Mitochondrial membrane stabilization by Angelica sinensis polysaccharide in murine aplastic anemia
2019-08-09PingZhongXingCui
Ping Zhong, Xing Cui
Mitochondrial membrane stabilization by Angelica sinensis polysaccharide in murine aplastic anemia
Ping Zhong1#, Xing Cui2#
1Department of rehabilitation medicine, The 960th hospital of the people's Liberation Army, Jinan, Shandong, China.2Department of Hematology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China.
In order to investigate the mechanism of mitochondrial membrane stabilization by Angelica sinensis polysaccharide (ASP) in murine aplastic anemia (AA).ICR mice were randomly divided into control, AA and ASP-treated groups. The AA group mice were treated with 60Coγand intraperitoneal injections of cyclophosphamide and chloramphenicol. The control animals were treated with lead shielding irradiation and saline injection. The treated AA mice were fed with ASP for 2 wk. Mitochondrial ultrastructure of the bone marrow was observed by transmission electron microscopy, and the transmembrane potential of bone marrow-nucleated cells (BMNC)was examined by fluorescence spectrophotometry. The Cox and MDH contents of the medium were also studied in the three groups.The mitochondrial number and transmembrane potential of BMNC in the bone marrow decreased in the AA group as compared to the control group, but improved in the ASP-treated group as compared to the AA group. Complete mitochondrial cleavage in the ASP-treated group was significantly delayed (< 0.05) as compared to the AA group. We conclude that ASP might improve mitochondrial membrane stabilization, and suppress the downregulation of transmembrane potential and apoptosis of BMNC in AA.
aplastic anemia, Angelica sinensis polysaccharide, mitochondria, membrane potential, ICR mice
Acquired deletions of mtDNA and abnormal mitochondrial function are crucial reasons in some blood disease include aplastic anemia. Angelica sinensis helps in tonifying the blood and promoting its circulation via anti-oxidative and neuroprotective effects. In this paper, we demonstrated that Angelica sinensis polysaccharide can improve improve the mitochondrial ultrastructure, and suppress the downregulation of transmembrane potential and apoptosis of myeloid element to cure bone marrow failure.
Background
Aplastic anemia (AA) is a blood disorder in which the bone marrow and the associated blood stem cells are damaged causing a deficiency of red blood cells, white blood cells, and platelets. These deficiencies are individually known as anemia, leucopenia and thrombocytopenia, respectively, and collectively known as pancytopenia. Exposure to chemicals, drugs, radiation, radioactive materials, radiation-producing devices, infection, immune disease, heredity (in 50% of the cases) and unknown etiology can also lead to the development of AA.
Mitochondria are considered to be the “powerhouses of the cell” because they produce adenosine triphosphate (ATP) by systematically extracting energy from nutrient molecules (substrates) [1]. Moreover, mtDNA is replicated with a high mutation rate since it lacks protective histones and an effective DNA repair system. Mutations in mtDNA are associated with hematological diseases such as acquired sideroblastic anemia, myelodysplastic syndromes and acquired AA [2-4]. Our previous study [5] showed that functional impairment of the mitochondrial respiratory chain induced by mutations might be involved in hematopoietic failure in AA patients. Irrespective of the morphological features of end-stage cell death (apoptotic, necrotic, autophagic, or mitotic), mitochondrial membrane permeabilization (MMP) is frequently the decisive event between survival and death [6].
According to traditional Chinese medicine, Angelica sinensis helps in tonifying the blood and promoting its circulation [7]. Recent studies have shown that extracts of Angelica sinensis have anti-oxidative and neuroprotective effects [8,9]. However, the anti-oxidative function of ASP remains unclear. This study examined the early cell damage using mitochondrial lysis time curve and the mitochondrial membrane stabilizing effect of ASP in AA.
Materials and Methods
Grouping of animals
Healthy ICR male mice, weighing 18-22 g, aged 6-8 weeks, were provided by the Experimental Animal Center of Shandong University (China). Animals were housed in a warm, quiet environment with free access to food and water, and acclimatized for one week before beginning the experiments.
The mice were randomly divided into three groups: normal group, AA group, and treated group, respectively. The aplastic anemia model was generated as previously described [10].
Briefly, the mice were irradiated with 2.0Gy 60Coγ and then treated with daily intraperitoneal injections of 40 mg/kg/day cyclophosphamide and 50 mg/kg/day chloramphenicol for the next three days. The treated group was intragastrically fed with ASP (200 mg/kg/d, according to the Chinese Medical Dictionary). The control group and the AA group mice were intragastrically fed with physiological saline (10 ml/kg/d) supplemented diet. Furthermore, all mice received a standard diet during the study. After treatment with ASP or physiological saline for 2 wk, the mice were sacrificed by cervical dislocation.
Hematological examination
The tail blood samples from the three groups were collected on the first and fourteenth day, respectively. WBC and platelets were counted in the peripheral blood samples, and Hb levels were determined. On completion of the experiments, the mice were sacrificed and femur smears were prepared for differential counting of bone marrow-nucleated cells (BMNCs) (Figure 1).
Transmission electron microscopy
The femoral marrow was smeared and sliced into ultrathin sections. Mitochondria of hematopoietic cells in the femoral marrow were analyzed and counted by transmission electron microscopy (JEM-2000EX, Japan) under 50 fields of vision.

Figure 1. Effects of ASP on the mitochondria of hematopoietic cells from AA mice.
After treatment with ASP or physiological saline for 2 wk, representative transmission electron micrographs (×25000) of mitochondrial structures from hematopoietic cells are shown (B and C). Panel A is the control group. Panel C shows more mitochondria in the ASP-treated group as compared to the AA group (Panel B).
Mitochondrial lysis time curve
Mitochondria were extracted from murine BMNCs according to the manufacturer’s protocol of the mitochondria isolation kit (Pierce Biotechnology Inc., USA). The concentration of monoamine oxidase (MAO) indicates the concentration of mitochondria, and was determined using 200U/ml mitochondrial suspensions from 20 serum cultures, within 12 hours at different time points (30 min intervals). The contents of cytochrome oxidase (Cox) and malate dehydrogenase (MDH) in the medium were also determined. The peak time analysis of the mitochondrial membrane and matrix specific enzyme concentration time curves showed the extent of mitochondrial membrane lysis.
Mitochondrial membrane potential
After pre-incubation with MAO or ASP, the isolated mitochondria from the three groups were resuspended in 0.5 ml phosphate‑buffered saline (Wuhan Boster Biotechnology, Ltd., China), and 10 μl rhodamine 123 working solution (Sigma-Aldrich, USA) was added and incubated at 37˚C in 5% CO2 for 15 min. The mitochondrial membrane potential was analyzed by flow cytometry using an excitation wavelength of 490 nm and emission wavelength of 520 nm.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 software. Data are expressed as means and standard deviations. Student’s t-test was used to compare the different groups.was considered to be statistically significant.
Results
Peripheral blood and BMC counts
Using a fully automatic blood cell analyzer, the number of peripheral blood cells and BMCs in the AA mice was found to be notably decreased (), indicating that the AA mouse model was successfully established (Table 1).

Table 1. Peripheral blood cell counts (x±SD) in the three groups of mice
*< 0.01, **< 0.05, as compared to the control group; △< 0.01, as compared to the AA group.
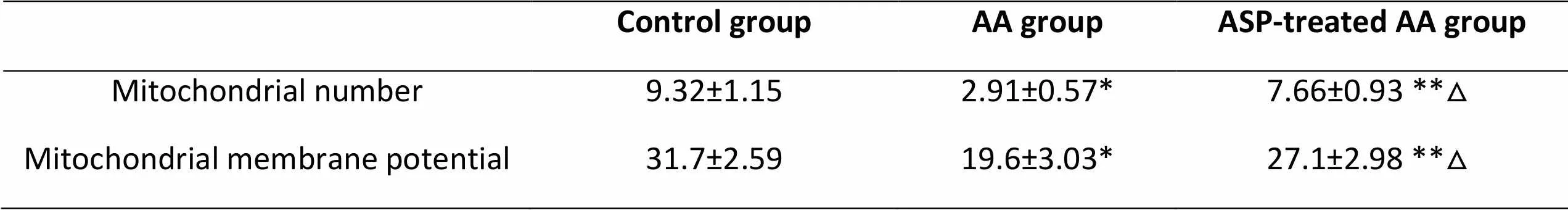
Table 2. Effect of ASP on the mitochondrial number and mitochondrial membrane potential(MMP)of bone marrow in mice(x±SD)
*< 0.01, **< 0.05, as compared to the control group; △< 0.01, as compared to the AA group.
Mitochondrial ultrastructure in aplastic anemia mice
Transmission electron microscopy of the bone marrow of AA mice showed that the mitochondrial ultrastructure significantly improved with ASP in the treated group (Table 2 and Figure 1), indicating that ASP stabilizes the mitochondria in AA mice. The size and shape of mitochondria of the control group were normal. In contrast, the mitochondria of the AA group had enlarged globular structures, along with the disruption or disappearance of cristae (Figure 1A–C).
Mitochondrial membrane potential (MMP) in aplastic anemia mice
As transmission electron microscopy revealed mitochondrial damage, we determined the effect of ASP on the MMP in AA mice by measuring the relative differences in the fluorescence of Rh 123 among the three groups using a fluorescence spectrophotometer.
The results showed that the Rh 123 fluorescence of bone marrow cells in the AA group (19.6±3.03) was lower than that in the control group (31.7±2.59,). However, the Rh 123 fluorescence of the ASP-treated AA group was higher than that in the AA group () (Table 2 and Figure 2), indicating that ASP facilitated recovery of the MMP in AA mice.
Early cell damage mitochondrial lysis time curve
In the control group, the contents of mitochondrial in vitro culture Cox and MDH took 3.5 hours to reach the peak. The MAO content in the culture did not change significantly over time, but the contents of COX and MDH gradually increased with time, reaching a peak, and then gradually declining. Thus, the complete mitochondrial contents released by cells took about 3.5 hours to reach the cleavage peak.
The COX and MDH peaks in the AA group appeared at 1.5 hours, 1.375U/l, 36.732U/l, respectively. The COX and MDH peaks in the treated group appeared at 5.5 hours, 6.5 hours, 1.341U/l, 33.994U/l, respectively. Two sets of data at each time point were used for statistical analysis. At 1.5 hours, the COX and MDH levels in the AA group were significantly higher than the treated group ().
Therefore, complete mitochondrial cleavage in the serum was significantly delayed after addition of ASP (), with a slight decrease in the peak (Figure 3).
Discussion
Aplastic anemia (AA) is a bone marrow failure syndrome characterized by peripheral pancytopenia and marrow hypoplasia. Mutations and instability of mtDNA have been demonstrated in several diseases. Mitochondrial dysfunction and decrease in the number of mitochondria may result in the reduction of mtDNA. Acquired deletions of mtDNA in the hematopoietic compartment have been found to occur in severe pancytopenia and reticulocytopenia [11]. Based on our previous research [5], we examined whether ASP can stabilize the mitochondrial membrane of AA mice.

Figure 2. Effects of ASP on the mitochondrial membrane potential (MMP) in bone marrow cells of aplastic anemia (AA) mice. Bone marrow cells from AA mice and ASP-treated AA mice were stained with rhodamine 123.
Figure 3. The concentration time curves of COX and MDH
Angelica sinensis polysaccharide-iron complex (APIC) not only has a superior therapeutic effect on IDA but also on supplementing blood and promoting blood circulation [12]. ASP may be useful for the treatment of diseases induced by hepcidin overexpression by preventing the janus-kinase (JAK), son of mothers against decapentaplegic (SMAD) and extracellular signal-regulated kinase (ERK) pathways to downregulate hepcidin expression in IDA rats [13]. Qin J found that ASP can improve proteoglycan (PG) synthesis of chondrocytes in rat OA model in vivo and IL-1β-stimulated chondrocytes in vitro by promoting the expression of aggrecan and GTs involved in PG synthesis [14].
In our study, the AA mouse model was induced by a combination of acetylphenylhydrazine, X-rays and cyclophosphamide. The AA mice showed statistically significant reductions in peripheral blood leucocytes, Hb and platelets (Table 1), and severe reductions in humeral marrow cells and marrow-committed progenitor cells, which are clinical characteristics of AA. The AA mice treated with ASP showed a progressive increase in BM cells as compared to the AA group. Additionally, the number of mitochondria in the hematopoietic cells was also affected. ASP resulted in significantly higher number of mitochondria in the treated group as compared to the AA group (Figure 1). These results showed that ASP could promote marrow nucleated cells proliferation, increase the number of mitochondria, and stabilize the mitochondrial membrane in AA mice.
Mitochondrial injury is reflected by mtDNA damage and a decline in the levels of mtRNA transcripts, protein synthesis, and mitochondrial function, which might result in decreased cellular energy, disruption of cell signaling, and interference with cellular differentiation and apoptosis. Furthermore, deficient mitochondrial ATP production might promote chromosomal instability [15]. Since mtDNA encodes components of four out of five mitochondrial respiratory complexes, alterations in mtDNA result in mitochondrial disease [16-18]. Apart from mitochondrial disease, mutations in mtDNA are linked to cancer, diabetes, cardiovascular diseases, neurodegenerative disorders, hematological diseases such as leukemia as well as the normal process of aging [19]. Importantly, mtDNA mutations as well as reduction in mtDNA copy number can be pathogenic [20, 21]. Understanding cellular mechanisms for the maintenance of mtDNA integrity and copy number is of utmost importance since it can provide targets for clinical interventions aimed at prevention and treatment of hematological diseases such as AA. These factors might also result in decreased energy metabolism, which will affect self-renewal and differentiation of the hematopoietic stem cells.
The findings of the present study demonstrate that ASP can improve the mitochondrial ultrastructure, and suppress the downregulation of transmembrane potential and apoptosis of myeloid element to cure bone marrow failure.
1. Chinnery PF, Schon EA. Mitochondria. J Neurol Neurosurg Psychiatry 2003, 74: 1188-1199.
2. Gattermann N. Mitochondrial DNA mutations in the hematopoietic system. Leukemia 2004, 18:18–22.
3. Gattermann N, Retzlaff S, Wang YL,Heteroplasmic point mutations of mitochondrial DNA affecting subunit I of cytochrome c oxidase in two patients with acquired idiopathic sideroblastic anemia. Blood 1997, 90: 4961–4972.
4. Kim HR, Shin MG, Kim MJ,Mitochondrial DNA aberrations of bone marrow cells from patients with aplastic anemia. Korean Med Sci 2008, 23:1062–1067.
5. X Cui, JQ Wang,ZGCai,Complete sequence analysis of mitochondrial DNA and telomere length in aplastic anemia. Int J Mol Med 2014, 34: 1309-1314.
6. Chiu TL, Su CC. Tanshinone IIA induces apoptosis in human lung cancer A549 cells through the induction of reactive oxygen species and decreasing the mitochondrial membrane potential.Int J Mol Med 2010, 25:231-236.
7. Liu PJ, Hsieh WT, Huang SH,Hematopoietic effect of water-soluble polysaccharides from Angelica sinensis on mice with acute blood loss. Exp Hematol 2010, 38:437-445.
8. Kuang X, Yao Y, Du JR,Neuroprotective role of Z-ligustilide against forebrain ischemic injury in ICR mice. Brain Res 2006, 1102:145-153.
9. Xin J, Zhang J, Yang Y,Radix Angelica Sinensis that contains the component Z-ligustilide promotes adult neurogenesis to mediate recovery from cognitive impairment. Curr Neurovasc Res 203, 10:304-315.
10. Chen YF,Wu ZM,Xie C,ExpressionlevelofIL-6secretedbybonemarrowstromalcellsinmicewithaplasticanemia. ISRN Hematol 2013:986219.
11. Hatfill SJ, La Cock CJ, Laubscher R,A role for mitochondrial DNA in the pathogenesis of radiation-induced myelodysplasia and secondary leukemia. Leuk Res 1993, 17: 907-913.
12. Wang PP, Zhang Y, Dai LQ,Effect of Angelica sinensis polysaccharide-iron complex on iron deficiency anemia in rats. Chin J Integr Med 2007, 13:297-300.
13. Zhang Y, Cheng Y, Wang N,The action of JAK, SMAD and ERK signal pathways on hepcidin suppression by polysaccharides from Angelica sinensis in rats with iron deficiency anemia. Food Funct 2014, 5:1381-1388.
14. Qin J, Liu YS, Liu J,Effect of angelica sinensis polysaccharides on osteoarthritisand: apossible mechanism to promote proteoglycans synthesis. Evid Based Complement Alternat Med 2013, 79476.
15. Gattermann N. Mitochondrial DNA mutations in the hematopoietic system. Leukemia 2004, 18: 18-22.
16. Holt IJ, Harding AE, Morgan-Hughes JA. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature 1988, 331:717-719.
17. Lestienne P, Ponsot G. Kearns-Sayre syndrome with muscle mitochondrial DNA deletion. Lancet 1988, 1:885.
18. Wallace DC, Singh G, Lott MT,Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science 1988, 242:1427-1430.
19. Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 2005, 39:359-407.
20. Clay Montier LL, Deng JJ, Bai Y. Number matters: control of mammalian mitochondrial DNA copy number. J Genet Genomics 2009, 36:125-131.
21. Rötig A, Poulton J. Genetic causes of mitochondrial DNA depletion in humans. Biochim Biophys Acta 2009, 1792:1103-1108.
为了证实当归多糖可以通过调控线粒体膜稳定性干预鼠再障模型设计了该实验。ICR小鼠随机分为对照组、再障组和当归多糖治疗组。其中再障小鼠使用60Coγ照射和腹腔注射环磷酰胺和环孢素的方法造模。对照组小鼠采用铅屏蔽照射。对照组和再障组小鼠使用生理盐水腹腔注射,治疗组小鼠口服当归多糖两周。分别检测骨髓单个核细胞的线粒体超微结构和膜电位。检测COX、MDH在三组中的寒凉差异。结果显示,再障组中线粒体数量和膜电位较对照组均有显著下降,应用当归多糖干预后,有不同程度的回升。治疗组的线粒体裂解时间较再障组大幅延迟(< 0.05)。我们认为当归多糖可以提升再障骨髓单个核细胞的线粒体膜稳定性,并可能抑制线粒体通路的凋亡。
再生障碍性贫血; 当归多糖; 线粒体; 膜电位; ICR小鼠
:Zhong P, Cui XMitochondrial membrane stabilization by Angelica sinensis polysaccharide in murine aplastic anemia. TMR Modern Herbal Medicine 2019, 2 (3): 151-157.
10.12032/TMRmhm2017A50.
Submitted: 17 April 2019,
19 June 2019,
Xing Cui, Department of Hematology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, China. E-mail: cdz45@163.com.
# Both authors contributed equally to this work.
20 June 2019.
Abbreviations:AA: Aplastic anemia, ATP: adenosine triphosphate, MMP: mitochondrial membrane permeabilization, BMNCs: bone marrow-nucleated cells, MAO: monoamine oxidase, JAK: janus-kinase, SMAD: son of mothers against decapentaplegic, ERK: extracellular signal-regulated kinase.
This study was supported by the National Natural Science Foundation of China (No. 81202839), the National Natural Science Foundation of China (No. 81774080), the “Taishan Scholar” Project Special Fund, the Study Abroad Funding by the Shandong health science and technology association and the Affiliated Hospital of Shandong University of Traditional Chinese Medicine.
Competing interests: The authors declare that there is no conflict of interests regarding the publication of this paper.
Executive Editor: Jing Sun
猜你喜欢
杂志排行
TMR Modern Herbal Medicine的其它文章
- Effect of Tanshinone IIA on LPS-induced inflammatory response in a ROS-NLRP3 inflammasome dependent manner in RAW264.7 cells
- The Protective Effect of Jiujiuguiyi, a Medicine and Food Homologous Formula, on Acute Alcohol Poisoning Mice
- Analysis of drug use law and mechanism of prostate cancer based on data mining and network pharmacology
- Erahertz spectral analysis of Xiling Zhimu with different geological conditions and plant age
- Application Progress of Porous Materials in Modern Pharmaceutical
