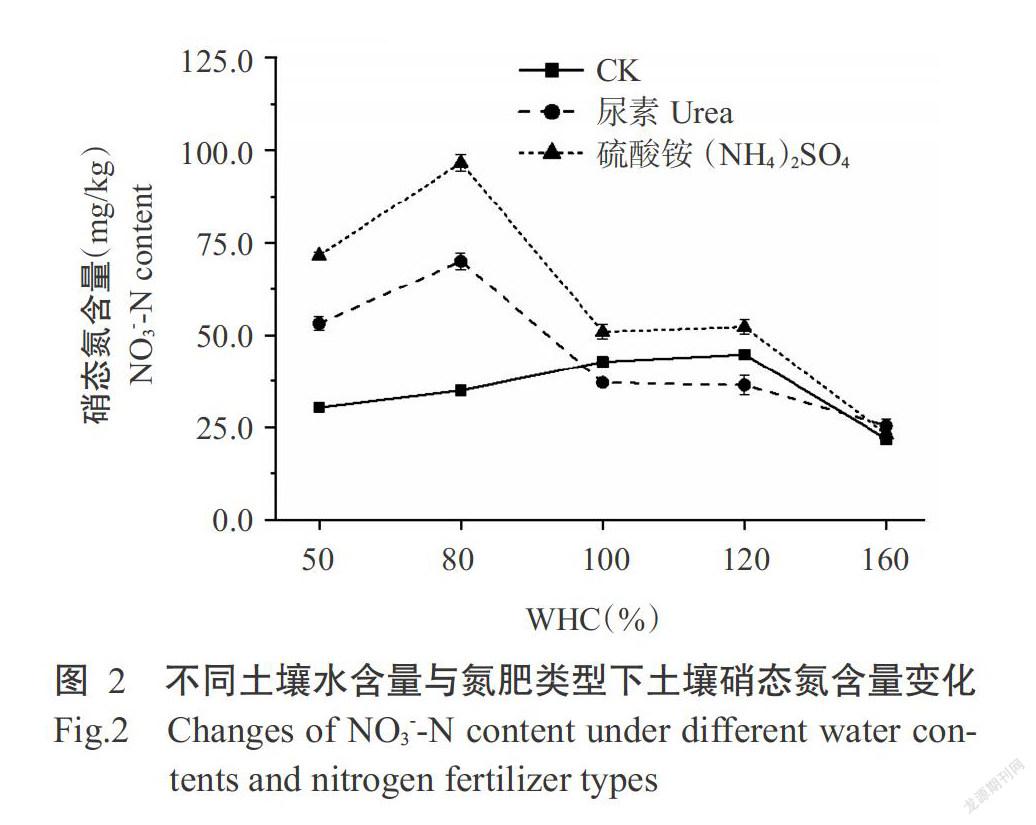
由图2可知,随着土壤水含量的增加,土壤硝态氮含量呈先增加后减少的变化趋势,CK的硝态氮含量在WHC为120%时达最大值,尿素和硫酸铵处理均在WHC为80%时达最大值,分别为96.6和70.0 mg/kg。
2. 2 氮肥类型对N2O排放途径的影响
2. 2. 1 N2O排放通量 由图3可看出,施用氮肥不仅增加了N2O排放总量,还增加了自养硝化和异养硝化的N2O排放量,自养硝化N2O排放量由CK的3.1 μg/(kg·h)提高到462.9 μg/(kg·h)(尿素)和800.8 μg/(kg·h)(硫酸铵),异养硝化由CK的6.2 μg/(kg·h)提高到31.8 μg/(kg·h)(尿素)和86.3 μg/(kg·h)(硫酸铵),其中尿素对N2O排放总量的增加作用高于硫酸铵,而在自养硝化和异养硝化方面,硫酸铵的增加量高于尿素。
2. 2. 2 N2O排放途径的相对贡献 由图4可看出,CK处理中硝化作用在N2O排放中占主导作用,而反硝化作用对N2O释放的贡献仅为6.0%,硝化作用中又以异养硝化为主,占63.0%,自养硝化仅占31.0%。与CK相比,添加氮肥后反硝化作用与自养硝化在N2O排放中占主导作用,而异养硝化的比例最小。对比不同氮肥类型处理,发现加入硫酸铵后提高了自养硝化与反硝化对N2O排放的贡献,自养硝化由CK的31.0%提高到49.0%,使自养硝化成为N2O排放的主导作用,异养硝化对N2O的贡献只有5.3%。尿素处理降低了自养硝化与异养硝化的贡献,分别由CK的31.0%和63.0%降低到25.0%和1.7%,反硝化作用占N2O释放的73.0%。
2. 2. 3 土壤铵态氮和硝态氮含量 从图5可看出,不同抑制处理铵态氮含量的变化趋势相同,均表现为处理Ⅲ>处理Ⅱ>处理Ⅰ,且不同抑制处理下施氮处理铵态氮含量表现为硫酸铵>尿素>CK,最大值分别为178.2、154.4和31.3 mg/kg。
从图6可看出,CK与施氮处理硝态氮含量均表现为处理Ⅱ>处理Ⅰ>处理Ⅲ,施氮后土壤硝态氮含量均在处理Ⅱ和处理Ⅲ达最大值和最小值,尿素处理分别为111.0和45.6 mg/kg,硫酸铵处理分别为69.0和30.0 mg/kg。
3 讨论
Gagnon等(2011)在玉米生长季节使用尿素、氨水和硝酸铵作为氮肥施入土壤,发现在不同施氮量情况下使用尿素替代部分硝酸铵后的N2O释放量均高于单施硝酸铵处理。席瑞泽等(2017)利用黄棕壤性水稻土进行室内培养试验,添加尿素和硫酸铵作为氮源,发现在不同水分条件下添加尿素后N2O释放速率均高于硫酸铵。本研究结果表明,不同土壤氮肥类型处理在培养期间N2O释放量不同,在不同水含量条件下均表现为尿素处理N2O释放量高于硫酸铵处理。不同肥料类型间的N2O释放差异可能是由于尿素使用为土壤中的反硝化微生物提供了碳源,刺激了反硝化微生物的活性,添加尿素后土壤N2O释放途径转变为反硝化占主导地位,表明尿素处理中N2O释放量的增加是由于反硝化作用增强所致。
郑欠等(2017)通过室内培养试验发现以硫酸铵作为氮源时,潮褐土在67%、80%和95% WFPS条件下N2O释放量逐渐增加,其中95% WFPS時N2O释放量约为67% WFPS时的15倍。本研究发现,土壤水含量显著影响N2O的释放速率,随着土壤水含量增加,N2O释放速率呈先增大后减小的变化趋势。N2O释放速率在100% WHC时达最大值,随后N2O释放速率随水含量增加而逐渐下降。这可能是由于土壤中厌氧条件进一步加强,当土壤水含量高于75% WHC时, N2O与N2的比值随着水含量增加而减少(Rudaz et al.,1999;Dalal et al.,2003)。
Zhang和Wienhold(2002)研究表明,WHC高于80%时土壤硝态氮浓度快速下降,而铵态氮浓度逐渐增加。在本研究中,硝态氮含量在80% WHC时达最大值,随后随水含量的增加逐渐下降,土壤中的铵态氮经硝化细菌的作用转换为硝态氮,硝态氮被反硝化细菌逐步还原为NO、N2O和N2,由于培养期间高水含量条件下厌氧环境逐渐增强,反硝化对硝态氮的消耗可能超过硝化作用中硝态氮的产生。
本研究中,尿素处理自养硝化和异养硝化的N2O释放量均低于硫酸铵处理,可能是由于试验供试土壤为碱性土壤,从而抑制了硝化微生物的活性。相关研究表明,加入尿素会使土壤pH升高,而硫酸铵可能会降低土壤pH(Zhao and Xing,2009;Tong and Xu,2012),当pH大于6.9时,随着pH升高,氨氧化古菌与氨氧化细菌的amoA基因丰度及转录活性逐渐降低(Nicol et al.,2008;苏瑜和王为东,2017),异养硝化细菌的硝化活性也会随着pH升高而降低(Duggin et al.,1991)。
王大鹏等(2018)研究表明,在35%~70% WFPS范围内,随着土壤水分的增加,土壤反硝化速率呈线性增加。Inubushi等(1996)通过添加硫酸铵或硝酸钠进行室内培养试验发现,当土壤WHC在60%或80%时,土壤有87%~92%的N2O由自养硝化过程释放,而当土壤WHC为100%时,土壤释放的N2O中96%~98%来自于反硝化过程。在本研究中,当土壤水含量为100% WHC时,土壤N2O释放量达最大值,其中由反硝化作用引起的N2O释放量占44.0%~73.0%,自养硝化占31.0%~49.0%,表明反硝化作用在100% WHC时主导土壤N2O的释放,而自养硝化作用是硝化作用N2O释放的主要来源。尿素处理中反硝化作用对N2O释放的贡献大于硫酸铵处理,可能是由于尿素为反硝化微生物提供了碳源,进而提高反硝化微生物的丰度及活性(Chen et al.,2018),且由于培养瓶中土层厚度仅有1 cm,在100% WHC时土壤表面仍可接触到O2,从而高估硫酸铵处理中硝化作用对N2O释放的贡献(Pihlatie et al.,2004)。Stange等(2013)在森林土壤中使用15N同位素示踪技术研究不同途径N2O释放贡献,发现异养硝化作用对N2O释放的贡献为48%~76%;而Rütting等(2010)使用牧场土壤进行的培养试验中,通过分析15N标记的NH4NO3与N2O的同位素特性,发现异养硝化作用对N2O释放的贡献达68.5%~90.6%。本研究中,异养硝化在施肥处理中的贡献仅为1.7%~5.3%,在CK中的贡献却高达63.0%,表明在无外源氮加入时,土壤本身异养硝化细菌通过利用土壤有机质进行的异养硝化过程是土壤N2O释放的主要来源。
本研究还发现,与处理Ⅰ相比,处理Ⅲ中使用C2H2抑制自养硝化作用,抑制了铵态氮的转换,而处理Ⅱ通过增加O2分压至100 kPa抑制了反硝化过程,导致硝态氮的积累(Taylor et al.,2013)。本研究为理解影响土壤N2O的释放因素提供了新见解,丰富了不同环境条件下制定减排措施的理论依据,但今后仍需进一步了解反硝化过程中间产物的分配及微生物在其中发挥的重要作用。
4 结论
碱性水稻土N2O释放速率随土壤水含量的增加呈先增大后减小的变化趋势,且施用硫酸铵的N2O排放速率低于尿素。添加氮肥后异养硝化对N2O排放的贡献降低,其中硫酸铵处理自养硝化起主导作用,尿素处理反硝化作用起主导作用。因此,旱地土壤施肥应选用反硝化起主导作用的尿素并配合使用硝化抑制剂,水田施肥则以铵态氮肥能更好地减少N2O释放。
参考文献:
苏瑜,王为东. 2017. 我国北方四类土壤中氨氧化古菌和氨氧化细菌的活性及对氨氧化的贡献[J]. 环境科学学报,37(9):3519-3527. [Su Y,Wang W D. 2017. Activity of AOA and AOB and their contributions to ammonia oxidization in four soils in North China[J]. Acta Scientiae Circumstantiae,37(9):3519-3527.]
王大鹏,郑亮,罗雪华,王文斌,张永发,薛欣欣,吴小平. 2018. 砖红壤不同温度、水分及碳氮源条件下硝化和反硝化特征[J]. 土壤通报,49(3):616-622. [Wang D P,Zheng L,Luo X H,Wang W B,Zhang Y F,Xue X X,Wu X P. 2018. Nitrification and denitrification under different temperature,moisture,carbon and nitrogen sources in latosols[J]. Chinese Journal of Soil Science,49(3):616-622.]
席瑞泽,付庆灵,杨永强,尤锦伟,朱俊,胡红青,叶磊. 2017. 氮肥品种和含水量对水稻土N2O排放速率及排放过程的影响[J]. 农业环境科学学报,36(12):2553-2560. [Xi R Z,Fu Q L,Yang Y Q,You J W,Zhu J,Hu H Q,Ye L. 2017. Effects of nitrogen fertilization and water content on the process and rate of N2O emission in paddy soils[J]. Journal of Agro-Environment Science,36(12):2553-2560.]
鄭欠,丁军军,李玉中,林伟,徐春英,李巧珍,毛丽丽. 2017. 土壤含水量对硝化和反硝化过程N2O排放及同位素特征值的影响[J]. 中国农业科学,50(24):4747-4758. [Zheng Q,Ding J J,Li Y Z,Lin W,Xu C Y,Li Q Z,Mao L L. 2017. The effects of soil water content on N2O emissions and isotopic signature of nitrification and denitrification[J]. Scientia Agricultura Sinica,50(24):4747-4758.]
Bateman E J,Baggs E M. 2005. Contributions of nitrification and denitrification to N2O emissions from soils at diffe-rent water-filled pore space[J]. Biology and Fertility of Soils,41:379-388.
Burger M,Venterea R T. 2011. Effects of nitrogen fertilizer types on nitrous oxide emissions[J]. ACS Symposium Series, 1072:179-202.
Cai Z C. 2012. Greenhouse gas budget for terrestrial ecosystems in China[J]. Science China Earth Sciences,55(2):173-182.
Chen S,Wang F,Zhang Y,Qin S,Wei S,Wang S,Hu C,Liu B. 2018. Organic carbon availability limiting microbial denitrification in the deep vadose zone[J]. Environmental Microbiology,20(3):980-992.
Dalal R C,Wang W J,Robertson G P,Parton W J. 2003. Nitrous oxide emission from Australian agricultural lands and mitigation options:A review[J]. Australian Journal of Soil Research,41:165-195.
Duggin J A,Voigt G K,Bormann F H. 1991. Autotrophic and heterotrophic nitrification in response to clear-cutting northern hardwood forest[J]. Soil Biology and Bioche-mistry,23(8):779-787.
Gagnon B,Ziadi N,Rochette P,Chantigny M H,Angers D A. 2011. Fertilizer source influenced nitrous oxide emissions from a clay soil under corn[J]. Soil Science Society of America Journal,75(2):595-604.
Galloway J N,Townsend A R,Erisman J W,Bekunda M,Cai Z,Freney J R,Martinelli L A,Seitzinger S P,Sutton M A. 2008. Transformation of the nitrogen cycle:Recent trends,questions,and potential solutions[J]. Science,320(5878):889-892.
Ge T D,Li B Z,Zhu Z K,Hu Y J,Yuan H Z,Dorodnikov M,Jones D L,Wu J S,Kuzyakov Y. 2017. Rice rhizodeposition and its utilization by microbial groups depends on N fertilization[J]. Biology and Fertility of Soils,53(1):37-48.
Inubushi K,Naganuma H,Kitahara S. 1996. Contribution of denitrification and autotrophic and heterotrophic nitrification to nitrous oxide production in andosols[J]. Biology and Fertility of Soils,23(3):292-298.
Ishii S,Ikeda S,Minamisawa K,Senoo K. 2011. Nitrogen cycling in rice paddy environments:Past achievements and future challenges[J]. Microbes and Environments,26(4):282-292.
Ke X,Angel R,Lu Y,Conrad R. 2013. Niche differentiation of ammonia oxidizers and nitrite oxidizers in rice paddy soil[J]. Environmental Microbiology,15(8):2275-2292.
Lan T,Han Y,Roelcke M,Nieder R,Cai Z C. 2014. Temperature dependence of gross N transformation rates in two Chinese paddy soils under aerobic condition[J]. Biology and Fertility of Soils,50(6):949-959.
Lebender U,Senbayram M,Lammel J,Kuhlmann H. 2014. Effect of mineral nitrogen fertilizer forms on N2O emissions from arable soils in winter wheat production[J]. Journal of Plant Nutrition and Soil Science,177(5):722-732.
Li Y L,Wang X X. 2013. Root-induced changes in radial oxygen loss,rhizosphere oxygen profile,and nitrification of two rice cultivars in Chinese red soil regions[J]. Plant and Soil,365:115-126.
Li Y,Watanabe T,Murase J,Asakawa S,Kimura M. 2014. Abundance and composition of ammonia oxidizers in response to degradation of root cap cells of rice in soil microcosms[J]. Journal of Soils and Sediments,14:1587-1598.
Liu R,Hayden H L,Suter H,Hu H W,Lam S K,He J Z,Mele P M,Chen D L. 2017. The effect of temperature and moisture on the source of N2O and contributions from ammonia oxidizers in an agricultural soil[J]. Biology and Fertility of Soils,53(1):141-152.
Liu X J,Mosier A R,Halvorson A D,Reule C A,Zhang F S. 2007. Dinitrogen and N2O emissions in arable soils:Effect of tillage,N source and soil moisture[J]. Soil Biology and Biochemistry,39(9):2362-2370.
Morse J L,Bernhardt E S. 2013. Using 15N tracers to estimate N2O and N2 emissions from nitrification and denitrification in coastal plain wetlands under contrasting land-uses [J]. Soil Biology and Biochemistry,57:635-643.
Müller C,Laughlin R J,Spott O,Rütting T. 2014. Quantification of N2O emission pathways via a 15N tracing model[J]. Soil Biology and Biochemistry,72:44-54.
Nicol G W,Leininger S,Schleper C,Prosser J I. 2008. The influence of soil pH on the diversity,abundance and transcriptional activity of ammonia oxidizing archaea and bacteria[J]. Environmental Microbiology,10(11):2966-2978.
Pihlatie M,Syv?salo E,Simojoki A,Esala M,Regina K. 2004. Contribution of nitrification and denitrification to N2O production in peat,clay and loamy sand soils under different soil moisture conditions[J]. Nutrient Cycling in Agroecosystems,70(2):135-141.
Ravishankara A R,Daniel J S,Portmann R W. 2009. Nitrous oxide(N2O):The dominant ozone-depleting substance emitted in the 21st Century[J]. Science,326(5949):123-125.
Rudaz A O,W?lti E,Kyburz G,Lehmann P,Fuhrer J. 1999. Temporal variation in N2O and N2 fluxes from a permanent pasture in Switzerland in relation to management,soil water content and soil temperature[J]. Agriculture,Ecosystems & Environment,73(1):83-91.
Rütting T,Clough T J,Mueller C,Lieffering M,Newton P C. 2010. Ten years of elevated atmospheric carbon dioxide alters soil nitrogen transformations in a sheep-grazed pasture[J]. Global Change Biology,16(9):2530-2542.
Sainju U M. 2016. A global meta-analysis on the impact of management practices on net global warming potential and greenhouse gas intensity from cropland soils[J]. PLoS One,11:e0148527.
Shcherbak I,Millar N,Robertson G P. 2014. Global metaanalysis of the nonlinear response of soil nitrous oxide(N2O)emissions to fertilizer nitrogen[J]. Proceedings of the National Academy of Sciences of the united States of Ame-rica,111:9199-9204.
Solomon S,Qin D,Manning M,Chen Z,Marquis M,Averyt K B,Tignor M,Miller H L. 2007. Contribution of wor-king group I to the fourth assessment report of the intergovernmental panel on climate change[M]. United Kingdom:Cambridge University Press:1-1538.
Stange C F,Spott O,Arriaga H,Menéndez S,Estavillo J M,Merino P. 2013. Use of the inverse abundance approach to identify the sources of NO and N2O release from Spa-nish forest soils under oxic and hypoxic conditions[J]. Soil Biology and Biochemistry,57:451-458.
Taylor A E,Vajrala N,Giguere A T,Gitelman A I,Arp D J,Myrold D D,Luis S S,Bottomley P J. 2013. Use of aliphatic n-alkynes to discriminate soil nitrification activities of ammonia-oxidizing thaumarchaea and bacteria[J]. Applied & Environmental Microbiology,79:6544-6551.
Tong D L,Xu R K. 2012. Effects of urea and(NH4)2SO4 on nitrification and acidification of Ultisols from Southern China[J]. Journal of Environmental Sciences,24(4):682-689.
Yao Z S,Zhou Z X,Zheng X H,Xie B H,Mei B L,Wang R,Butterbach-Bahl K,Zhu J G. 2010. Effects of organic matter incorporation on nitrous oxide emissions from rice-wheat rotation ecosystems in China[J]. Plant and Soil,327:315-330.
Zhang R,Wienhold B J. 2002. The effect of soil moisture on mineral nitrogen,soil electrical conductivity,and pH[J]. Nutrient Cycling in Agroecosystems,63(2-3):251-254.
Zhao X,Xing G X. 2009. Variation in the relationship between nitrification and acidification of subtropical soils as affected by the addition of urea or ammonium sulfate [J]. Soil Biology and Biochemistry,41(12):2584-2587.
Zhu G,Wang S,Wang Y,Wang C,Risgaard-Petersen N,Jetten M S,Yin C. 2011. Anaerobic ammonia oxidation in a fertilized paddy soil[J]. The ISME Journal,5(12):1905-1912.
(責任编辑 王 晖)
