过表达ApGSMT2和ApDMT2基因的拟南芥和玉米耐盐性分析
2019-08-16王娟关海英董瑞刘春晓刘强刘铁山汪黎明何春梅
王娟 关海英 董瑞 刘春晓 刘强 刘铁山 汪黎明 何春梅


摘要:玉米是对土壤盐渍化中度敏感的作物,易受盐碱危害。甘氨酸甜菜碱作为一种主要的渗透保护物质,能够提高植物对多种非生物胁迫(如盐碱、干旱、低温等)的抗性。本工作前期从嗜盐隐杆藻中克隆得到两个参与甘氨酸甜菜碱合成的甲基转移酶基因ApGSMT2和ApDMT2,利用农杆菌介导法,将两个基因分别在拟南芥和玉米中共同过表达,获得转基因阳性株,收获T1代转基因种子,经自交后得到T2代种子。以拟南芥T2代种子为试材,设置0、50、100、150、200 mmol/L NaCl处理,进行种子萌发试验,结果显示,不同盐浓度处理下,转基因拟南芥种子的萌发率显著高于未转基因对照植株,说明过表达ApGSMT2和ApDMT2基因对于提高拟南芥的耐盐性具有显著效果。进一步对T2代转基因玉米株系幼苗的耐盐性进行试验,结果表明,180 mmol/L NaCl处理后,未转基因对照植株萎蔫,而转基因株系长势良好,其株高、根长、叶片相对含水量和鲜重显著高于对照,说明过表达ApGSMT2和ApDMT2基因显著提高了玉米对盐胁迫的耐受性,为利用基因工程技术创制玉米耐盐种质提供了理论依据。
关键词:玉米;拟南芥;ApGSMT2;ApDMT2;甘氨酸甜菜碱;耐盐性
中图分类号:S513.034+Q949.748.306 文献标识号:A 文章编号:1001-4942(2019)06-0010-07
Abstract Maize is a moderately sensitive crop to soil salinization and is vulnerable to saline-alkali damage. Glycine betaine (GB), as a major osmotic protective solute, has shown the ability to improve plant resistance to a variety of abiotic stresses, such as salinity, drought and low temperature. Two methyltransferase genes, ApGSMT2 and ApDMT2, which are involved in the synthesis of GB, were cloned from Aphanothece halophytica in our previous studies. The two genes were overexpressed in Arabidopsis and maize through Agrobacterium-mediated method, and the transgenic positive strains were obtained. The T2 generation was obtained through selfing-cross from T1 generation. With the T2 seeds of Arabidopsis as materials, the germination test was conducted by setting the treatments of 0, 50, 100, 150 and 200 mmol/L NaCl. The germination rate of transgenic Arabidopsis seeds was significantly higher than that of wild-type plants under various concentrations of salt treatment. It indicated that overexpressing ApGSMT2 and ApDMT2 could significantly enhance the salt tolerance of Arabidopsis. The test was further conducted on the salt tolerance of T2 maize seedlings. Under the treatment of 180 mmol/L NaCl, the transgenic maize seedlings developed better, while the control plants wilting. The plant height, root length, leaf relative water content and fresh weight of transgenic lines were significantly higher than those of untransformed control plants. These results demonstrated that ApGSMT2 and ApDMT2 overexpression significantly increased the tolerance of Arabidopsis and maize to salt stress.
Keywords Maize; Arabidopsis; ApGSMT2;ApDMT2; Glycine betaine; Salt tolerance
土壤鹽渍化是造成作物减产的主要因素之一[1]。玉米既是重要的粮食和饲料作物,又可作为医药和工业原料。由于玉米属于中度盐敏感植物,耐盐能力比较低,因此其种植面积和产量受到一定的限制[2, 3]。随着生物技术的迅速发展,利用基因工程技术培育转基因玉米已成为提高玉米耐盐性和解决其在盐碱化土壤上种植的有效途径之一[4, 5]。
甘氨酸甜菜碱(glycine betaine,GB)作为一种主要的渗透保护物质,能够提高植物对多种非生物胁迫(如盐碱、干旱、低温等)的抗性[6-10]。在自然界中,已知的甘氨酸甜菜碱的生物合成途径主要有两种,即胆碱氧化途径和甘氨酸甲基化途径[11-13]。以甘氨酸为底物合成甜菜碱的途径首先是在两种极嗜盐的微生物中发现的,由甘氨酸经过连续三步N-甲基化生成甜菜碱,该途径由依赖S-腺苷甲硫氨酸(SAM)的甘氨酸肌氨酸甲基转移酶(glycine sarcosine methyltransferase,GSMT)和依赖SAM的肌氨酸二甲基甘氨酸甲基转移酶(sarcosine dimethylglycine methyltransferase,SDMT)分别催化完成[13]。将该甘氨酸甲基化途径引入作物中可显著增加GB的累积并提高作物的耐逆性[14-17]。2005年,Waditee等从耐盐藻青菌(Aphanothece halophytica)中克隆出ApGSMT和ApDMT基因并共转化到淡水藻青菌(Synechococcus sp. PCC7942)和拟南芥中,发现在0.5 mol/L NaCl胁迫下,转ApGSMT和ApDMT基因的淡水藻青菌细胞内的GB浓度高达200 mmol/L,比转胆碱氧化途径基因的细胞GB含量高了5倍,使其耐盐能力足够在海水中生活;GB在转ApGSMT/ApDMT基因拟南芥的根、茎、叶和花等中都有积累,转基因植株的耐盐、耐冷和抗旱性与转胆碱氧化途径基因的拟南芥相比都有明显提高[15]。Niu等在水稻中共表达ApGSMT和ApDMT基因,转基因株系体内积累了较高的GB含量且耐盐耐冷性得到了显著性提高[18]。山东大学He等从南京大学提供的一株被命名为Aphanothece halophytica GR20的嗜盐隐杆藻中克隆出ApGSMT2和ApDMT2基因,并在烟草中验证了其功能,确定了共表达ApGSMT2和ApDMT2的转基因烟草耐旱性大幅度提高[19]。Song等的研究表明,共表达密码子经过优化的ApGSMT2g和ApDMT2g基因显著提高了转基因棉花在盐碱地的抗性及产量[20]。此外,共表达ApGSMT2和ApDMT2的转基因玉米体内积累了较高的GB含量且耐旱性得到显著提高[21],目前,关于引入甘氨酸甲基化途径基因ApGSMT2和ApDMT2对玉米耐盐性的影响还未见相关报道。
本试验将ApGSMT2和ApDMT2基因重组到植物表达载体中,利用农杆菌介导法分别转化拟南芥和玉米,以T2代转基因株系为材料,通过分析不同浓度NaCl处理下拟南芥种子的萌发率来明确过表达ApGSMT2和ApDMT2基因的耐盐效果,并进一步对NaCl胁迫下转基因玉米幼苗的生长发育和基因表达情况进行分析,探讨过表达ApGSMT2和ApDMT2基因与玉米耐盐性的关系,以期为玉米耐盐育种提供理论基础和潜在优异耐盐种质资源。
1 材料与方法
1.1 试验材料与试剂
以本实验室前期构建的植物双元表达载体p3300-ApGSMT2-ApDMT2-bar为基础,通过农杆菌介导的幼胚遗传转化方法分别转化拟南芥Col-0和玉米自交系HiII,获得转基因阳性植株,收获T1代转基因种子,经自交后得到T2代种子,以此为材料,进行耐盐性鉴定。
RNAiso Plus和PrimeScript RT reagent Kit with gDNA Eraser试剂盒购自大连宝生物工程公司;2×Taq Plus Master Mix购自南京诺唯赞生物科技有限公司;其余试剂均为进口或国产分析纯。
1.2 试验方法
1.2.1 转基因阳性植株的PCR鉴定 采用CTAB法提取拟南芥或玉米叶片基因组DNA[22],进行PCR检测,以确定转基因阳性植株。PCR鉴定所用引物为UBI-F:5′-CTTTTTGTTCGCTTGGTTGTGATGA-3′和G-R:5′-CGCGCTTGCCAATTGATTAAC-3′(扩增产物560 bp)。PCR反应体系为20 μL,包括:2×Taq Plus Master Mix 10 μL,上下游引物(10 μmol/L)各0.8 μL,基因组DNA(50 ng/μL)2 μL,灭菌水6.4 μL。PCR反应程序为:94℃预变性5 min;94℃变性30 s,56℃退火30 s,72℃延伸30 s,循环35次;72℃过度延伸5 min。
1.2.2 盐胁迫下拟南芥转基因株系的种子萌发试验 随机选取5个拟南芥T2代转ApGSMT2和ApDMT2基因株系(L2~L6),以拟南芥Col-0为对照(WT),种子置于4℃处理48 h,然后经次氯酸钠消毒后,分别播种于含0、50、100、150、200 mmol/L NaCl的1/2MS固体培养基(pH 5.8)上。培养温度为22℃,光周期为16 h光照、8 h黑暗。每个处理重复3次。处理12 d后,统计种子萌发率。
1.2.3 玉米转基因株系的基因表达量鉴定 取转ApGSMT2和ApDMT2玉米T2代株系及对照HiII(WT)的幼苗叶片,液氮速冻,按照RNAiso Plus试剂盒说明书提取总RNA,参照PrimeScript RT reagent Kit with gDNA Eraser试剂盒说明书进行反转录合成cDNA。
表达量鉴定采用半定量RT-PCR方法,PCR所用ApGSMT2引物为GT-F: 5′-GCAAGCGCG
ATCGACCAGTGA-3′和GT-R: 5′-CCCGTTCCCGTGGCAGCATCT-3′;ApDMT2引物为DT-F: 5′-TGCGAGTGTGCGTACCGTTGC-3′和DT-R: 5′-TGCCATATAACGAGCGGAGCC-3′;内参FPGS[23]引物為:FP-F: 5′-ATCTCGTTGGGGATGTCTTG-3′ 和FP-R: 5′-AGCACCGTTCAAATGTCTCC-3′。半定量PCR反应程序为:94℃预变性5 min;94℃变性30 s,56℃退火30 s,72℃延伸15 s,循环28次;72℃过度延伸5 min。
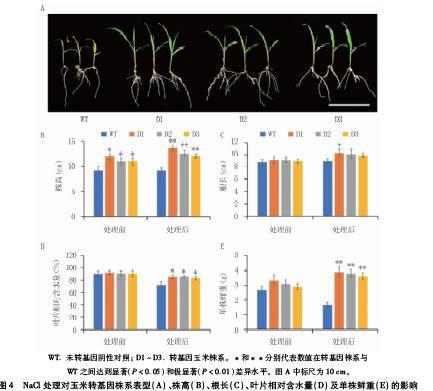
1.2.4 玉米转基因株系苗期的耐盐性鉴定 选取3个T2代转ApGSMT2和ApDMT2基因玉米株系(D1~D3),以玉米自交系HiII(WT)为对照,幼苗经1/2MS营养液水培一周后,转移至含180 mmol/L NaCl的1/2MS营养液中盐处理7 d。分别对处理前后的玉米株系拍照并测量株高、根长、叶片相对含水量和单株鲜重,取3株植株进行重复。
叶片相对含水量的测定:称取玉米幼苗第二片全展叶约0.1 g,浸入去离子水中约4 h至恒重,取出用滤纸吸去表面水分,称饱和鲜重;再将叶片放入烘箱于70℃烘干至恒重,称干重。
1.3 数据处理与统计分析
利用软件Microsoft Excel 2019和SigmaPlot 12.5进行数据的统计分析和作图,采用One-Way ANOVA方法进行差异显著性分析。数据结果以3次重复的平均值±标准差表示。
2 结果与分析
2.1 转基因阳性植株的分子鉴定
通过农杆菌介导法将植物双元表达载体p3300-ApGSMT2-ApDMT2-bar分别转化拟南芥Col-0和玉米自交系HiII,ApGSMT2基因由玉米Ubiquitin1启动子pUbi驱动表达,而ApDMT2基因由烟草花叶病毒(CaMV)35S启动子驱动表达。根据载体T-DNA区结构图(图1A),设计载体启动子pUbi上的正向引物和ApGSMT2基因特异反向引物进行PCR鉴定。拟南芥转基因PCR鉴定结果如图1B所示,玉米转基因鉴定结果如图3A所示。结果表明,拟南芥转基因株系L2~L6和玉米转基因株系D1~D3均扩增出与重组质粒同等大小的基因片段,而对照(WT)则未扩增出目的基因片段,说明以上株系均为转基因阳性株系。
2.2 不同浓度NaCl处理对转ApGSMT2和ApDMT2拟南芥种子萌发率的影响
将转ApGSMT2和ApDMT2基因拟南芥株系和WT种子播于含不同浓度NaCl(0、50、100、150、200 mmol/L)的1/2MS培养基上培养12 d,观察各株系表型。结果表明,在不含NaCl的1/2MS培养基上进行培养时,转基因拟南芥与WT均萌发和生长正常;但随着盐浓度的提高,各株系的生长均受到明显抑制,与WT相比,转基因株系生长受抑制程度较低,在100、150 mmol/L NaCl处理时差异非常明显(图1C)。
2.3 玉米转基因株系中ApGSMT2和ApDMT2基因的表达丰度分析
利用半定量RT-PCR方法对玉米转基因株系中ApGSMT2和ApDMT2基因表达丰度的鉴定结果(图3A、B)表明,WT中无目的基因表达,而各转基因株系中ApGSMT2和ApDMT2均有较高表达;内参基因FPGS在WT及各转基因株系中表达稳定,无差异,说明ApGSMT2和ApDMT2基因在各个转基因株系中均成功表达。
2.4 NaCl处理对转ApGSMT2和ApDMT2基因玉米表型的影响
对萌发后水培一周的转基因玉米株系及WT幼苗进行盐胁迫(180 mmol/L NaCl)处理,结果(图3C、图4A)表明,处理前各株系长势良好,转基因株系稍高于对照植株,说明转ApGSMT2和ApDMT2基因对玉米的生長具有促进作用。盐处理7 d后,WT叶片黄化和萎蔫严重,茎秆因失水而变细倒伏,且根部生长受到抑制;而转基因株系叶片仅出现轻微黄化和萎蔫,茎秆较粗壮,根系发达,整体长势仍然良好。
3 讨论与结论
盐胁迫能显著影响植物的生长和发育。本试验中,随着盐浓度的增加,未转基因拟南芥种子的萌发率降低,而过表达ApGSMT2和ApDMT2基因显著提高了不同盐浓度处理下拟南芥种子的萌发率,对提高拟南芥的耐盐性具有明显效果。比较盐处理前后玉米转ApGSMT2和ApDMT2基因株系与未转基因对照的表型及生理指标发现,NaCl处理前,除株高外,根长、叶片相对含水量和鲜重两者间均没有显著差异;但是在盐胁迫条件下,转基因株系的株高和鲜重极显著高于对照,叶片相对含水量和D1株系的根长显著高于对照,说明转ApGSMT2和ApDMT2基因玉米株系在盐胁迫条件下维持了较好的长势。
玉米是易受盐碱危害的作物,由于玉米种质资源缺乏和田间选择困难等原因,常规育种选育玉米耐盐品种的工作收效不大。本研究发现转ApGSMT2和ApDMT2基因可以显著提高玉米对盐胁迫的耐受性,利用基因工程技术结合常规育种有望在较短时间内创造出玉米耐盐新种质[24, 25],这不仅可以扩大玉米的种植面积,而且可以提高玉米的稳产性,对于我国农业生产有重大意义。
参 考 文 献:
[1] Deinlein U, Stephan A B, Horie T, et al. Plant salt-tolerance mechanisms [J]. Trends in Plant Science, 2014, 19(6): 371-379.
[2] Farooq M, Hussain M, Wakeel A, et al. Salt stress in maize: effects, resistance mechanisms, and management. A review [J]. Agronomy for Sustainable Development, 2015, 35(2): 461-481.
[3] 赵韦. 土壤盐碱化对玉米胁迫的研究进展[J]. 黑龙江农业科学,2019(1): 140-142.
[4] Sakamoto A, Murata N. The role of glycinebetaine in the protection of plants from stress: clues from transgenic plants [J]. Plant Cell and Environment, 2002, 25(2): 163-171.
[5] 郭嘉,孫传波,杨向东,等. 耐盐碱转基因玉米的获得及其抗性分析[J]. 玉米科学,2016,24(6): 24-29.
[6] Hayashi H, Alia A, Mustardy L A, et al. Transformation of Arabidopsis thaliana with the codA gene for choline oxidase; accumulation of glycinebetaine and enhanced tolerance to salt and cold stress [J]. The Plant Journal, 1997, 12(1): 133-142.
[7] Chen T H H, Murata N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes [J]. Current Opinion in Plant Biology, 2002, 5(3): 250-257.
[8] Chen T H H, Murata N. Glycinebetaine: an effective protectant against abiotic stress in plants [J]. Trends in Plant Science, 2008, 13(9): 499-505.
[9] Yang X, Liang Z, Lu C. Genetic engineering of the biosynthesis of glycinebetaine enhances photosynthesis against high temperature stress in transgenic tobacco plants [J]. Plant Physiology, 2005, 138(4): 2299-2309.
[10] Wani S H, Singh N B, Haribhushan A, et al. Compatible solute engineering in plants for abiotic stress tolerance — role of glycine betaine [J]. Current Genomics, 2013, 14(3): 157-165.
[11] Rhodes D, Hanson A D. Quaternary ammonium and tertiary sulfonium compounds in higher plants [J]. Annual Review of Plant Physiology and Plant Molecular Biology, 1993, 44:357-384.
[12] Rathinasabapathi B, Burnet M, Russell B L, et al. Choline monooxygenase, an unusual ironsulfur enzyme catalyzing the first step of glycine betaine synthesis in plants: prosthetic group characterization and cDNA cloning [J]. Proceedings of the National Academy of Sciences of the United States of America, 1997, 94(7): 3454-3458.
[13] Nyyssola A, Kerovuo J, Kaukinen P, et al. Extreme halophiles synthesize betaine from glycine by methylation [J]. The Journal of Biological Chemistry, 2000, 275(29): 22196-22201.
[14] Waditee R, Tanaka Y, Aoki K, et al. Isolation and functional characterization of N-methyltransferases that catalyze betaine synthesis from glycine in a halotolerant photosynthetic organism Aphanothece halophytica[J]. The Journal of Biological Chemistry, 2003, 278(7): 4932-4942.
[15] Waditee R, Bhuiyan M N, Rai V, et al. Genes for direct methylation of glycine provide high levels of glycinebetaine and abiotic-stress tolerance in Synechococcus and Arabidopsis [J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(5): 1318-1323.
[16] Waditee-Sirisattha R, Singh M, Kageyama H, et al. Anabaena sp. PCC7120 transformed with glycine methylation genes from Aphanothece halophytica synthesized glycine betaine showing increased tolerance to salt [J]. Archives of Microbiology, 2012, 194(11): 909-914.
[17] Lai S J, Lai M C, Lee R J, et al. Transgenic Arabidopsis expressing osmolyte glycine betaine synthesizing enzymes from halophilic methanogen promote tolerance to drought and salt stress [J]. Plant Molecular Biology, 2014, 85(4/5): 429-441.
[18] Niu X, Xiong F, Liu J, et al. Co-expression of ApGSMT and ApDMT promotes biosynthesis of glycine betaine in rice(Oryza sativa L.) and enhances salt and cold tolerance [J]. Environmental and Experimental Botany, 2014, 104: 16-25.
[19] He Y,He C M,Li L H,et al.Heterologous expression of ApGSMT2 and ApDMT2 genes from Aphanothece halophytica enhanced drought tolerance in transgenic tobacco [J]. Molecular Biology Reports, 2011, 38(1): 657-666.
[20] Song J, Zhang R, Yue D, et al. Co-expression of ApGSMT2g and ApDMT2g in cotton enhances salt tolerance and increases seed cotton yield in saline fields [J]. Plant Science, 2018, 274: 369-382.
[21] He C, He Y, Liu Q, et al. Co-expression of genes ApGSMT2 and ApDMT2 for glycinebetaine synthesis in maize enhances the drought tolerance of plants [J]. Molecular Breeding, 2013, 31(1): 559-573.
[22] 何春梅,王娟,董瑞,等. 玉米ZmGS5基因的克隆及其對转基因拟南芥种子发育的影响[J]. 浙江农业学报,2019,31(4): 513-518.
[23] Manoli A, Sturaro A, Trevisan S, et al. Evaluation of candidate reference genes for qPCR in maize [J]. Journal of Plant Physiology, 2012, 169(8): 807-815.
[24] Apse M P, Blumwald E. Engineering salt tolerance in plants [J]. Current Opinion in Biotechnology, 2002, 13(2): 146-150.
[25] Agarwal P K, Shukla P S, Gupta K, et al. Bioengineering for salinity tolerance in plants: state of the art [J]. Molecular Biotechnology, 2013, 54(1): 102-123.
