Effect of Tanshinone IIA on LPS-induced inflammatory response in a ROS-NLRP3 inflammasome dependent manner in RAW264.7 cells
2019-08-09DanLiShanGaoSarheneMichaelYuYingGuoHaoDengShiXinXuGuanWeiFan
Dan Li, Shan Gao, Sarhene Michael,Yu-Ying Guo, Hao Deng, Shi-Xin Xu, Guan-Wei Fan*
Effect of Tanshinone IIA on LPS-induced inflammatory response in a ROS-NLRP3 inflammasome dependent manner in RAW264.7 cells
Dan Li1,2, Shan Gao1,2, Sarhene Michael1,2,Yu-Ying Guo1,2, Hao Deng1,2, Shi-Xin Xu1,2, Guan-Wei Fan1,2*
1First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China.2Tianjin Key Laboratory of Translational Research of TCM Prescription and Syndrome,Tianjin, China.
Emerging evidence has demonstrated that Tanshinone IIA (Tan IIA) prevents cardiomyocytes injury, cardiac fibroblasts and atherosclerosis. However, the molecular mechanism underlying the effects of Tan IIA is still unclear. To investigate the role of Tan IIA in inflammatory response in a ROS-NLRP3 inflammasome dependent manner, RAW264.7 cells stimulated with LPS were recruited to produce a cell model of inflammatory response. Our results indicated that the production of NO was significantly increased after stimulated by LPS, and Tan IIA treated significantly decreased the level of NO. The mRNA expression of NLRP3, IL-1β and TNF-α was significantly inhibited by Tan IIA compared with LPS treated cells. The protein expression of NLRP3, IKBα, pp65/p65 and pp38/p38 was significantly decreased by Tan IIA, compared with LPS or LPS+ATP stimulated groups. Meanwhile, Tan IIA significantly inhibited the level of ROS induced by LPS+ATP. And NAC, a ROS inhibitor, could also inhibit the protein expression of NLRP3. Based on these findings, it could be speculated that the mechanism underlying the effect of Tan IIA may involve the regulation of ROS-NF-κB/ P38-NLRP3 pathway. This study further characterized the molecular mechanism of Tan IIA, and provided new thoughts to its clinical therapy.
Tanshinone IIA, Inflammation, NLRP3 inflammasome, ROS
Tan IIA could inhibit the inflammatory response and NLRP3 expression stimulated by LPS or LPS+ATP. Acetylcysteine (N-acetyl-l-cysteine, NAC), a ROS inhibitor, could inhibit LPS+ATP-induced increase in NLRP3 level. The mechanism underlying the effects of Tan IIA may involve the regulation of ROS-NF-κB/ P38-NLRP3 pathway. This study further characterized the molecular mechanism of Tan IIA, and provided new thoughts to its clinical therapy.
Background
Macrophages play an important role in keeping cardiac homeostasis and orchestrating tissue repair as well as remodeling. In heart failure patients, elevated serum proinflammatory cytokines are supposed to be predictive biomarkers of worsened clinical outcomes, and inflammation may contribute to the progression of heart failure [1]. Though inflammation has emerged as a therapeutic target to treat cardiovascular diseases, it however seems that broad immunosuppression failed to improve clinical outcomes either after myocardial infarction (MI) or in heart failure [2, 3].
Several clinical studies have shown that inhibition of an inflammatory pathway, such as the inflammatory cytokine, IL-1β, could effectively reduce recurrent cardiovascular events [4, 5]. It can be speculated that the immunomodulation of inflammatory response has important diagnostic and therapeutic significance in the process of cardiovascular diseases.
NLRP3 inflammasome is the most extensively studied of all kinds of inflammasomes. It has been documented that NLRP3 inflammasome plays a crucial part in many diseases, such as multiple sclerosis (MS), Alzheimer disease (AD), atherosclerosis, obesity and type 2 diabetes mellitus [6], and more and more evidence indicates that NLRP3 inflammasome is also involved in myocardial ischemia/reperfusion injury (MI/RI) [7, 8]. The mechanisms of NLRP3 activation may include the generation of reactive oxygen species (ROS), potassium efflux and the release of cathepsins into the cytosol after lysosomal destabilization. When cells were stimulated by pathogens or danger signals, NLRP3 was activated and recruited pro-caspase-1 through the adaptor protein ASC. NLRP3, ASC and pro-caspase-1 made NLRP3 inflammasome complex assembly. Under the action of NLRP3 inflammasome, pro-caspase-1 was activated to be caspase-1, eventually leading to the secretion of IL-1β and IL-18, and causing a series of inflammatory reactions [9, 10].
Tan IIA is a liposoluble constituent of Danshen (), possessing various biological properties and taking part in a series of biochemical reactions. Tan IIA has anti-inflammatory, antioxidant and promotes angiogenesis effect, and has also been widely used for the treatment of disease of cardio-cerebrovascular system [11, 12]. Emerging evidence has demonstrated that Tan IIA prevents cardiomyocytes injury, cardiac fibroblasts, atherosclerosis and neuroinflammation [13-16]. However, the molecular mechanism underlying the effects of Tan IIA is still unclear.
During ischemia/reperfusion injury, monocyte/macrophage, neutrophils and endothelial cells initiate the inflammatory response, inducing the release of a variety of inflammatory cytokines, and resulting in increased vascular permeability, tissue edema and damage. Macrophage is an important inflammatory regulation cell type among all the inflammatory cell species, and plays a crucial role in the process of myocardial infarction [17]. Monocyte/macrophage recruited to the damaged myocardium can further release ROS, inflammatory mediators and proteases which aggravates myocardial injury [18].
For the reason that macrophages play an important role in keeping cardiac homeostasis and orchestrate tissue repair, we recruited RAW264.7 cells stimulated with LPS to produce a cell model of inflammatory response. The present study investigated whether Tan IIA has an effect on LPS stimulated macrophages, and the mechanisms underlying the effects of Tan IIA on macrophage in a ROS-NLRP3 inflammasome dependent manner.
Materials and Methods
Cell Culture and Treatment
RAW264.7 cell was purchased from Cell Culture Center of Chinese Academy of Medical Sciences (Beijing, China). Cells were cultured in DMEM medium (high glucose), added 10 % fetal bovine serum (FBS), 100 U/mL penicillin, and100 μg/mL streptomycin. Cells were cultured at 37 °C in a fully humidified atmosphere with 5 % CO2. For all experiments, cells were treated at 80–90% cell confluence. The cells were treated with different concentrations of Tan IIA (at 10, 1, and 0.1 μM) for different time, with or without 1 μg/mL LPS. Meanwhile, cells in the control group were treated with equal volume 0.1 % DMSO.
Measurement of NO production
NO Assay Kit (Beyotime, China) was utilized to measure the production of NO according to the manufacturer’s instructions. Briefly, the standards were diluted with DMEM+10%FBS and 50μL standard or cell culture supernatant was added to each well of the 96-well plate. Then 50μL each of Griess Reagent I and II were pipetted into each well of the plate and the optical density (OD) was detected by Varioskan LUX multimode microplate reader at 540 nm.
RNA Extraction
RAW264.7 cells were plated at a density of 1×106 cells/well with 10 % FBS DMEM in six-well plates. 24 hours later, the medium was removed and added with serum-free, antibiotic-free DMEM medium instead. RAW264.7 cells were treated with DMSO (control), LPS (1 μg/mL) and LPS + Tan IIA (10, 1, and 0.1 μM) for 6 h. Then total RNA was extracted to detect the mRNA level of NLRP3, IL-1β and TNF-α. Total RNA was extracted by use of the TRIzol reagent (Ambion). RNA concentrations were measured by an ultramicrofluorescence spectrophotometer (DeNovix). HiFiScript cDNA synthesis kit (cwbiotech) was used for reverse transcription according to the manufacturer’s instructions. 1 μg of RNA samples were reverse-transcribed, and the reaction steps were as follows: 42°C for 50 min, 85 °C for 5 min and then maintained at 4 °C.
RT-PCR
Quantitative PCR (QPCR) assay was conducted with a Q-PCR instrument (Lightcycler 96, Roche), using UltraSYBR mixture (cwbiotech). The PCR protocol was 95℃ for 10min, followed by 40 cycles of 95℃ for 10s, 56 for 30s, and 72 for 32s. The primers used for qRT-PCR are shown as follows: GADPH-Forward: 5′- AACTTTGGCATTGTGGAAGG-3′;GADPH-Reverse: 5′- ACACATTGGGGGTAGGAACA-3′;NLRP3-Forward: 5′- ATGCTGCTTCGACATCTCCT -3′;NLRP3-Reverse: 5′- AACCAATGCGAGATCCTGAC -3′;IL-1β-Forward: 5′- GCCCATCCTCTGTGACTCAT -3′;IL-1β-Reverse: 5′- AGGCCACAGGTATTTTGTCG -3′;TNF-α-Forward: 5′-GCAGATGGGCTGTACCTTATC-3′;TNF-α-Reverse: 5′-GCAGATGGGCTGTACCTTATC-3′. Gene expression level was calculated using the 2−ΔΔCt method.
Western blotting
Western blotting was conducted with the following antibodies: anti- NLRP3 antibody (CST), anti- pp38 antibody (CST), anti- p38 antibody (CST) , anti- pp65 antibody (CST), anti- GADPH antibody (proteintech), anti-β-tubulin antibody(proteintech) , anti- HMGB1 antibody (proteintech), anti- p65 antibody (proteintech), anti- IKB-α antibody (proteintech). Protein fractions were extracted from the lysates of cultured cells and an Enhanced BCA Protein Assay Kit (Beyotime, China) was utilized to measure the protein concentrations. Proteins were denatured and resolved on SDS-PAGE, then transferred to a PVDF membrane. After blocking with QuickBlock™ Blocking Buffer (Beyotime, China) for 1 h, the PVDF membranes were then incubated with primary antibodies overnight at 4 °C. Then the membranes were washed with Western Wash Buffer (Beyotime, China) for three times, and incubated with HRP conjugated secondary antibodies for 1 h at room temperature. A BeyoECL Plus (Beyotime, China) kit was utilized to detect the proteins according to the manufacturer’s instructions. A C-DiGit 3600 (Li-Cor, USA) was employed to image and the bands were quantitated by Image J Software.
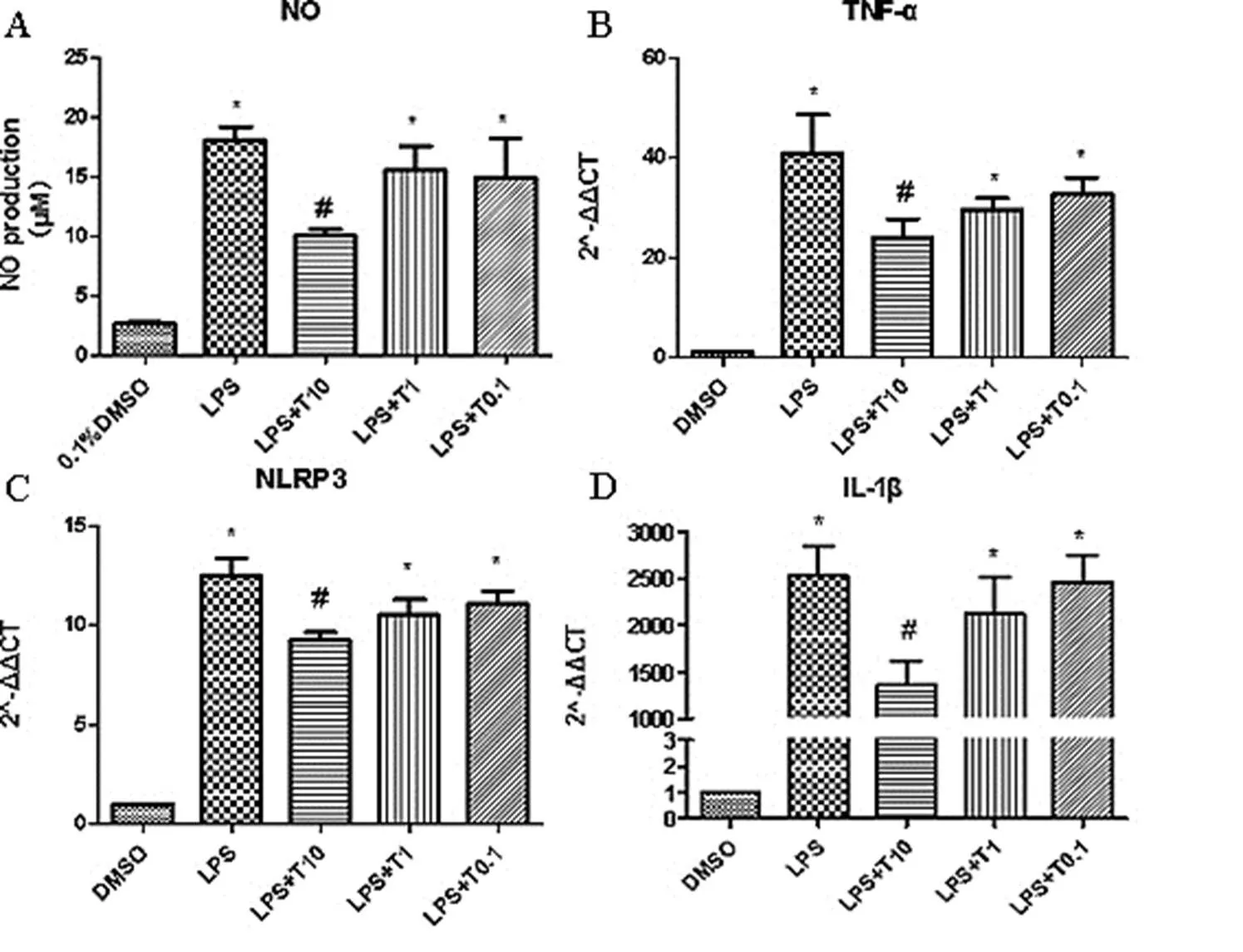
Figure 1. Tan IIA possessed regulatory effect on NO production, NLRP3, IL-1β, TNF-α mRNA expression
A. RAW264.7 Cells NO production of different groups.The cells were pretreated with Tan IIA 10µM, 1µM, and 0.1µM for 30 min and stimulated with LPS (1 μg/mL) for 24 h. *vs ctrl, #vs LPS. B-D. Tan IIA possessed regulatory effect on mRNA expression of TNF-α, NLRP3 and IL-1β.The cells were pretreated with Tan IIA 10µM, 1µM, and 0.1µM for 30 min and stimulated with LPS (1 μg/mL) for 6 h. *vs ctrl, #vs LPS.
Measurement of ROS accumulation
A DCFH-DA probe Reactive Oxygen Species Assay Kit (Beyotime, China) was used to measure intracellular ROS level in RAW264.7 cells according to the manufacturer’s instructions. Briefly, DCFH-DA was diluted with serum-free DMEM medium and the final concentration was adjusted to 10μM/L. After incubation with 10 μM DCFH-DA dye for 30 min at 37 °C, RAW264.7 cells were washed thrice with serum-free DMEM medium, so as to remove the excess DCFH-DA. For positive control well, 1μL of Rosup was added to the cells. The fluorescence was quantified using a Nikon IX73 inverted microscope. The images of cells were measured using Image J Software.
Statistical Analysis
All the experiments were repeated at least three times. The SPSS software was used for all analyses, and the GraphPad Prism 5 was performed for the graphics drawing. All results were given as the mean value ± SD. One-way ANOVA was used to analyze data among groups, and paired comparison using SNK-q test. A valuewas considered statistically significant in all cases.
Results
The Effect on RAW264.7 Cells NO production of Tan IIA Treatment
NO, a second messenger molecule, performs various functions in cell physiological and pathological processes. NO secreted by macrophages plays an important role in inflammatory, immune and anti-viral reactions [19, 20]. Due to these, NO can be used to estimate the effects of LPS on immune and inflammatory response. The cells were treated with different concentrations of Tan IIA (at 10, 1, and 0.1 μM) with or without 1 μg/mL LPS for 24h, and the cells in the control group were treated with equal volume of 0.1 % DMSO. As shown in Figure 1A, compared with the control group, in the model group stimulated with LPS for 24h, the production of NO markedly increased (); And various concentrations of TanIIA treatment (at 10, 1, and 0.1 μM) significantly decreased the NO production () of the cells stimulated with LPS.

Figure 2. Tan IIA possessed regulatory effect on protein expression of NLRP3, IKBα, pp65/p65 and pp38/p38.
The cells were pretreated with Tan IIA 10µM for 30 min and stimulated with LPS (1 μg/mL) for 5.5 h. ATP was added 30 min before cells were harvested. A. Representative Western blot of NLRP3, IKBα, pp65/p65 and pp38/p38. B-F. Quantitative data of average across three separate experiments. *< 0.05 vs ctrl, #< 0.05 vs LPS/LPS+ATP.
The Effect on NLRP3, IL-1β and TNF-α mRNA expression of Tan IIA Treatment
RAW264.7 cells stimulated with LPS could induce the activation of inflammatory response, and it could be ameliorated by Tan IIA, including the mRNA expression of NLRP3, IL-1β and TNF-α. As shown in Figure 1B-D, compared with the control group, in the model group stimulated with LPS, the mRNA expression of NLRP3, IL-1β and TNF-α were markedly increased (); And various concentrations of Tan IIA treatment (at 10, 1, and 0.1 μM) significantly decreased the mRNA expression of NLRP3, IL-1β and TNF-α () in cells stimulated with PS.
The Effect on NLRP3 protein expression of Tan IIA Treatment
ATP, a second signal molecule, is an activator required for NLRP3 inflammasome activation. So, ATP was used to induce the activation of NLRP3 [21, 22]. Our results showed that LPS+ATP-induced the activation of NLRP3 protein expression and it could be ameliorated by Tan IIA. As shown in Figure 2, compared with the control group, in the model group induced by LPS+ATP, the protein expression level of NLRP3 was markedly increased (); and TanIIA treatment (at 10μM) significantly decreased the protein expression of NLRP3 () in cells stimulated with LPS+ATP.
LPS-induced activation of NF-κB and p38 pathway could be inhibited by Tan IIA
As shown in Figure 2, compared with the control group, in the cells stimulated with LPS or LPS+ATP, the protein expression of IKBα, pp65/p65 was markedly increased (); and Tan IIA treatment (at 10μM) significantly decreased the upregulation. The protein level of pp38/p38 was upregulated in cells stimulated with LPS+ATP, and the upregulation was inhibited by Tan IIA (10μM) (Figure 2). However, the protein level of pp38/p38 did not change significantly in cells stimulated with LPS. Furthermore, the protein expression of HMGB1 was also enhanced in cells induced with LPS or LPS+ATP, but showing no statistical difference. These results suggest that LPS or LPS+ATP induced activation of NF-κB and p38 pathway can be inhibited by Tan IIA.

Figure 3. Tan IIA possessed regulatory effect on ROS expression (scale bar 50μm).
The cells were pretreated with Tan IIA 10µM for 30 min and stimulated with LPS (1 μg/mL) for 5.5 h. ATP was added 30 min before cells were harvested. A. Representative fluorescence images of cell ROS expression. B. Quantitative data of average across three separate experiments. *vs ctrl, #vs LPS/LPS+ATP.
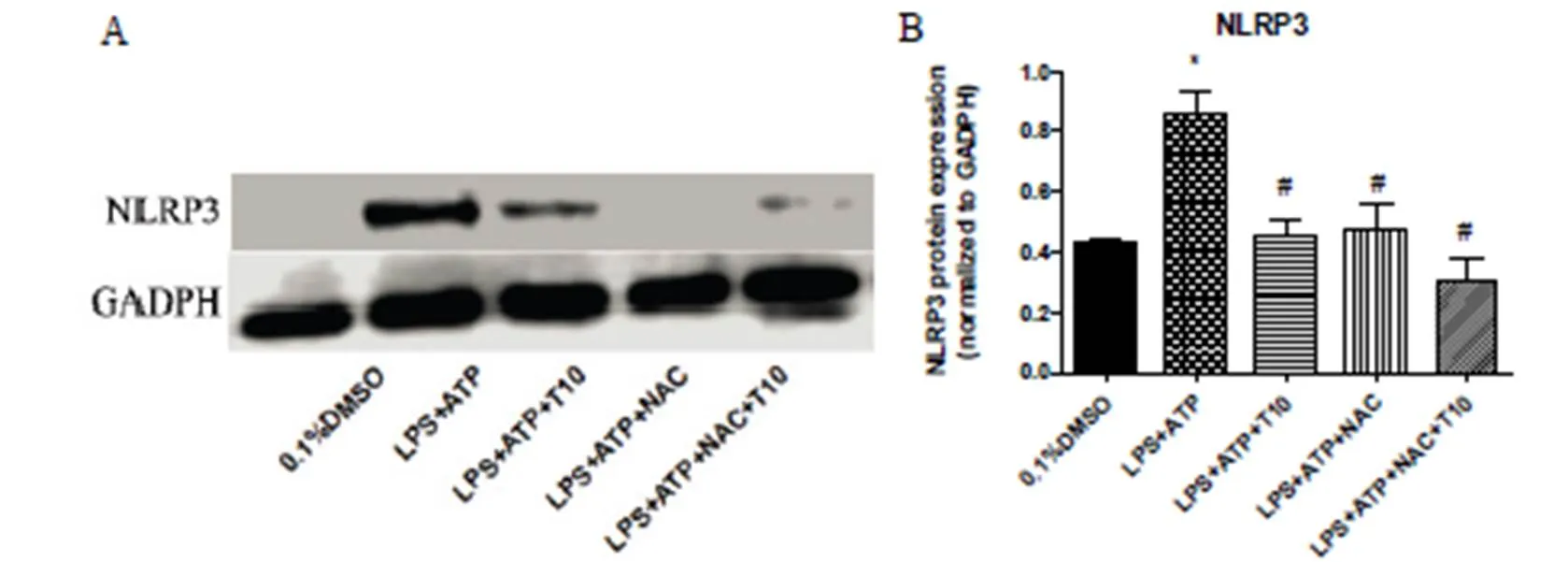
Figure 4. NAC possessed regulatory effect on protein expression of NLRP3.
The cells were pretreated with Tan IIA 10µM for 30 min and stimulated with LPS (1 μg/mL) /LPS (1 μg/mL) +NAC (1mM) for 5.5 h. ATP was added 30 min before cells were harvested. A. Representative Western blot of NLRP3. B. Quantitative data of average across three separate experiments. *vs ctrl, #vs LPS+ATP.
Tan IIA possessed regulatory effect on ROS
To confirm whether Tan IIA could scavenge intracellular ROS, the production of intracellular ROS was evaluated by fluorescence microscopy using a Reactive Oxygen Species Assay Kit. The RAW264.7 cells were exposed to LPS for 6 h, and 1 mM ATP was added 30min before the cells were harvested. The ROS positive cells were visualized as bright green spots, and LPS+ATP treatment increased the level of intracellular ROS significantly, compared with that in the control group (Figure 3). Meanwhile, treatment with Tan IIA inhibited the LPS+ATP-induced increase in intracellular ROS significantly (Figure 3). These results suggested that Tan IIA had antioxidant capacity.
NAC possessed regulatory effect on NLRP3
Acetylcysteine (N-acetyl-l-cysteine,NAC), a scavenger of ROS, could inhibit the protein expression of NLRP3. As previously mentioned, the protein expression of NLRP3 increased significantly after stimulated with LPS+ATP for 6h, and treatment with Tan IIA inhibited the LPS+ATP-induced increase in NLRP3 protein level. Furthermore, treatment with NAC could also inhibit LPS+ATP-induced increase in NLRP3 protein level significantly (Figure 4). These results showed that the regulatory effect of Tan IIA on NLRP3 may depend on a ROS-NLRP3 pathway.
Discussion
Macrophages participate in the process of inflammatory response, and play a crucial part in the process of myocardial infarction. Monocyte/macrophage recruited to the damaged myocardium can further release ROS, inflammatory mediators and protease, which aggravates myocardial injury [23]. RAW264.7 cells have been regarded as a dominating experimental macrophage model, and play an important role in the research of macrophage relevant inflammatory signaling pathways [24]. Macrophages play a vital role in keeping cardiac homeostasis and orchestrating tissue repair as well as remodeling. LPS-stimulated RAW264.7 cells have been extensively used to study drugs possessing anti-inflammatory properties and the underlying mechanisms [25, 26].
The NLRP3 inflammasome is a vital mediator of inflammatory responses, and there has been increasing evidence that NLRP3 inflammasome plays a crucial part in heart diseases. In human arterial vessels, NLRP3 high expression was correlated with risk factors of atherosclerotic diseases and the severity of coronary artery disease [27], and the level of NLRP3 inflammasome-related genes was associated with atherosclerotic plaque [28]. Activation of the NLRP3 inflammasome has also been associated with adverse cardiac remodeling. NLRP3 was activated in cardiomyocytes in transverse aortic constriction (TAC) induced mice adverse cardiac remodeling model, and the activation of NLRP3 inflammasome brought about the transformation of pro-caspase-1 to its active form [29]. MCC950, a NLRP3 inflammasome inhibitor, could inhibit atherosclerotic effect, probably in a way of inhibiting the adhesion of monocytes and reducing intraplaque macrophages accumulation [30].
Tan IIA is a major monomer extracted from Danshen, possessing various biological properties. Tan IIA has anti-inflammatory, antioxidant and promotes angiogenesis effect, and has been used widely for the treatment of diseases of cardio-cerebrovascular system [11]. Emerging evidence has demonstrated that Tan IIA prevented cardiomyocytes injury, cardiac fibroblasts, atherosclerosis and neuroinflammation [13-16]. Our previous studies have shown that Tan IIA exerted an anti-inflammatory effect by inhibiting the TLR4-MyD88-NF-κB signaling pathway and adjusting related miRNA expression in LPS-stimulated RAW264.7 cells [31]. In addition, Tan IIA could promote macrophage polarization from a proinflammatory M1 phenotype toward an anti-inflammatory M2 phenotype [32]. However, the molecular mechanism underlying the effects of Tan IIA was still unclear.
Our results have shown that the production of NO markedly increased in RAW264.7 cells stimulated with LPS, and Tan IIAtreatment (at 10, 1, and 0.1 μM) significantly decreased the NO production of the cells. RAW264.7 cells stimulated with LPS could induce the activation of inflammatory response, and it could be ameliorated by Tan IIA, including the mRNA expression of NLRP3, IL-1β and TNF-α. ATP is a second signal molecule of NLRP3 inflammasome activation. In the study, ATP was used to induce the activation of NLRP3. Results showed that LPS+ATP-induced the activation of NLRP3 protein expression and it could be ameliorated by Tan IIA. In the cells stimulated with LPS or LPS+ATP, the protein expression of IKBα, pp65/p65 was markedly increased; and Tan IIA treatment (at 10μM) significantly decreased the upregulation. The protein level of pp38/p38 was upregulated in cells stimulated with LPS+ATP, and the upregulation was inhibited by Tan IIA (10μM). Furthermore, the protein expression of HMGB1 was also enhanced in cells induced with LPS or LPS+ATP, but showing no statistical difference. These results suggested that LPS or LPS+ATP induced activation of NF-κB and p38 pathway could be inhibited by Tan IIA.
LPS+ATP treatment increased the level of intracellular ROS significantly, and Tan IIA inhibited the LPS+ATP-induced increase in intracellular ROS significantly. Acetylcysteine (N-acetyl-l-cysteine, NAC) is a scavenger of ROS. NAC could inhibit LPS+ATP-induced increase in NLRP3 protein level significantly. These results suggested that Tan IIA had antioxidant capacity and that the regulatory effect of Tan IIA on NLRP3 might depend on a ROS-NLRP3 pathway.
To sum up, Tan IIA could inhibit the inflammatory response and NLRP3 expression stimulated by LPS or LPS+ATP, and NAC could inhibit LPS+ATP-induced increase in NLRP3 level. Hereby, it could be speculated that the mechanism underlying the effects of Tan IIA may involve the regulation of ROS-NF-κB/ P38-NLRP3 pathway. This study further characterized the molecular mechanism of Tan IIA, and provided new thoughts to its clinical therapy.
1. M. DeBerge, S.J. Shah, L. Wilsbacher,Macrophages in heart failure with reduced versus preserved ejection fraction. Trends Mol. Med 2019, 25: 328-340.
2. G.H. Gislason, S. Jacobsen, J.N. Rasmussen,Risk of death or reinfarction associated with the use of selective cyclooxygenase-2 inhibitors and nonselective nonsteroidal antiinflammatory drugs after acute myocardial infarction. Circulation 2006, 113: 2906-2913.
3. D.M. McNamara, R. Holubkov, R.C. Starling,Controlled trial of intravenous immune globulin in recent-onset dilated cardiomyopathy. Circulation 2001, 103: 2254-2259.
4. P.M. Ridker, B.M. Everett, T. Thuren,Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017, 377: 1119-1131.
5. P.M. Ridker, J.G. MacFadyen, T. Thuren,Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017, 390: 1833-1842.
6. H. Guo, J.B. Callaway, J.P. Ting. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 2015, 21: 677-687.
7. L. Minutoli, D. Puzzolo, M. Rinaldi,ROS-mediated NLRP3 inflammasome activation in brain, heart, kidney, and testis ischemia/reperfusion injury. Oxid Med Cell Longev 2016, 2016: 2183026.
8. M. Kawaguchi, M. Takahashi, T. Hata,Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 2011, 123: 594-604.
9. G. Trendelenburg. Molecular regulation of cell fate in cerebral ischemia: role of the inflammasome and connected pathways. J Cereb Blood Flow Metab 2014, 34: 1857-1867.
10. C. Jin, R.A. Flavell. Molecular mechanism of NLRP3 inflammasome activation. J Clin Immunol 2010, 30: 628-631.
11. Y. Cai, W. Zhang, Z. Chen,Recent insights into the biological activities and drug delivery systems of tanshinones. Int J Nanomedicine 2016, 11: 121-130.
12. M. Akaberi, M. Iranshahi, S. Mehri. Molecular signaling pathways behind the biological effects of salvia species diterpenes in neuropharmacology and cardiology. Phytother Res2016, 30: 878-893.
13. D. Wang, Y. Liu, G. Zhong,Compatibility of tanshinone IIA and astragaloside IV in attenuating hypoxia-induced cardiomyocytes injury. J Ethnopharmacol 2017, 204: 67-76.
14. W. Chen, X. Li, S. Guo,Tanshinone IIA harmonizes the crosstalk of autophagy and polarization in macrophages via miR-375/KLF4 pathway to attenuate atherosclerosis. Int Immunopharmacol 2019, 70: 486-497.
15. Y.T. Tsai, S.H. Loh, C.Y. Lee,Tanshinone IIA inhibits high glucose-induced collagen synthesis via nuclear factor erythroid 2-related factor 2 in cardiac fibroblasts. Cell Physiol Biochem 2018, 51: 2250-2261.
16. N. Huang, Y. Li, Y. Zhou,Neuroprotective effect of tanshinone IIA-incubated mesenchymal stem cells on Abeta25-35-induced neuroinflammation. Behav Brain Res 2019, 365: 48-55.
17. T. Heidt, G. Courties, P. Dutta,Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res 2014, 115: 284-295.
18. S. Steffens, F. Montecucco, F. Mach. The inflammatory response as a target to reduce myocardial ischaemia and reperfusion injury. Thromb Haemost 2019, 102: 240-247.
19. J. MacMicking, Q.W. Xie, C. Nathan Nitric oxide and macrophage function Annu Rev Immunol 1997, 15: 323-350.
20. S.H.H.H. W., W. U.. NO at Work. Cell 1994, 78: 919-925.
21. L. Franchi, T. Eigenbrod, R. Munoz-Planillo,The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 2009, 10: 241-247.
22. X. Dong, Z. Zheng, P. Lin,ACPAs promote IL-1beta production in rheumatoid arthritis by activating the NLRP3 inflammasome. Cell Mol Immunol 2019.
23. P.J. Murray, T.A. Wynn. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011, 11: 723-737.
24. G. Pascual, A.L. Fong, S. Ogawa,A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature 2005, 437: 759-763.
25. X. Zhang, G. Wang, E.C. Gurley,Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. PLoS One 2014, 9: e107072.
26. Z. Yuan, M.A. Syed, D. Panchal,Curcumin mediated epigenetic modulation inhibits TREM-1 expression in response to lipopolysaccharide. Int J Biochem Cell Biol 2012, 44: 2032-2043.
27. F. Zheng, S. Xing, Z. Gong,NLRP3 inflammasomes show high expression in aorta of patients with atherosclerosis. Heart Lung Circ 2013, 22: 746-750.
28. G. Paramel Varghese, L. Folkersen, R.J. Strawbridge,NLRP3 inflammasome expression and activation in human atherosclerosis. J Am Heart Assoc 2015, 5.
29. T. Suetomi, A. Willeford, C.S. Brand,Inflammation and NLRP3 inflammasome activation initiated in response to pressure overload by Ca(2+)/calmodulin-dependent protein kinase II delta signaling in cardiomyocytes are essential for adverse cardiac remodeling. Circulation 2018, 138: 2530-2544.
30. T. van der Heijden, E. Kritikou, W. Venema,NLRP3 inflammasome inhibition by MCC950 reduces atherosclerotic lesion development in apolipoprotein E-deficient mice-brief report. Arterioscler Thromb Vasc Biol 2017, 37: 1457-1461.
31. G. Fan, X. Jiang, X. Wu,Anti-inflammatory activity of tanshinone IIA in LPS-stimulated RAW264.7 macrophages via miRNAs and TLR4-NF-kappaB pathway. Inflammation 2016, 39: 375-384.
32. S. Gao, Y. Wang, D. Li,Tanshinone IIA alleviates inflammatory response and directs macrophage polarization in lipopolysaccharide-stimulated RAW264.7 cells. Inflammation 2019, 42: 264-275.
:探讨丹参酮IIA抑制LPS诱导的Raw264.7细胞炎症反应作用机制。: LPS刺激RAW264.7细胞构建炎症细胞模型,筛选丹参酮IIA药物适宜浓度,利用DCFH-DA荧光探针检测细胞ROS水平变化,通过实时荧光定量PCR检测NLRP3,IL-1βmRNA水平变化, Western Blot检测p-p65/p65,p-p38/p38,NLRP3,HMGB1蛋白表达。:丹参酮IIA能够抑制LPS诱导的RAW264.7细胞炎性反应递质NO及炎症因子TNF-α表达,抑制NLRP3及其下游基因IL-1β mRNA水平表达。丹参酮IIA能够显著抑制LPS+ATP刺激细胞后ROS表达水平,抑制LPS或LPS+ATP刺激RAW264.7细胞NLRP3、NF-κB通路相关蛋白及P38蛋白水平表达,包括IKBα,pp65/p65,pp38/p38蛋白水平表达。LPS或LPS+ATP刺激RAW264.7细胞后,晚期炎症标志因子HMGB1蛋白水平变化没有显著差异。而ROS抑制剂NAC可以抑制LPS+ATP刺激RAW264.7细胞诱导的NLRP3表达增加。丹参酮IIA可以抑制LPS或LPS+ATP刺激RAW264.7细胞诱导的炎症反应,其机制可能涉及丹参酮IIA对ROS-NF-κB/ P38-NLRP3通路的调控。
丹参酮IIA,炎症,NLRP3炎性体,ROS
:Li D, Gao S,Effect of Tanshinone IIA on LPS-induced inflammatory response in a ROS-NLRP3 inflammasome dependent manner in RAW264.7 cells. TMR Modern Herbal Medicine, 2019, 2(3): 131-139.
10.12032/TMRmhm2017A48.
Guan-Wei Fan, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China. E-mail: fgw1005@hotmail.com.
Submitted: 10 July 2019, Accepted: 19 July 2019, Online: 20 July 2019.
Abbreviations:MI: myocardial infarction, MS: multiple sclerosis, AD: Alzheimer disease, MI/RI: myocardial ischemia/reperfusion injury, ROS: reactive oxygen species。
This study was funded by the Tianjin Outstanding Youth Science Foundation (No. 17JCJQJC46200), the National Natural Science Foundation of China (No.81774050), the Natural Science Foundation of Tianjin (17JCYBJC29000) and the Foundation of First Teaching Hospital of Tianjin University of Traditional Chinese Medicine (No. 201703).
Competing interests: The authors declare that there is no conflict of interests regarding the publication of this paper.
Executive Editor: Jing Sun
猜你喜欢
杂志排行
TMR Modern Herbal Medicine的其它文章
- Erahertz spectral analysis of Xiling Zhimu with different geological conditions and plant age
- Analysis of drug use law and mechanism of prostate cancer based on data mining and network pharmacology
- The Protective Effect of Jiujiuguiyi, a Medicine and Food Homologous Formula, on Acute Alcohol Poisoning Mice
- Mitochondrial membrane stabilization by Angelica sinensis polysaccharide in murine aplastic anemia
- Application Progress of Porous Materials in Modern Pharmaceutical
