2型糖尿病进程中小鼠下丘脑室旁核和视上核nesfatin`-1表达
2019-09-10李晓玲刘媛宋莉敏董静
李晓玲 刘媛 宋莉敏 董静
[摘要]目的探讨2型糖尿病进程中nesfatin`-1在小鼠下丘脑室旁核和视上核中的表达。方法将雄性昆明小鼠随机分为对照组(普通饮食喂养)、高脂饮食组和糖尿病组。糖尿病组小鼠腹腔注射链脲佐菌素,并给予高脂饮食喂养诱导2型糖尿病模型。比较3组小鼠血糖水平、稳态模型的胰岛素抵抗指数(HOMA`-IR)及摄食量。分别于造模成功后的2、4、6、8、10、12周,采用免疫荧光组织化学技术检测各组小鼠下丘脑室旁核和视上核组织中nesfatin`-1表达。结果糖尿病组小鼠血糖较高脂饮食组和对照组明显增高,且出现明显的胰岛素抵抗,同时摄食量增多。糖尿病组小鼠室旁核和视上核nesfatin`-1阳性神经元数量在造模后前6周逐渐增加,后6周逐渐降低,总体nesfatin`-1表達水平低于高脂饮食组和对照组。结论2型糖尿病小鼠下丘脑室旁核和视上核中的nesfatin`-1表达水平降低,其表达变化呈前期上升、后期下降的趋势。
[关键词]nesfatin`-1;糖尿病,2型;下丘脑室旁核;视上核;小鼠
[ABSTRACT]ObjectiveTo investigate the expression of nesfain`-1 in the hypothalamic paraventricular nucleus (PVN) and the supraoptic nucleus (SON) in mice during the course of type 2 diabetes. MethodsMale Kunming mice were randomly divi`-ded into control group with a normal diet, high`-fat diet group, and diabetes group. The mice in the diabetes group were given intrape`-ritoneal injection of streptozotocin and a high`-fat diet to induce diabetes. The three groups were compared in terms of the level of blood glucose, homeostasis model assessment of insulin resistance (HOMA`-IR), and food intake. At 2,4,6,8,10, and 12 weeks after the model was successfully established, immunofluorescence assay was used to measure the expression of nesfatin`-1 in the PVN and the SON. ResultsCompared with the high`-fat diet group and the control group, the diabetes group had significantly higher blood glucose level, HOMA`-IR, and food intake. In the diabetes group, there was a gradual increase in the number of nesfatin`-1`-positive neurons in the PVN and the SON during the first 6 weeks, followed by a gradual reduction in the next 6 weeks, and the diabetes group generally had lower expression of nesfatin`-1 than the high`-fat diet group and the control group. ConclusionThere is a reduction in the expression of nesfatin`-1 in the hypothalamic PVN and the SON in mice with type 2 diabetes, and the expression of nesfatin`-1 increases in the early stage and then decreases in the late stage.
[KEY WORDS]nesfatin`-1; diabetes mellitus, type 2; paraventricular hypothalamic nucleus; supraoptic nucleus; mice
2型糖尿病表现为代谢功能障碍。高脂血症以及高糖血症是影响2型糖尿病病人许多组织器官(包括心脏、肝脏、肾脏和眼血管内皮等)并发症的触发因素[1`-2]。Nesfatin`-1作为一种厌食肽,通过中枢机制参与能量稳态的调节。有研究结果表明,2型糖尿病病人的血浆nesfatin`-1水平升高,并与体质量指数(BMI)、血浆胰岛素水平和稳态模型的胰岛素抵抗指数(HOMA`-IR)有关[3]。最近的研究结果揭示了nesfatin`-1在体内葡萄糖平衡中的抗高糖血症作用[4]。中枢nesfatin`-1在标准饮食和高脂饮食的大鼠中均能够显著抑制肝脏磷酸烯醇丙酮酸羧激酶(PEPCK)表达水平,而且 nesfatin`-1在高糖血症条件下可增加β细胞的胰岛素释放。外周nesfatin`-1可通过激活2型糖尿病小鼠的AMP`-ACC途径参与脂肪代谢调节[5]。但nesfatin`-1在2型糖尿病进程中是否有变化及其所起的作用目前尚不清楚,本研究旨在进一步探讨2型糖尿病进程中小鼠中枢核团室旁核(PVN)和视上核(SON)nesfatin`-1的表达变化,为糖脂代谢下丘脑调节机制的深入研究提供实验依据。现将结果报告如下。
1材料和方法
1.1动物及分组
实验所用3周龄雄性昆明小鼠90只,均由青岛市药品检验所提供。将小鼠随机分为对照组(Con组)、高脂饮食组(HFD组)以及糖尿病组(DM组)。Con组小鼠喂普通鼠粮,HFD组和DM组小鼠喂高脂食物(59%普通鼠粮,20%糖,18%猪油和3%蛋黄)。小鼠饲养于标准动物房,室温(23±2)℃,湿度50%~60%,7:00—19:00进行光照,严格12 h昼夜循环,自由饮水和进食。处理小鼠的规程经青岛大学动物保护和使用委员会认证。本研究符合国立卫生研究院《实验室护理与使用指南》的指导方针。
1.2主要试剂
小鼠胰岛素ELISA试剂盒(R&D Systems),兔抗鼠nesfatin`-1抗体购于美国 Phoenix公司,荧光标记的山羊抗兔IgG购于北京中杉金桥公司,链脲佐菌素(STZ)购于Sigma公司,生理盐水(NS)购于青岛大学校医院,水合氯醛购于天津市瑞金特化学品有限公司。
1.32型糖尿病小鼠模型构建
在6和9周龄时,DM组的小鼠进行两次低剂量STZ(100 mg/kg,将STZ溶解于pH值为4.5的0.1 mol/L柠檬酸盐缓冲液中)腹腔注射,Con组和HFD组仅注射等剂量的缓冲液。在11周龄末进行空腹血糖以及空腹血浆胰岛素水平检测,空腹血糖水平高于12 mmol/L且HOMA`-IR异常增高,则认为2型糖尿病造模成功[6]。
1.4小鼠血糖水平、胰岛素抵抗和摄食量测定
于造模成功后2、4、6、8、10、12周末,从小鼠尾静脉采血,用德国贝朗倍佳血糖仪测定空腹血糖水平,用ELISA试剂盒测定空腹血浆胰岛素水平。通过以下公式计算HOMA`-IR[7]:HOMA`-IR=空腹血浆胰岛素(mU/L)×空腹血糖(mmol/L)/22.5。每周周日19:00开始测12 h累积摄食量。
1.5免疫荧光法检测小鼠下丘脑核团nesfatin`-1阳性神经元表达
于造模成功后2、4、6、8、10、12周末尾静脉取血后,腹腔注射80 g/L水合氯醛0.4 g/kg麻醉小鼠,进行心脏灌注,迅速断头取脑置40 g/L多聚甲醛溶液中固定,于4 ℃冰箱中固定24 h后,再用300 g/L蔗糖溶液脱水24 h。根据小鼠大脑立体定位图谱分别切取下丘脑PVN和SON进行冷冻切片(切片厚度为10~15 μm),将切片置于载玻片上。切好的脑片置于60 ℃温箱中烤片4 h,用PBST溶液浸泡20 min,置于修复液内,用微波炉95 ℃中火修复抗原10 min,待自然冷却后以PBST溶液洗3次,每次7 min。用小牛血清室温封闭2 h,兔抗鼠nesfatin`-1抗体(1∶500)4 ℃孵育脑片12 h,以PBST溶液冲洗3次,用荧光标记的山羊抗兔IgG(1∶100)室温避光孵育2 h,以PBST溶液洗片后用封片液(PBST和甘油体积比为1∶1)封片固定脑片,在荧光显微镜下观察nesfatin`-1的表达。采用Image`-Pro Plus 6.0软件进行阳性神经元计数。
1.6统计学分析
实验所得计量数据以±s表示,使用SPSS 20.0软件、采用析因设计的方差分析进行显著性检验,以P<0.05为差异有统计学意义。
2结果
2.12型糖尿病小鼠血糖水平、胰岛素抵抗和摄食量改变
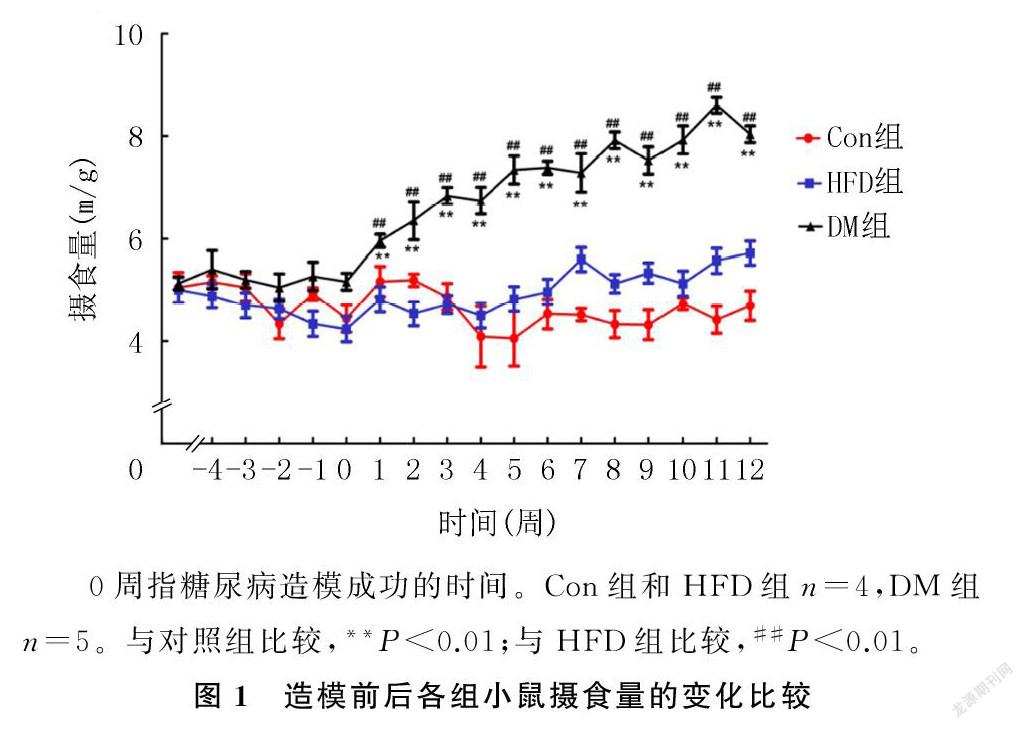
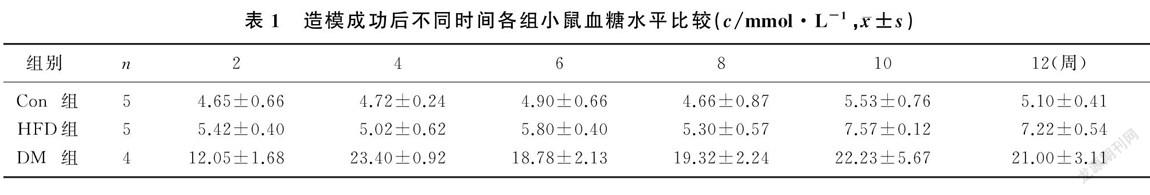
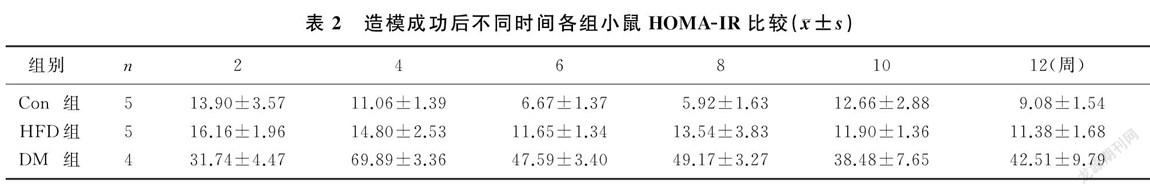
造模成功后小鼠摄食量持续增加,并且显著高于HFD和Con组小鼠(F=5.151~101.225,P<0.01)。见图1。小鼠血糖水平组别的主效应有统计学意义(F=155.922,P<0.01),DM组小鼠不同时间的血糖水平均显著高于HFD组和Con组(F=14.435~253.832,P<0.01);时间的主效应也有统计学意义(F=2.844,P<0.05),DM组小鼠造模成功后2周的血糖水平低于4、6、8、10、12周(F=2.578,P<0.05);两因素间的交互效应无统计学意义(P>0.05)。见表1。小鼠胰岛素抵抗水平组别的主效应有统计学意义(F=142.257,P<0.01),DM组小鼠各时间的HOMA`-IR较HFD和Con组明显增加(F=6.905~150.941,P<0.05);时间的主效应也有统计学意义(F=3.546,P<0.05),DM组小鼠造模成功后4周的HOMA`-IR较其他时间明显增高(F=5.122,P<0.05);兩因素间的交互效应有统计学意义(F=3.899,P<0.01)。见表2。
2.22型糖尿病小鼠下丘脑PVN和SON中nesfatin`-1阳性神经元表达
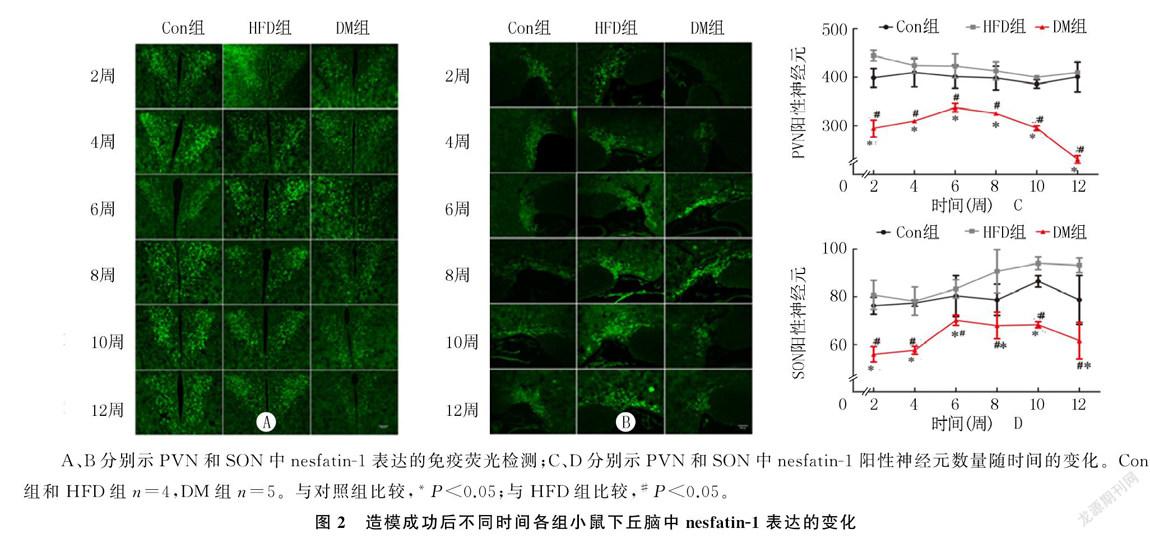
免疫荧光检测显示,小鼠PVN中nesfatin`-1表达水平组别的主效应有统计学意义(F=64.747,P<0.01),DM组小鼠各时间nesfatin`-1阳性神经元的数量明显少于HFD组和Con组(F=4.917~30.321,P<0.05);时间的主效应没有统计学意义(P>0.05),但DM组小鼠nesfatin`-1阳性神经元的数量在造模成功后前6周呈上升趋势,之后呈下降趋势;两因素间的交互效应没有统计学意义(P>0.05)。SON中阳性nesfatin`-1神经元数量的变化呈现出与PVN大致相同的趋势。小鼠PVN中nesfatin`-1表达水平组别的主效应有统计学意义(F=29.622,P<0.01),DM组小鼠各时间nesfatin`-1阳性神经元的数量明显少于HFD组和Con组(F=5.185~31.538,P<0.05);时间的主效应无统计学意义(P>0.05);两因素间的交互效应无统计学意义(P>0.05)。见图2。
3讨论
Nesfatin`-1是由前体蛋白核苷结合蛋白2水解产生的含有82个氨基酸的多肽[8],可产生厌食的效应[9]。Nesfatin`-1诱导的摄食抑制可能是通过抑制促食欲神经元来介导的[10]。Nesfatin`-1分布于中枢神经系统如PVN和SON等,以及外周如胃黏膜、胰腺、脂肪组织等[11]。在下丘脑和内分泌器官中的广泛分布意味着nesfatin`-1在能量稳态和代谢调节中起关键作用。有研究结果表明,nesfatin`-1可影响PVN中大部分不同亚群神经元的兴奋性,作为上游调节因子,能激活PVN缩宫素神经元的黑素皮质素通路,从而揭示了厌食性缩宫素的神经通路[12]。PVN和SON中的nesfatin`-1神经元可在餐后调节摄食行为,在能量稳态中起重要作用[13]。已有研究表明,nesfatin`-1能够增加能量消耗[14],并诱导动物心血管改变,升高血压[15`-17],增加胰岛素敏感性[18]。
Nesfatin`-1作为一种厌食肽,通过中枢机制参与能量稳态的调节[19`-20]。Nesfatin`-1可在大脑中发挥上调胰岛素敏感性的作用[18],并能增加β细胞胰岛素的释放,从而降低升高的血糖[21]。Nesfatin`-1也被发现可抑制中枢神经系统调节的食物摄入[8]。Nesfatin`-1在肥胖动物中的表达增加,并且随着摄食和饥饿状态的不同而改变[22]。2型糖尿病常发生于肥胖人群[23],胰岛素功能障碍[24]、饮食失调[25]和炎症[26]等因素也可引发2型糖尿病。2型糖尿病通常与葡萄糖代谢改变、胰岛素抵抗、空腹血糖异常和葡萄糖耐量降低有关。本研究中糖尿病模型小鼠摄食量增加,体质量下降,表现为明显的胰岛素抵抗。有研究提出,糖尿病性多食是由循环nesfatin`-1水平降低引起的[27]。还有研究表明,nesfatin`-1可以刺激脂质代谢表现出抗炎作用[5]。目前总体来说,nesfatin`-1是肥胖(机体能量代谢)[28]、糖尿病(葡萄糖代谢)[29]和抑郁症[30]等的极为重要的肽激素。
有研究显示,血浆nesfatin`-1水平在2型糖尿病病人中升高[3,31],与BMI、血浆胰岛素和HOMA`-IR有关;并且发现只有新诊断的2型糖尿病病人的循环nesfatin`-1水平增加。但是也有研究显示,糖尿病病人的nesfatin`-1水平较低[27,32],认为结果不一致是由病人的BMI和胰岛素抵抗的差异引起的。本研究比較了糖尿病小鼠与高脂饮食及正常饮食小鼠下丘脑核团nesfatin`-1的表达水平,结果显示,与HFD组和Con组相比,DM组小鼠PVN和SON中nesfatin`-1的表达水平显著降低,表明nesfatin`-1在2型糖尿病的发展中起重要作用。本研究还观察到,在2型糖尿病病程进展过程中,PVN和SON中nesfatin`-1的表达量呈早期逐渐升高后期逐渐降低的趋势。这可能是由于糖尿病早期血糖升高,营养物质相对充足,无需摄入食物来供能,表现为代偿性nesfatin`-1水平升高;但在糖尿病的后期,随着胰岛素抵抗的加重,细胞缺乏足够的能量,刺激摄食中枢,引起摄食的增加,nesfatin`-1水平降低。这与循环中nesfatin`-1水平与2型糖尿病关系的Meta分析结果相一致[33],即接受抗糖尿病治疗或疾病持续时间长的2型糖尿病病人循环nesfatin`-1水平较低,早期2型糖尿病与nesfatin`-1水平升高相关,可能是由于血糖和食物摄入的补偿机制。
中枢神经系统可以通过下丘脑`-垂体`-肾上腺皮质轴来调控外周器官功能活动,近年来,下丘脑对内脏功能的调节一直受到关注。有文献报道,下丘脑nesfatin`-1活性是由ERK信号传导介导的,ERK进而调节自主神经系统[34]。先前的研究发现,许多不同的下丘脑细胞内信号,如AMPK、mTOR等通过介导nesfatin`-1影响交感神经系统[18]。但中枢nesfatin`-1到底如何改善体内代谢紊乱目前仍不是非常明确,还需进一步探讨nesfatin`-1是否通过交感神经调节外周代谢以及如何调节。
综上所述,STZ和高能量摄食诱导的2型糖尿病小鼠下丘脑PVN和SON中的nesfatin`-1表达降低,nesfatin`-1的表达变化呈前期上升、后期下降的趋势。表明nesfatin`-1在2型糖尿病的进程中发挥了重要作用,有望成为抗糖尿病治疗的新靶标。本文结果为糖脂代谢的神经调节研究提供了依据。
[参考文献]
[1]GUILHERME A, VIRBASIUS JV, PURI V, et al. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes[J]. Nature Reviews Molecular Cell Biology, 2008,9(5):367`-377.
[2]LEWIS G F, CARPENTIER A, ADELI K, et al. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes[J]. Endocrine Reviews, 2002,23(2):201`-229.
[3]ZHANG Z, LI L, YANG M, et al. Increased plasma levels of nesfatin`-1 in patients with newly diagnosed type 2 diabetes mellitus[J]. Experimental and Clinical Endocrinology & Diabetes, 2012,120(2):91`-95.
[4]SU Y, ZHANG J, TANG Y, et al. The novel function of nesfatin`-1:anti`-hyperglycemia[J]. Biochemical and Biophysical Research Communications, 2010,391(1):1039`-1042.
[5]DONG Jing, XU Huan, XU Huan, et al. Nesfatin`-1 stimulates fatty`-acid oxidation by activating AMP`-activated protein kinase in STZ`-induced type 2 diabetic mice[J]. PLoS One, 2013,8(12):e83397.
[6]XIA Yunqiu, LI Qing, ZHONG Weizhen, et al. L`-carnitine ameliorated fatty liver in high`-calorie diet/STZ`-induced type 2 diabetic mice by improving mitochondrial function[J]. Diabetology & Metabolic Syndrome, 2011,3:31.
[7]ZHANG Xingguang, ZHANG Yanqi, CHENG Qianpeng, et al. The impact of insulin pump therapy to oxidative stress in patients with diabetic nephropathy[J]. European Journal of Medical Research, 2018,23(1):7.
[8]OH I S, SHIMIZU H, SATOH T, et al. Identification of nes`-
196青島大学学报(医学版)55卷
fatin`-1 as a satiety molecule in the hypothalamus[J]. Nature, 2006,443(7112):709`-712.
[9]SHIMIZU H, OH I S, HASHIMOTO K, et al. Peripheral administration of nesfatin`-1 reduces food intake in mice:the leptin`-independent mechanism[J]. Endocrinology, 2009,150(2):662`-671.
[10]PRICE C J, SAMSON W K, FERGUSON A V. Nesfatin`-1 inhibits NPY neurons in the arcuate nucleus[J]. Brain Research, 2008,1230:99`-106.
[11]STENGEL A, GOEBEL M, YAKUBOV I, et al. Identification and characterization of nesfatin`-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa[J]. Endocrinology, 2009,150(1):232`-238.
[12]MAEJIMA Y, SEDBAZAR U, SUYAMA S, et al. Nesfatin`-1`-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin`-independent melanocor`-tin pathway[J]. Cell Metabolism, 2009,10(5):355`-365.
[13]STENGEL A, TACHE Y. Minireview:nesfatin`-1-an emerging new player in the brain`-gut, endocrine, and metabolic axis[J]. Endocrinology, 2011,152(11):4033`-4038.
[14]WERNECKE K, LAMPRECHT I, JOHREN O, et al. Nesfatin`-1 increases energy expenditure and reduces food intake in rats[J]. Obesity(Silver Spring, Md), 2014,22(7):1662`-1668.
[15]TANIDA M, MORI M. Nesfatin`-1 stimulates renal sympathetic nerve activity in rats[J]. Neuroreport, 2011,22(6):309`-312.
[16]YOSTEN G L, SAMSON W K. The anorexigenic and hypertensive effects of nesfatin`-1 are reversed by pretreatment with an oxytocin receptor antagonist[J]. American Journal of Phy`-siology Regulatory, Integrative and Comparative Physiology, 2010,298(6):R1642`-1647.
[17]YOSTEN G L, SAMSON W K. Neural circuitry underlying the central hypertensive action of nesfatin`-1:melanocortins, corticotropin`-releasing hormone, and oxytocin[J]. American Journal of Physiology Regulatory, Integrative and Comparative Physiology, 2014,306(10): R722`-R727.
[18]YANG Mengliu, ZHANG Zhihong, WANG Chong, et al. Nesfatin`-1 action in the brain increases insulin sensitivity through Akt/AMPK/TORC2 pathway in diet`-induced insulin resistance[J]. Diabetes, 2012,61(8):1959`-1968.
[19]KOHNO D, NAKATA M, MAEJIMA Y, et al. Nesfatin`-1 neurons in paraventricular and supraoptic nuclei of the rat hypothalamus coexpress oxytocin and vasopressin and are activated by refeeding[J]. Endocrinology, 2008,149(3):1295`-1301.
[20]STENGEL A, TACHE Y. Brain peptides and the modulation of postoperative gastric ileus[J]. Curr Opin Pharmacol, 2014,19:31`-37.
[21]NAKATA M, MANAKA K, YAMAMOTO S, et al. Nesfatin`-1 enhances glucose`-induced insulin secretion by promoting Ca(2+) influx through L`-type channels in mouse islet beta`-cells[J]. Endocrine Journal, 2011,58(4):305`-313.
[22]LI Ziru, XU Geyang, LI Yin, et al. mTOR`-dependent modulation of gastric nesfatin`-1/NUCB2[J]. Cellular Physiology and Biochemistry, 2012,29(3`-4):493`-500.
[23]DEFRONZO R A. Lilly lecture 1987. The triumvirate:beta`-cell, muscle, liver. A collusion responsible for NIDDM[J]. Diabetes, 1988,37(6):667`-687.
[24]NISKANEN L. Insulin treatment in elderly patients with non`-insulin`-dependent diabetes mellitus. A double`-edged sword[J]? Drugs & Aging, 1996,8(3):183`-192.
[25]HERPERTZ S, WAGENER R, ALBUS C, et al. Diabetes mellitus and eating disorders:a multicenter study on the comorbidity of the two diseases[J]. Journal of Psychosomatic Research, 1998,44(3`-4):503`-515.
[26]SCHERNTHANER G H, SCHERNTHANER G. Insulin resistance and inflammation in the early phase of type 2 diabetes:potential for therapeutic intervention[J]. Scandinavian Journal of Clinical and Laboratory Investigation Supplementum, 2005,240:30`-40.
[27]LI Qingchun, WANG Haiyan, CHEN Xi, et al. Fasting plasma levels of nesfatin`-1 in patients with type 1 and type 2 diabetes mellitus and the nutrient`-related fluctuation of nesfatin`-1 level in normal humans[J]. Regulatory Peptides, 2010,159(1`-3):72`-77.
[28]SHAPARENKO O V, KRAVCHUN P G, KRAVCHUN P P, et al. Nesfatin`-1 role in remodeling of the left ventricle myocardium in patients with arterial hypertension and obesity[J]. Wiadomosci Lekarskie (Warsaw, Poland:1960), 2018,71(5):1006`-1009.
[29]MASUO K. Nesfatin`-1 could be a strong candidate obesity or diabetes medication, if blood pressure elevation can be controlled[J]. Hypertension Research:Official Journal of the Ja`-panese Society of Hypertension, 2014,37(2):98`-99.
[30]ALGUL S, OZCELIK O. Evaluating the levels of nesfatin`-1 and ghrelin hormones in patients with moderate and severe major depressive disorders[J]. Psychiatry Investigation, 2018,15(2):214`-218.
[31]GUO Y, LIAO Y, FANG G, et al. Increased nucleobindin`-2(NUCB2)transcriptional activity links the regulation of insulin sensitivity in type 2 diabetes mellitus[J]. Journal of Endocrinological Investigation, 2013,36(10):883`-888.
[32]ALGUL S, OZKAN Y, OZCELIK O. Serum nesfatin`-1 levels in patients with different glucose tolerance levels[J]. Physiological Research, 2016,65(6):979`-985.
[33]ZHAI Ting, LI Shizhen, FAN Xintong, et al. Circulating nesfatin`-1 levels and type 2 diabetes:a systematic review and Meta`-analysis[J]. 2017, 2017:7687098.
[34]TANIDA M, GOTOH H, YAMAMOTO N, et al. Hypothalamic nesfatin`-1 stimulates sympathetic nerve activity via hypothalamic ERK signaling[J]. Diabetes, 2015,64(11):3725`-3736.
