The Protective Effect of Jiujiuguiyi, a Medicine and Food Homologous Formula, on Acute Alcohol Poisoning Mice
2019-08-09YangFanZhouTianZeZhangSiQiDengMengZhangYuJiaoZhanYiFangLiRongRongHe
Yang-Fan Zhou, Tian-Ze Zhang, Si-Qi Deng, Meng Zhang, Yu-Jiao Zhan, Yi-Fang Li*, Rong-Rong He*
The Protective Effect of Jiujiuguiyi, a Medicine and Food Homologous Formula, on Acute Alcohol Poisoning Mice
Yang-Fan Zhou1,2,3#, Tian-Ze Zhang1,2,3#, Si-Qi Deng1,2,3, Meng Zhang1,2,3, Yu-Jiao Zhan1,2,3, Yi-Fang Li1,2,3*, Rong-Rong He1,2,3*
1Guangdong Province Research and Development Center for Chinese Medicine in Disease Susceptibility, College of Pharmacy, Jinan University, Guangzhou, China.2International Cooperative Laboratory of Traditional Chinese Medicine Modernization and Innovative Drug Development of Chinese Ministry of Education (MOE), College of Pharmacy, Jinan University, Guangzhou, China.3Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research,College of Pharmacy, Jinan University, Guangzhou, China.
: Nowadays, acute alcoholic intoxication has become the third public problem in China, and the anti-inebriation products mainly aimed at increasing the activity of enzyme involved in the alcohol metabolism, which is a single mechanism that can accelerate alcohol metabolism. Thus, a new formula, Jiujiuguiyi (JJGY) which could protect liver, relieve the abnormal excitability of the center and improve muscle retardation at the same time is designed by us.: The model of acute alcoholic intoxication was established by intragastric administration with 0.12 ml/10g 50% alcohol in mice. JJGY was orally administrated (gavage) once a day for 20 consecutive days before the establishment of acute alcoholic model. Mice were randomly divided into 8 groups with 8 each: blank control group (CON), model group (M), Haiwangjinzun positive control group (HWJZ), experimental groups (AL, AH, BL, BH, AB). Giant, crawling time on the rota-rod, the activities of aspartate amino trans- ferase (AST), alanine amino transferase (ALT) and superoxide dismutase (SOD) in both liver and serum, alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH), glutathione peroxidase (GSH-PX) in liver as well as the HE staining of liver slices, the formation of malondialdehyde (MDA) in serum were determined after acute alcoholic intoxication.: Compared with model group, JJGY significantly decreased the AST and ALT activity in liver and serum and MDA activity in serum. Meanwhile, it enhanced the ADH and ALDH level in liver as well as the hepatic and serous SOD activity, indicating more efficient metabolism of alcohol and less hepatic injury. HE staining results also proved that JJGY could reduce alcoholic liver cell injury, and the effect was more obvious in the group medicated before alcohol administration. Moreover, JJGY significantly prolonged the crawling time on the rota-rod and improved the gait of mice and the effect was proved to be better than the widely used health product Haiwangjinzun.: This study suggests that JJGY is able to protect liver, relieve the abnormal excitability of the center and improve muscle retardation after acute alcoholic intoxication. Its liver protection effect is likely related to its modulation on the alcohol metabolizing and antioxidant enzymes.
Jiujiuguiyi (JJGY), acute alcoholic intoxication of mice, medicine and food homology, ethology test
In the present study, a new compound Chinese herbal medicine formula, Jiujiuguiyi, was designed by using the medicine and food homology theory. The formula aims at protecting liver, relieving the abnormal excitability of the center and improving muscle retardation at the same time. Acute alcoholic intoxication model in mice was built, then the ethology test and biochemical test were conducted to exam the efficacy of the formula in different preparations. The results suggest that JJGY can protect the acute alcoholic intoxication mice through multiple mechanisms, providing a new way to develop antialcoholismic drug homologous food.

Background
Acute alcohol poisoning is caused by the accumulation of ethanol at one time, exceeding the oxidation rate of human body [1].Alcohol poisoning is a multi-system disease that affects the whole body, not just the liver [2]. Alcohol can easily go through the blood-brain barrier and act on the cerebral cortex, disrupting the delicate balance between γ-aminobutyric acid (GABA) [3], then cause the inhibitions of subcortical center, cerebellum, medulla, vasomotor center and respiratory center [4].Respiratory and circulatory failure may occur in severe cases. Alcohol intoxication can also lead to alcoholic myopathy and amyotrophy [5-7], resulting in muscle weakness and pain for weeks or months [8]. Also, drinking alcohol can directly damage the lipoprotein layer of the gastric mucosa, causing damage to the gastric mucosal barrier [9]. In recent years, due to social needs and increased stress in life, the alcohol consumption group in China has exceeded 500 million. Alcohol and alcoholism have become the third public health problem all over the world [10], after cardiovascular disease and cancer [11].
Traditional Chinese medicine (TCM) has potential therapeutic value in the prevention and treatment of alcoholism [12]. For centuries, herbal medicines and food supplements have been used in China and east Asia to prevent and treat alcohol-related diseases [13]. In the present study, we developed a new compound Chinese herbal medicine formula calledwhich is able to protect liver, to relieve the abnormal excitability of the center and to improve muscle retardation. Aimed at improving acute alcoholic intoxication in different periods more accurately, we designed two different formulas--Formula A is designed to be taken before drinking the alcohol and Formula B is designed to be taken after drinking the alcohol [14]. Formula A containsextractand, focusing on preventing acute alcoholic intoxication.Formula B containsand, focusing on improving the symptoms of acute alcoholic intoxication [15]
is a small molecular weight antioxidant [16].andhave the effect of sober-up [17, 18] and attendant liver function [19], according to.is reported able to resist oxidation and inflammation [20]. Bothand[21]can tranquilize mind. Milk powder and konjaku flour are aimed at protecting gastric mucosa and reducing the absorption of alcohol.[22]and[23]can promote the excretion of alcohol metabolites.
To access the function of, and whether prevention or remediation is more effective, we established the model of acute alcoholic intoxication in mice [24, 25].
Materials and Methods
Drugs and major reagents
(batch no: 171103151),(batch no: 170806301),(batch no: 170800501),(batch no: 171008681),(batch no: 170902361),(batch no: 170703411),(batch no: 170905201) were bought from Kangmei Pharmaceutical Co., Ltd. (Shenzhen, China).was picked from Nankun mountain, Longmen county, Guangdong province.(batch no: XSD2018010205) was bought from Qixiang Biotechnology Co., Ltd. (Guangzhou, China). Haiwangjinzun was bought from Neptunus Medicine Group Co., Ltd. (Shenzhen, China).
Formula A (mass ratio):::::::6 : 15 : 15 : 9 : 12 : 9 : 2
Formula B (mass ratio):::::::= 6 : 15 : 15 : 9 : 9 : 9 : 9
Add 8 times of water and cook the above-mentioned herbs for 2 times, each time for 1 hour. Concentrate the filtrate to liquid equivalent to 1 g/ml of crude drugs, and cool it for later use.
Animals
SPF Kunming mice (18-22 g, male) were obtained from Guangdong Medical Laboratory Animal Center (License No: SCXK 2013-0002). All animals were acclimatized housing environment with 23 ± 2 °C and 12 : 12 h light-dark cycle for one week before the experiments. They were maintained on standard chow pellet and water ad libitum. All animal care and experimental procedures were approved by the Laboratory Animal Ethics Committee of Jinan University, and were in accordance with the National Institute of Health’s Guide for the Care and Use of Laboratory Animals (the 7th edition, USA).
Laboratory apparatus
Enzyme-labeled instrument (MK3, Labsystem, Finland), ultra-pure water system (Milli Q Plus, Millipore, USA), freezing microtome (Leica CM1950, Leica, Germany), optical microscope (Olympus BX50, Olympus, Japan), electronicbalance (BS 210S, Sartorius, Germany), rota-rod analyzer (ZH, Anhui Zhenghua Biologic Apparatus Facilities, China), catwalk analysis system (catwalk XT 9, Noldus, Holland), homogenizer (IKA labortechnik, Stanfen, Germany), refrigerated centrifuge (3-18K, Sartorius, Germany)
Group and administration
SPF Kunming mice (18-22 g) were divided randomly into 8 groups with 8 each: blank control group (CON), model group (M),(HWJZ) positive control group, experimemtal group (EP) containing Formula A high dose group (AH), Formula A low dose group (AL), Formula B high dose group (BH), Formula B low dose group (BL), Formula A plus Formula B group (AB). Mice in these groups were administered with 0.1 ml/10 g purified water for both CON and M groups,(3 g/10 ml before the administration of alcohol) for positive control group, and Formula A or/and B (high dose group, 0.04 g/10 g; low dose group, 0.02 g/10 g) for EP group separately. On the 21st day. mice in CON, M, AL and AH group were administered with 0.12 ml/10g 50% alcohol 30 min after administered with 0.1 ml/10 g Formula A (dd H2O). Mice in BH and BL groups were administered with 0.1 ml/10 g Formula B 30 min after administered with 0.12 ml/10 g 50% alcohol. Mice in AB group were administered 0.12 ml/10 g 50% alcohol 15 min after administered with 0.1 ml/10 g Formula A and 15 min before administered with Formula B.
Ethology analysis
30 minutes after the asministration, the mice were placed in the catwalk analysis system. After the environment was adapted, the test started, and the gait was recorded. Each group of mice was tested alternately to avoid the influence of time on the spontaneous activity of the mice.
Then, the mice were placed on a rota-rod analyzer, and the test was started after the environment was adapted (1 min). The crawling time of the mice on the rota-rod was recorded, and each mouse was tested 3 times. Each group of mice was tested alternately to avoid the influence of time on the spontaneous activity of the mice.
Biochemical test
After finishing enthology analysis the mice were fasted for 12 hours. Then the eye blood was collected and the mice were sacrificed to collect the liver. The blood was centrifuged at 2500 rpm for 15 min and the serum was collected. The activities of serum aspartate transaminase (AST), alanine aminotransferase (ALT), malondialdehyde (MDA) and superoxide dismutase (SOD) were determined by kits provided by Nanjing Jiancheng Bioengineering Institute. Part of the liver were used for hepatic histology test, and the others were homogenized at 1:9 in cold normal saline (NS), and centrifuged at 3000 rpm for 15 min, and the supernatant was collected for the analysis of transaminase (AST), alanine aminotransferase (ALT), superoxide dismutase (SOD), glutathione peroxidase (GSH-XP), alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) by kits provided by Nanjing Jiancheng Bioengineering Institute.All tests were performed according to the kit instructions.
Observation of histopathological changes
The rest of the mouse livers from different group were fixed in 10% formalin solution for 48 h. After standard steps of frozen section and hematoxylin-eosin (HE) staining, conventional HE staining sections were prepared and histopathological changes were observed under optical microscope.
Statistical analysis
The data of ethology test were expressed as Mean ± SEM. The rest of the data were expressed as Mean ± SD. All the data were analyzed by one-way ANOVA using Graphpad Prism 5 software and P < 0.05 was considered statistically significant.
Results
Effect of JJGY on hepatic enzyme activity
The increasing of hepatic AST and ALT usually indicates the injury of liver. In Figure 1, compared with model group, the hepatic AST and ALT activity of AH, BH, AB groups and HWJZ group were significantly decreased (Figure 1a, 1b). The AST activity of AH group reduced by 18.32 U/mL compared with the model group, reaching 37.08 U/mL, and the ALT activity reached 119.20 U/mL, better than(AST 39.40 U/mL, ALT 137.7 U/mL).
ADH and ALDH constitute the ethanol dehydrogenase system, which is involved in ethanol metabolismfor humans and animals. As a key enzyme for the main short-chain alcohol metabolism in organisms, it plays an important role in many physiological processes, such as being the major way to metabolize alcohol in the liver. After administrating alcohol, the ADH and ALDH level in the mice (Figure 1c, 1d) increased significantly, which can be obtained from the difference between the control group and model group. AH, BL, BH and AB group obtained higher enzyme level comparing with the model group and even HWJZ group, suggesting higher efficiency in metabolizing alcohol.
GSH-PX is able to eliminate hydrogen peroxide in the body. The hepatic GSH-PX activity were little influenced by BL and BH treatment. AL, AH, AB and HWJZ groups, however, showed significant increasing (), compared with model group (Figure 1e), with AH group (1324U/L) increased the most.
SOD can specifically remove superoxide anion radicals produced in the oxidative metabolism of the body, thus delaying senility and enhancing autoimmunity. The results of the hepatic SOD activity showed that AH, BL and AB groups were significantly different from model group (), and the degree was greater than HWJZ group (Figure 1f).
Taking the above biochemical data into consideration, AH and AB group was superior to the HWJZ group, indicating thathas a certain effect in reducing hepatic cell damage and increase the alcoholic metabolism, and is superior to the positive drug.
Effect ofon hepatic tissue
The outcomes of the HE staining of liver slices from different group are shown in Figure 2. In the control group, the liver tissue structure was complete and clearly displayed, and there was no significant degeneration, necrosis or inflammatory cell infiltration. However, in the model group, the liver tissue structure of mice changed significantly--- hepatocytes were swollen and deformed, necrosis and fat vacuoles of different sizes were observed locally. The alcoholic liver injury was significantly improved in the AL, AH and HWJZ groups as most of the cell morphology maintained basically normal. Compared with the result of the group medicated before alcohol, local necrosis, deformation of hepatocytes, and inflammatory infiltration in livers were observed in the BL and BH group. Additionally, higher dosage of each formula all resulted in a greater effect of protecting the liver cells from alcoholic injury.

Figure 1. Effect ofJJGY on hepatic AST, ALT, ADH, ALDH,GSH-PX and SOD
The livers were collected from acute alcohol poisoningmice after ethology test, then they were homogenized at 1:9 in cold normal saline (NS), and centrifuged at 3000 rpm for 15 min, and the supernatant was collected for the analysis of transaminase (AST, 1a), alanine aminotransferase (ALT, 1b), glutathione peroxidase (GSH-PX, 1e), superoxide dismutase (SOD, 1f), alcohol dehydrogenase (ADH, 1c) and aldehyde dehydrogenase (ALDH, 1d)with Mk3 fluorescence microplate System. Compared with control group, **, ***, compared with model group, #, ##, ###.

Figure 2. Effect of JJGY on hepatic tissue
Mouse livers from different groups were fixed in 10% formalin solution and processed by hematoxylin-eosin (HE) staining. Conventional HE staining sections were prepared and histopathological changes were observed under optical microscope.
Effect of JJGY on serous enzyme activity
Hepatic injury can also be determined by serous AST, ALT, MDA and SOD activity. In Figure 3, except for AL and BL group in ALT activity test, all the experimental groups’ serous AST and ALT activity have significant difference to model group (Figure 3a, 3b). The greatest reduction is AH group with its serous AST and ALT activity reaching 41.33 U/mL and 1.70U/Ml, better than the positive drug(AST 47.29 U/mL, ALT 26.72 U/mL). Compared with model group, the serous MDA contents in all the experimental groups and HWJZ group were significantly decreased (Figure 3c,), and the serous SOD activity in AL, AH, BH AB and HWJZ groups were significantly increased (Figure 3d,or). SOD activity in AH group (317.8 U/L) increased more than HWJZ group (281.3 U/L).These indicate thatcan effectively reduce the hepatic cell damage, and is superior to the positive drug.

Figure 3.Effect ofJJGY on serum AST, ALT, MDA and SOD
Wholebloodwas collected from the orbit of acute alcohol poisoningmice after ethology test. The blood was centrifuged at 2500 rpm for 15 min and the serum was collected. The activities of serum aspartate transaminase (AST, 3a), alanine aminotransferase (ALT, 3b), malondialdehyde (MDA, 3c) and superoxide dismutase (SOD, 3d) were analyzed with Mk3 fluorescence microplate System.
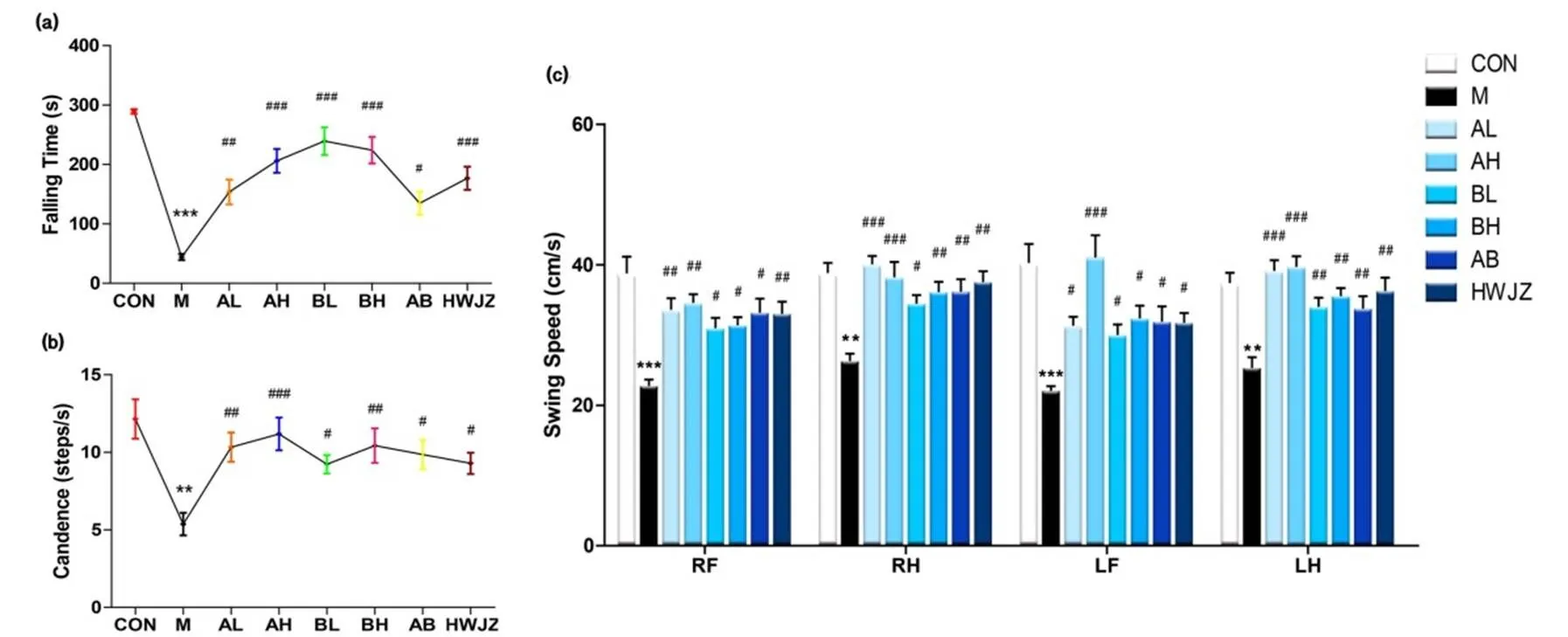
Figure 4.Effect of JJGY on motor coordination.
After setting up the acute alcoholic intoxication model, the mice were placed on a rota-rod analyzer to record the Falling Time (4a) after adapting the environment. After plenty of rest, the mice were placed in the catwalk analysis system for recording Candence (4b) and Swing Speed (4c) after adapting the environment. Compared with control group, **, ***, compared with model group, #, ##, ###.

Figure 5.Effect ofJJGY on gaits of mice.
After motor coordination test, mice got plenty of rest. Then, the mice were placed in the catwalk analysis system for recording Run Duration (5a), Walking Speed (5b), Step Cycle (5c), Timing View (5d, RF, right front; RH, right hind; LF, left front; LH, left hind) and Footfall Patterns (5e, Aa, RF-RH-LF-LH; Ab, LF-RH-RF-LH; Ca, RF-LF-RH-LH; Cb, LF-RF-LH-RH; Ra, RF-LF-LH-RH; Rb, LF-RF-RH-LH). The giant of mice should have a certain regulation.
Compared with control group, ** P < 0.01, *** P<0.001, compared with model group, # P < 0.05, ## P < 0.01, ### P<0.001.
Effect of JJGY on ethology
The ethology test could reflect the abnormal excitability of the center and muscle retardation. In Figure 4, experimental groups and HWJZ group successfully increased Falling Time, Candence and Swing Speed, showing significant difference to model group. Among these, mice of BL group () stayed longest on the rota-rod analyzer (Figure 4a). Mice of AH group () had the greatest improvement in Candence (Figure 4b) and Swing Speed (Figure 4c).
As shown in Figure 5, compared with model group, the figures of Run Duration, Walking Speed Variation and Step Cycle in experimental groups were significantly decreased. Mice of AL group had the best improvement of Run Duration () and Walking Speed Variation () (Figure 5a, 5b), while mice of AL group (showed the greatest improvement in Step Cycle (Figure 5c). Representative illumination of Timing View (Figure 5d) shows the stand and swing duration of each mice. The gait of control group mice obtained obvious regularity while the limbs of mice in model group had longer contact time with the glass plate and shorten suspending time, suggesting the negative effects of alcohol on the gait. The gait regularity of the experiment group had different degrees of improvement, especially the AH group. Footfall patterns (Figure 5e) shows the order of the mice placing its four paws on the ground. Compared with control, the footfall patterns recognized by the system were significantly decreased in model group and had many red patterns. Footfall patterns in experimental groups were recognized more than model group, and had less red patterns. Among all the experimental groups, AH group showed the best improvement in footfall patterns.
In conclusion, data from both Figure 4 and Figure 5 indicate thatcan significantly regulate the slowness of movement and gait imbalance caused by acute alcohol poisoning, and improve central disorder, better than the positive drug.
Taking all of the experimental results into consideration, Formula A high dose (AH) group has the best effect in protecting liver and gastric mucosa, relieving the abnormal excitability of the center and improving muscle retardation.
Discussion
This study mainly usesexperiment to develop and investigate the therapeutic effect of a new type of natural anti-alcoholic formula,. The formula ofuses the theory of medicine and food homology. The movement coordination evaluation of acute alcohol poisoned mice and their liver function evaluation are used to determine the effectiveness of the prepared formula and compare the difference between administration before or after alcohol assumption.
Initially, when a large amount of alcohol enters the human body in a short time, it first undergoes enzyme metabolism in liver. When the amount of alcohol in the human body exceeds the oxidant metabolism ability of the liver, it will accumulate in the brain tissue. In this process,,can be used to protect alcoholic liver injury. The effect of the herbs is positive shown by the result that in the liver of the experimental group, AST and ALT activities significantly decreased while ADH, ALDH, GSH-PX and SOD activities increased; the contents of AST, ALT and MDA in serum decreased largely while SOD activity increased, proving less hepatocyte necrosis and more efficient alcoholic metabolism in rodents. The result of the HE staining of liver slices further demonstrated the efficacy of JJGY in liver protection.
Present studies have find out thatcan protect liver from two aspects: in terms of oxidative stress, it can improve the antioxidant capacity of liver tissue, reduce the concentration of endotoxin (LPS) and inhibit the expression of cytochrome P4502E1 (CYP2E1), thus reduce the production of free radicals as well as the liver injury caused by oxidative stress; in terms of lipid metabolism, under the action of AMPK 2 activation,can inhibit the transcriptional activity of sterol regulator binding protein 1c (srebp-1c) in a large extent, and further inhibit the synthesis of triglycerides (TG) [26]. Other study on the anti-liver fibrosis mechanism ofextract showed that it can reduce the expression of TIMP-1 MRNA in the liver of liver fibrosis rates and gradually restore the liver collagen degradation system, thereby reversing the liver fibrosis and playing a role in protecting the liver [27].
Besides hepatic damage, the greatest influence from excessive alcohol is in the central nervous system. Alcohol affects the nervous system differently depending on the amount of alcohol consumed and the individual's tolerance after alcohol entering the brain through the blood-brain barrier easily. Alcohol belongs to the central nervous system inhibitor and excessive drinking can cause acute poisoning syndrome. The rota-rod test and the Catwalk gait analysis method, which can reflect the disease or damage in the central nervous system as well as the influence of drugs on the motor coordination function, show positive result in the rodents treated byin the form of step order, gait, duration, step length of each paw, paw pressure and other parameters. The improvement is due to that the ingredient ofextra andare able to shut down abnormal central excitability whileis responsible for easing muscle retardation.also has protective effect on alcoholic liver injury [28]. This is the first application of using, instead of ordinary tea, to achieve the substitution of caffeine, which can effectively reduce the adverse reactions caused by caffeine [29]. A number of studies on the sedative and hypnotic effects ofhave found that its sedative effect is related to the regulation on related neurotransmitters, such as reducing GLU level, increasing 5-ht, GABA level, and regulating the expression of GLU receptor, etc., suggesting thatcan react on multiple receptors, which reflects the characteristics of multi-target and synergistic effects of traditional Chinese medicine [30].is also the first to use carnosine to improve the gait instability after drinking since emerging evidence shows that carnosine acts as a cytoplasmic Ca-H exchanger and forms stable conjugates with exercise-induced reactive aldehydes [31]. Altogether they can reduce the excessive excitement on cerebral cortex caused by alcohol, the obstacle of reason and the inhibition in vasomotor center, which may further prevent occurrence of stiff muscle, convulsive etc.
Additionally, reducing gastrointestinal alcohol absorption is achieved by the addition of milk powder and Konjac flour. Because oral intake of alcohol can directly damage the lipoprotein layer of gastric mucosa epithelium, thus damaging the gastric mucosal barrier. At the same time, hydrogen ions in gastric juice can infiltrate into the mucosal epithelium, causing various gastric diseases such as mucosal hyperemia and ulcer [32]. Further addition of,[33],[34],[35] is to promote diuresis and accelerate alcohol excretion, which may facilitate the anti-inebriation effect described above as well as decrease the injury caused by the alcohol finally.
Conclusion
There are many anti-inebriation products at home and abroad. This product can protect the acute alcoholic intoxication mice through multiple mechanisms, realizing protecting liver, relieving the abnormal excitability of the center and improving muscle retardation at the same time. The usage of medicine and food homology theoryproviding a new way to develop antialcoholismic drug homologous food and Chinese herbal medicine.
1. Gao WL, XH Wang. Drug Therapy of Alcohol Induced Acute Intoxication: Review of the Literature. Pharmaceutical and Clinical Research 2015, 23: 59-61.
2. González-Reimers E,. Alcoholism: a systemic proinflammatory condition. World Journal of Gastroenterology: WJG 2014, 20: 14660.
3. Liang J, RW. OLSEN. Alcohol use disorders and current pharmacological therapies: the role of GABA A receptors. Acta Pharmacologica Sinica 2014, 35: 981.
4. Harper C. The neuropathology of alcohol-related brain damage. Alcohol & Alcoholism 2009, 44: 136-140.
5. Fernandez-Sola` J,. Molecular and cellular events in alcohol‐induced muscle disease. Alcoholism: Clinical and Experimental Research 2007, 31: 1953-1962.
6. Preedy VR. The importance of alcohol-induced muscle disease. Journal of MuscleResearch & Cell Motility 2003, 24: 55-63.
7. Simon L,. Chronic binge alcohol consumption alters myogenic gene expression and reduces in vitro myogenic differentiation potential of myoblasts from rhesus macaques. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 2014, 306: 837-844.
8. L S, JS E, MP E. Alcoholic myopathy: pathophysiologic mechanisms and clinical implications. Alcohol research: current reviews 2017, 38: 207.
9. L B. The effects of alcohol consumption upon the gastrointestinal tract. American Journal of Gastroenterology 2001, 95.
10. O’Shea RS, S Dasarathy, AJ McCullough. Alcoholic liver disease. Hepatology 2010, 51: 307-328.
11. Ao X, Z Shu. Progress in studies of anti-inebriation products. Chinese Journal of Microecology 2012, 24: 1146-1149.
12. Kim MS, M Ong, X Qu. Optimal management for alcoholic liver disease: Conventional medications, natural therapy or combination. World journal of gastroenterology 2016, 22: 8.
13. Wen DC,. Effect of ASF (a compound of traditional Chinese medicine) on behavioral sensitization induced by ethanol and conditioned place preference in mice. Evidence-Based Complementary and Alternative Medicine 2014, 2014.
14. Wei X. The study of animal experiment on the sobering up effect of the common material for regulating ethyl alcohol metabolism and their products, in Hunan Agricultural University 2006, Hunan Agricultural University: Changsha.
15. Hongwei L,. Influence of complex prescription antialcoholismic drugon acute ethanol intoxication. Modern Journal of Integrated Traditional Chinese and Western Medicine 2007: 3310-3311+3422.
16. Nagasawa T,. In vitro and in vivo inhibition of muscle lipid and protein oxidation by carnosine %J Molecular and Cellular Biochemistry 2001, 225: 1-2.
17. Jing W,. Puerarin extraction and identification of solution wine to protect liver function in mice. Journal of Regional Anatomy and Operative Surgery 2015, 24: 358-361.
18. Gao XQ,. Sober-Up Effect of Radix Puerariae and Flos Pueraria for Treating Acute Alcohol Poisoning Mice. Journal of Food Science and Biotechnology 2012, 31: 621-627.
19. Du JA,. Semen Hoveniae extract protects against acute alcohol-induced liver injury in mice. Pharmaceutical Biology 2010, 48: 953-958.
20. Gao X,. Cellular antioxidant, methylglyoxal trapping, and anti-inflammatory activities of cocoa tea (Camellia ptilophylla Chang). Food & Function 2017, 8: 2836-2846.
21. Zhai X,. The sedative and hypnotic effection and effects on EEG in insomnia rats of raw and processed Semen Ziziphi spinosae. Pharmacology and Clinics of Chinese Materia Medica 2015, 31: 94-97.
22. Chen LY,. Protective Effects of Different Extracts of Imperatae Rhizoma in Rats with Adriamycin Nephrosis and Influence on Expression of TGF-beta1, and NF-kappaB p65. Zhong yao cai = Zhongyaocai = Journal of Chinese medicinal materials 2015, 38: 2342-2347.
23. Yimam M,. A Standardized Composition from Extracts of Myristica Fragrans, Astragalus Membranaceus, and Poria Cocos Protects Liver from Acute Ethanol Insult. Journal of Medicinal Food 2016, 19: 780.
24. Li Y,. Effects of Puerariae Decoction Granules and Extracts of Pueraria Lobata on Acute Alcoholic Liver Injury in Mice. Pharmaceutical Journal of Chinese People's Liberation Army 2018, 34: 127-130.
25. Shuai TG, M Wang, G Zhong. Protective Effect of Konjac Flour on Acute Alcohol-Induced Brain Injury in Mice. Food Science 2018, 39: 207-213.
26. Qing GX. Effects of pueraria root and pueraria flower on alcohol and liver protection and its mechanism, in Jiangnan university 2013, Jiangnan university.
27. Liu X, H Zhang, F Wang. The effect of FHD seed extract on the expression of timp-1 and mmp-13 in liver tissue of experimental rats. Chinese journal of traditional Chinese medicine 2006, 31: 1097-1100.
28. Liu WH, TC Liu, MC Yin. Beneficial effects of histidine and carnosine on ethanol-induced chronic liver injury. Food and chemical toxicology 2008, 46: 1503-1509.
29. Xu J. Comparison of four phytochemical constituents of tea group and study on the effect of theacrine on central nervous system, in Shenyang pharmaceutical university 2006, Shenyang pharmaceutical university.
30. Bo GH, C Zhang, H Wang. Research progress of central pharmacological experiment of suanzaoren decoction. Henan traditional Chinese medicine 2019, 39: 307-311.
31. Matthews JJ,. The Physiological Roles of Carnosine and β-Alanine in Exercising Human Skeletal Muscle %J Medicine & Science in Sports & Exercise 2019.
32. Tarnawski A,. Alcohol injury to the normal human gastric mucosa: endoscopic, histologic and functional assessment. Clinical and investigative medicine. Medecine clinique et experimentale 1987, 10: 259-63.
33. Rigelsky JM, BV Sweet. Hawthorn: Pharmacology and therapeutic uses. American Journal of Health-System Pharmacy 2002, 59: 417-422.
34. Shun LL, WJ Shi, FC Wang. A Review of Chemical Constituents, Medicinal Function of Rhizoma Imperatae and Their Application in Health Care Products Development. Journal of Anhui Science and Technology University 2011, 25: 61-64.
35. Rios JL. Chemical Constituents and Pharmacological Properties of Poria cocos. Planta Medica 2011, 77: 681-691.
:急性酒精中毒已成为当代中国的第三大社会问题,而目前市面上的主流解酒产品主要是单一机制,通过提高与酒精代谢有关的酶活性来加速酒精代谢。因此,我们研发了一款可以同时做到保肝、减缓中枢异常兴奋、改善肌肉松弛的新型解酒配方——九酒归一。:给予0.12 ml/10g 50%乙醇灌胃,建立小鼠急性酒精中毒模型。连续20天给予九酒归一灌胃。接着,将小鼠随机分成8组,每组8只,分别为对照组(CON)、模型组(M)、海王金樽阳性对照组(HWJZ)、实验组(AL, AH, BL, BH, AB)。检测各组小鼠的步态、转棒爬行时间、肝脏和血清中的AST、ALT、SOD活性、血清中的MDA活性、肝脏中的ADH、ALDH、GSH-PX活性以及肝脏切片。:与模型组相比,九酒归一使肝脏和血清中的AST、ALT活性、血清中的MDA活性明显降低,同时使肝脏中的ADH、ALDH以及肝脏和血清中的SOD活性明显升高,显示肝脏代谢酒精能力增强、肝损伤降低。HE染色同样证明了九酒归一可以减少干细胞损伤,并且先药后酒组效果更好。而且,九酒归一明显延长了急性酒精中毒小鼠的转棒爬行时间,改善了急性酒精中毒小鼠的步态。:本研究证明,九酒归一可以有效保肝、减缓中枢异常兴奋、改善肌肉松弛。其作用机制主要是调节酒精代谢酶和抗氧化酶活性.
九酒归一、急性酒精中毒小鼠、药食同源、行为学检测
:Zhou YF, Zhang TZ,The Protective Effect of Jiujiuguiyi, a Medicine and Food Homologous Formula, on Acute Alcohol Poisoning Mice. TMR Modern Herbal Medicine, 2019, 2(3): 121-130.
10.12032/TMRmhm2017A49.
Submitted: 13 June 2019,
19 June 2019,
Rong-Rong He & Yi-Fang Li, Institute of Traditional Chinese Medicine & Natural Products, College of Pharmacy, Jinan University, Guangzhou 510632, P. R. China, Tel (Fax): +86-20-85221559, E-mail: rongronghe@jnu.edu.cn (R.-R. He) & liyifang706@jnu.edu.cn (Y.-F. Li).
Abbreviations:JJGY: Jiujiuguiyi (JJGY), AST: aspartate transaminase, ALT: alanine aminotransferase, MDA malondialdehyde, SOD: s uperoxide dismutase, GSH-XP: glutathione peroxidase.
This work was Financial supported by China College Students' Innovation and Entrepreneurship Project (Grant NO. CX18012 and 201910559129S)
Competing interests: The authors declare that there is no conflict of interests regarding the publication of this paper.
Executive Editor: Jing Sun
# Both authors contributed equally to this work.
20 June 2019.
猜你喜欢
杂志排行
TMR Modern Herbal Medicine的其它文章
- Erahertz spectral analysis of Xiling Zhimu with different geological conditions and plant age
- Analysis of drug use law and mechanism of prostate cancer based on data mining and network pharmacology
- Mitochondrial membrane stabilization by Angelica sinensis polysaccharide in murine aplastic anemia
- Effect of Tanshinone IIA on LPS-induced inflammatory response in a ROS-NLRP3 inflammasome dependent manner in RAW264.7 cells
- Application Progress of Porous Materials in Modern Pharmaceutical
