Impeding anion exchange to improve composition stability of CsPbX3(X =Cl,Br)nanocrystals through facilely fabricated Cs4PbX6 shell∗
2019-08-16ZhaohuiShen申朝晖PengjieSong宋鹏杰BoQiao乔泊JingyueCao曹靖玥QiongyuBai白琼宇DandanSong宋丹丹ZhengXu徐征SulingZhao赵谡玲GaoqianZhang张高倩andYuanjunWu吴元均
Zhaohui Shen(申朝晖), Pengjie Song(宋鹏杰), Bo Qiao(乔泊), Jingyue Cao(曹靖玥),Qiongyu Bai(白琼宇), Dandan Song(宋丹丹), Zheng Xu(徐征), Suling Zhao(赵谡玲),†,Gaoqian Zhang(张高倩), and Yuanjun Wu(吴元均)
1Key Laboratory of Luminescence and Optical Information(Beijing Jiaotong University),Ministry of Education,Beijing 100044,China
2Institute of Optoelectronics Technology,Beijing Jiaotong University,Beijing 100044,China
3Shenzhen China Star Optoelectronics Technology Co.,Ltd.,Shenzhen 518132,China
Keywords: CsPbX3 @Cs4PbX6 ,anion exchange,composition stability,core-shell constructure
1. Introduction
Inorganic lead halide perovskite nanocrystals(NCs)have emerged as an attractive semiconductor material and been widely used in various fields, such as solar cells,[1]lightemitting diodes (LEDS),[2]lasers,[3]and photodetectors.[4]The studies of Yang et al. showed that inorganic lead halide perovskite NCs are very soft,[5,6]and can easily exchange ions with other salts or other halogen perovskites.[7,8]Therefore,the energy levels and related photoelectronic features of perovskite NCs can be easily tuned in this way. Although this property is valuable for adjusting photoelectronic features of NCs, it also induces the composition stability problems. For example,anion exchange with other halide salts and halide elements in the environment during storage or application can change the material features and limit the application of NCs.The loss of an anion is caused by the high ions mobility of halide anions. Though improving the composition stability has become increasingly important and urgent, there are few reports about how to imped the anion exchange to improve the composition stability and reduce the loss of anion.
Previous efforts mainly focus on improving water and oxygen stability and crystalline phase stability of perovskite NCs. Alkyl phosphinic acid, a kind of traditional surface ligand, was used to replace oleic acid and effectively prevented α-CsPbI3from transforming into δ-CsPbI3.[9]However,surface ligands have no effect on anion protection. Using poly (methylmethacrylate) (PMMA) to encapsulate CsPbBr3can obviously protect CsPbBr3, because PMMA has a low ion diffusion rate and water-resistance ability.[10]However,those coated NCs are less likely to be applied in the field of optoelectronics due to PMMA’s insulation characteristics.Hydrolyzing (3-aminopropyl) triethoxysilane (APTES) can form a fully covered shell to improve NCs’ stability significantly, but the silicon shell usually encapsulates a large number of NCs together.[11]CsPb2Br5decorated on the surface of CsPbBr3NCs can extend the life of coated NCs in water.[12]CsPbBr3/ZnS and CsPbBr3/Ta2O5NCs heterostructures have been synthesized and the stability of CsPbBr3NCs was improved,[13,14]but CsPbBr3NCs were not completely covered. More importantly,these approaches are complex and difficult to be used in mass production.
Cs4PbM6(M =Cl, Br, I) NCs, indirect bandgap semiconductor materials,have become hotspots in recent years.[15]Differing from CsPbM3NCs, 0-dimensional Pbis separated in Cs4PbM6and the bond energy of Cs4PbM6NCs is stronger than that of CsPbM3NCs.[15,16]Recent studies have demonstrated that Cs4PbBr6coated CsPbBr3can form the first type of quantum well, but the size uniformity of coated NCs is poor and the prepared products have large particle size.[17]Li’s group treated CsPbBr3NCs as seeds to form Cs4PbBr6shells by adding Cs2CO3and ZnBr2.[18]But ZnBr2is likely to undergo cation exchange and introduces impurities into products.[7]
Here, we propose the concept of using Cs4PbX6shell to impede the anion exchange of CsPbX3NCs,and present a new method to build a Cs4PbX6shell on the surface of CsPbX3NCs. CsPbX3NCs are synthesized by high temperature injection method and are cooled to room temperature after being bathed in water. Then CsX and oleylamine (OLM) are added into the solution of CsPbX3NCs to synthesize Cs4PbX6shells. X-ray diffraction (XRD) peaks of both CsPbX3and Cs4PbX6are obtained. Anion exchange experiments prove that Cs4PbX6shells are fully covered on the surface of CsPbX3NCs and could significantly improve the composition stability of coated CsPbX3NCs and reduce the loss of an anion.
2. Material and methods
2.1. Chemicals
Cs2CO3(99.995%), PbBr2(99.999%), CsBr (99.999%),ethyl acetate(anhydrous,98%),octadecene(ODE,anhydrous,90%), hexane (HEX, anhydrous 99%), oleylamine (OLAM,anhydrous, 70%), and oleic acid (OA, anhydrous 90%) were purchased from Sigma-Aldrich. All chemicals were used without any further purification.
2.2. Synthesis of CsPbX3 NCs
To prepare Cs-oleate solution, 0.4-g Cs2CO3, 15-mL ODE,and 1.2-mL OA were mixed in a 100-mL three-necked flask with vigorous stir and dried under Ar flow at 120°C for 30 min, and then the solution was subsequently heated at 150°C under Ar flow for at least 30 minutes. 0.188-mmol PbX2and 5-mL ODE were degassed in another 100-mL threenecked flask and stirred at 120°C for 30 minutes under Ar flow. After being added with 0.5-mL OA and 0.5-mL OLM at 120°C,the PbX2solution was kept at 150°C for 30 minutes under magnetic stir. 0.6-mL Cs-oleate solution was quickly injected into PbX2solution to synthesize CsPbX3NCs. The reaction mixture was cooled by an ice-water bath after 5 s.
2.3. Synthesis of Cs4PbX6 shells
When the solution temperature of CsPbX3NCs declined to room temperature, 0.229-mmol CsX and 3.75-mL OLM were added into NCs solution.Then,the 100-mL three-necked flask containing NCs solution and CsX was put to an oil bath pot that has been heated to 100°C.The reaction process lasted for 20 min with vigorous stir and Ar flow. Then,the obtained NCs were cooled by water bath and centrifuged at 6500 rpm for 8 min with ethyl acetate. The core-shell structure NCs were redispersed in 3-mL hexane.
2.4. Characterization
The prepared NCs were characterized by XRD, transmission electron microscopy (TEM), and photoluminescence(PL) measurements as in the previous experiments.[19]The XRD patterns were collected with a D/max 2200 V x-ray powder diffractometer with Cu Kα radiation (λ =1.540 ˚A). The TEM images were obtained from a JEOL JEM-1400 microscope with a thermionic gun operating at a 100 kV acceleration voltage. High resolution transmission electron microscopy(HRTEM) measurements and energy dispersive spectrometer(EDS)element mapping were carried out by a JEM-2100F operating at 200 kV.Steady-state PL spectra were collected with a Hitachi F4500 fluorescence spectrophotometer with an Xe lamp that was equipped with a monochromator. The ultraviolet (UV)-absorption spectra were measured on a Shimadzu UV-3101 PC spectrophotometer. The PL decay process was carried out on a Horiba Fluorolog phosphorescence lifetime system equipped with a 373 nm,45-ps pulse laser and a time corrected single-photon counting(TCSPC)system. The samples of PL emission spectra, UV-absorption spectra, and PL decay were prepared by adding 100-µl crude NCs solution in 2900-µl n-hexane. All of the spectral measurements were performed at room temperature.
3. Results and discussion
Core CsPbX3NCs are synthesized based on the report of Protesecu et al.[20]and treated as seeds for the growth of Cs4PbX6. The growth process of Cs4PbX6shell over CsPbX3NCs is shown in Fig. 1(a). As CsPbX3and Cs4PbX6possess different element mole ratios,CsX needs to be added into the prepared CsPbX3NCs solution to construct a suitable atomic ratio.
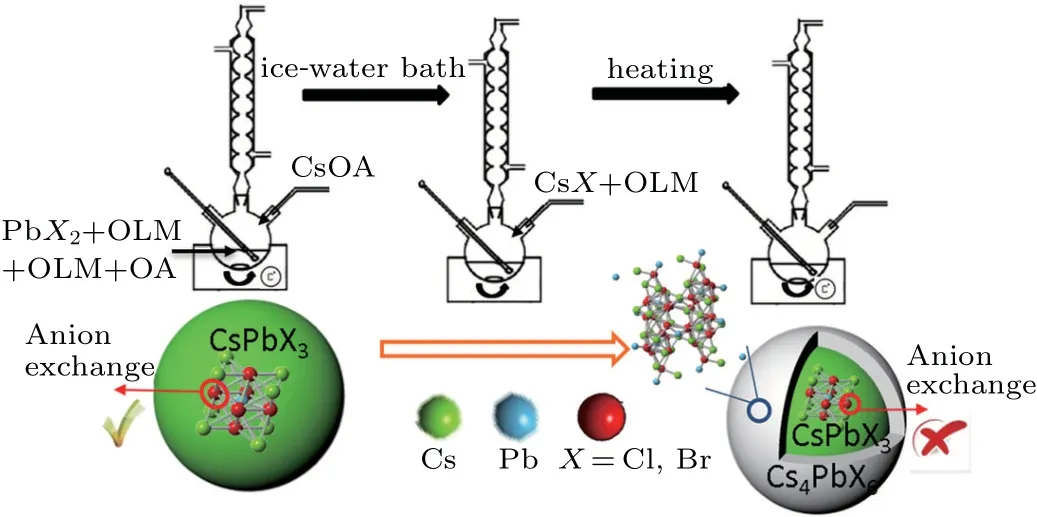
Fig.1. Schematic diagram showing the growth process and effect of the core-shell structure.
As shown in Figs. 2(a) and 2(b), the prepared CsPbBr3NCs are monodisperse with a uniform size ranging from 9 nm-13 nm, and the size of CsPbCl3NCs is close to 10 nm. Because of a large amount of unreacted PbBr2when CsPbX3NCs are prepared by high temperature injection, no more PbBr2needs to be added. OLM is utilized because Cs4PbX6NCs are likely to be synthesized in a more OLM reaction environment.[16]Comparing Fig.2(a)with Fig.2(c),Fig.2(b)with Fig. 2(d), it is easy to find that the size of coated NCs becomes bigger than before. The bigger size and shadowy outline in Figs. 2(c) and 2(d) are caused by the growth of Cs4PbX6shells. The formation of Cs4PbX6shells can also be inferred from XRD patterns in Fig.2(e)which match well with CsPbX3and Cs4PbX6standard cards, respectively. The UV-absorption peak near 300 nm in Fig. 3(a) also demonstrates that Cs4PbX6is formed after adding OLM and CsX. The possibility that CsPbX3may convert to Cs4PbX6when CsPbX3reacts with OLM and CsX is denied,because the transforming from CsPbX3to Cs4PbX6requires a large amount of OLM.However, the amount of OLM in this experiment is far less than the required quantity.[21]

Fig. 2. The TEM images of (a) CsPbCl3, (b) CsPbBr3, (c) CsPbCl3@Cs4PbCl6, and (d) CsPbBr3@Cs4PbBr6 NCs. (e) XRD of CsPbX3@Cs4PbX6 NCs.
It is expected to improve the composition stability of CsPbX3NCs by coating them with Cs4PbX6, because it is very difficult to break chemical bonds of Cs4PbX6and cause ion exchange.[15]In order to confirm that the core-shell structure has been prepared and Cs4PbX6shell can be used to reduce ions loss, anion exchange experiments before and after wrapping CsPbX3NCs are performed. The luminescence of CsPbX3NCs before and after anion exchange is detected and shown in Fig. 3(b). After mixing CsPbCl3and CsPbBr3NCs up, the luminous peak of alloy CsPbClxBr3-xNCs appears rapidly, while the PL emission locked at 408 nm and 512 nm vanishes. Comparing Fig. 3(c) with Fig. 3(d), slight peak position shifts from 408 nm of CsPbCl3to 410 nm of CsPbCl3@Cs4PbCl6and from 512 nm of CsPbBr3to 515 nm of CsPbBr3@Cs4PbBr6prove that the size of the core CsPbX3barely changes after being coated. The bigger size of NCs after encapsulated is due to the shell of Cs4PbX6which has no contribution to peak positions. As shown in Fig. 3(c), the peak wavelength of alloy CsPbClxBr3-xNCs does not move after 30 s, indicating that anion exchange between CsPbCl3and CsPbBr3NCs already finishes within few seconds. However,anion exchange among coated NCs is impeded successfully, because there is blue emission of CsPbCl3@Cs4PbCl6and green emission of CsPbBr3@Cs4PbBr6in Fig. 3(e) after mixing both coated NCs for 11 min. The intensity of CsPbX3@Cs4PbX6NCs just changes slightly. More importantly,even after mixing CsPbX3@Cs4PbX6NCs for 3 hours,the blue and green emission in Fig.3(d)still exist at the same time,and there are no significant changes compared with their initial peak positions. We do not conduct experiments about CsPbI3due to poor crystalline phase stability.
The above discussion leads to a conclusion that the Cs4PbX6shell is completely covered on the surface of seeds NCs and can obviously prevent anion movement and reduce anion loss. This facile method is potentially used for building other kinds of shells and forming different heterojunctions. It is also possible to realize multilayer coatings. The NCs coated by Cs4PbX6shell can be used to fabricate pure white optical devices by mixing CsPbM3@Cs4PbM6(M = Cl, Br, and I)NCs and improve the stability of inorganic lead halide perovskite NCs solar cells and LEDS. Cs4PbX6coated CsPbX3NCs can form the first type of quantum well and are likely to improve the external quantum efficiency of quantum dot light emitting diodes LEDs.

Fig.3. (a)UV-absorption spectra of CsPbX3 and CsPbX3@Cs4PbX6 NCs. (b)PL of CsPbX3 NCs before and after anion exchange. (c)Peak positions of CsPbX3 NCs before and after anion exchange. (d)PL of mixed CsPbX3@Cs4PbX6 NCs. (e)Peak positions of CsPbX3@Cs4PbX6 NCs before and after mixing coated NCs up.
4. Conclusion
The core-shell structure CsPbX3@Cs4PbX6NCs has been successfully synthesized by adding CsX and OLM into prepared CsPbX3NCs. The absorption spectra and XRD have shown that core CsPbX3NCs are fully covered by Cs4PbX6.The anion exchange experiments have not only demonstrated the distribution of Cs4PbX6but they have also proven that shells can effectively improve composition stability of CsPbX3(X =Cl,Br)NCs and block the influence of the external environment.This new coating method provides a way to form different core-shell structures or other forms of heterojunctions to improve the stability of inorganic perovskite NCs and expand its application fields. With additional research in the future,CsPbX3@Cs4PbX6NCs are likely to fabricate pure white optical devices and quantum dot light emitting diodes LEDs with high external quantum efficiency.
猜你喜欢
杂志排行
Chinese Physics B的其它文章
- Lorentz transmission electron microscopy for magnetic skyrmions imaging∗
- Spin transport in antiferromagnetic insulators∗
- First-principles study of the band gap tuning and doping control in CdSexTe1-x alloy for high efficiency solar cell∗
- Non-Stokes drag coefficient in single-particle electrophoresis:New insights on a classical problem
- SymTopo: An automatic tool for calculating topological properties of nonmagnetic crystalline materials∗
- Tunable coupling between Xmon qubit and coplanar waveguide resonator∗
