AuPd和AgPd枝晶纳米催化剂催化甲酸分解制氢
2019-08-08谢佳琦吴新华兰立新
刘 军 谢佳琦 吴新华 李 容 兰立新
(湖南化工职业技术学院,制药与生物工程学院,株洲 412000)
0 Introduction
Among various power sources,hydrogen energy is attracting increasing attention on account of its cleanness and sustainability[1].However,storage and transfer of hydrogen gas are difficult with current technology because hydrogen gas is flammable and has a poor volumetric energy density.Noteworthily,formic acid has been considered as a promising in situ hydrogen source by catalytic decomposition[1-19]because it offers relatively high volumetric energy density,is non-toxic,can be safely handled in liquid and in aqueous solution,and can be derived from hydrogenation of CO2[20-21].Decomposition of formic acid through two possible pathways is as follows[2,6]:

Reaction (1)is desired for hydrogen production and Reaction (2)should be avoided because CO is highly poisonous to catalysts.The content of carbon monoxide in hydrogen gas should be less than 10-3%for direct use in fuel cells[2].
Recently,considerable progress has been made to obtain ultrapure hydrogen gas effectively from formic acid with different catalysts.For example,homogenous catalystsof metallic complexes(e.g.Ru and Fe complexes)showed excellent catalytic activities at ambient conditions towards the decomposition of formic acid in an inert atmosphere and in the presence of formate[10-11],amine[12-15]or in an organic solvent[16].On the other hand,much more efforts have also been made recently in developing heterogenous catalysis of formic acid decomposition to surpass the efficiency by homogenous catalysis.Some nanocatalysts dispersed in aqueous solution of formic acid containing formate have also shown high activity for hydrogen production from decomposition of formic acid.Core-shell Ag@Pd/C nanoparticles[2],Pd/C catalysts[3],Pd-B-P/C[4],Au/ZrO2catalyst[5],Pd/C[6],TiO2-supported AgPd@Pd nanocatalysts[9],AgPd nanoparticles[17],AuPd/C[18],and agglomerated Ag-Pd catalyst[22]are such representativenanocatalysts.However,further improvements of nanocatalysts in activity and selectivity are still requested to obtain high-quality hydrogen gas by decomposition of formic acid at room temperature.
As far as we knew,nanoporous alloy materials like AuPd[23],PdAg[24],PtNi[25],PtFe[26],PtCo[27],and PtRu[28-29]prepared by dealloying or hydrothermal method have also received considerable attention for electrocatalytic oxidation of small organic molecules.We first demonstrated that noble metal alloy foam materials of AuPt[30]with nanodendritic structures prepared by electrodeposition with a template of in situ produced hydrogen bubbles have high electrocatalytic activity for the oxidation of formic acid.Since the pioneer work on the electrodeposition of Cu and Sn foams[31],the hydrogen bubble dynamic template approach has been employed to prepare a lot of monometallic and bimetallic 3D porous foams,including Au[32-33],Ag[34-35],Ni[36],Bi[37],CuSn[38],PtPd[39],CuPd[40],Pt@AuxCu100-x[41],CoNi[42],and NiCu[43].This is a very facile,fast and environment-friendly method for the preparation of metal foams without using protective reagents and additional template reagents.
In this work,we made detailed investigation for the first time on the preparation of nanodendritic AuPd and AgPd foam film catalysts with different atomic ratios by direct electrodeposition using hydrogen bubble dynamic templates and on their performance in hydrogen production from decomposition of formic acid at room temperature.These nanodendritic foam film catalysts show excellent activity for the decomposition of formic acid under room temperature and have great potential for practical applications in fuel cells.
1 Experimental
1.1 Reagents and materials
HAuCl4·4H2O,AgNO3,Pd(C2H3O2)2and PdCl2were obtained from Sinopharm Chemical Reagent Co.,Ltd.(Shanghai,China).A gold disk (1 mm diameter,purity 99.99%),a platinum foil (geometric area 1 cm2)and Ti plates (geometric area 1 or 2 cm2)were purchased from Tianjin Ai Da Heng Sheng Technology Development Co.,Ltd. (Tianjin,China).Sulfuric acid (H2SO4),formic acid(HCOOH)and sodium formate (HCOONa)were purchased from the Factory of Hunan Normal University.All the chemicals were of analytical grade and were used as received.Milli-Q water with a resistivity of greater than 18 MΩ·cm was used in the preparation of aqueous solutions.
1.2 Electrodeposition of alloy foam film s
In order to save Au and Pd precursors,exploratory electrodeposition of prous foams were performed firstly on a CHI 660C electrochemical workstation(Chenhua Instruments,Shanghai,China)with a small gold disk (1 mm diameter,purity 99.99%)or a Ti disk (2 mm diameter,purity 99.99%)purchased from Good Fellows as the substrate (working electrode).A platinum foil (geometric area 1 cm2),and a saturated mercurous sulfate electrode(SMSE)were employed as the counter and reference electrode,respectively.Prior to use,the working electrode was polished with 2 000 grit carbimet paper,followed by rinsing in Millipore water under ultrasonic waves.Then the electrode was electrochemically pretreated by cycling the potential between-0.7 and 1.1 V in 0.5 mol·L-1H2SO4at a scan rate of 100 mV·s-1until a stable voltammogram was obtained.According to our previous experience for theelectrodeposition of AuPt[30]alloy foams,electrodeposition of AuPd foam films were performed under a constant potential of-4 V for 300 s in a stationary solution of 2 mmol·L-1HAuCl4+0.58 mmol·L-1PdCl2+2 mol·L-1H2SO4,1 mmol·L-1HAuCl4+1 mmol·L-1PdCl2+2 mol·L-1H2SO4,0.5 mmol·L-1HAuCl4+1.5 mmol·L-1PdCl2+2 mol·L-1H2SO4,and we denoted the deposited foam films as Au100Pd29,Au1Pd1,and Au1Pd3,respectively,according to the molar ratios of HAuCl4/PdCl2in the feeding solution.For comparison,monometallic Au and Pd foam films were deposited in the same way by substituting the mixed precursors with 1 mmol·L-1HAuCl4and 2 mmol·L-1PdCl2,respectively.AgPd foam films were also prepared by electrodeposition under-4 V for 300 s with precursor solutions of 1 mmol·L-1AgNO3+1 mmol·L-1Pd(C2H3O2)2+2 mol·L-1H2SO4.
An alternative two-step method was also employed to fabricate Pd-covered Au foams (denoted as Pd/Au).First,the gold foam was deposited under 4 V for 300 s in a solution of 2 mmol·L-1HAuCl4+2 mol·L-1H2SO4,then,immersed in Milli-Q water for 10 min to remove the absorbed AuCl4-.Finally,the gold foam film electrode was transferred into a solution of 5 mmol·L-1of PdCl2+0.1 mol·L-1HClO4to deposit Pd on the surface by galvanic replacement reaction for 45 min.The obtained Au/Pd bimetallic foam electrode was taken out and immersed into 100 mL Milli-Q water for 30 min to remove the absorbed precursor ions.
For convenience and practical applications,the alloy foam catalysts for the decomposition of formic acid were prepared with a direct current (DC)power (KXN-1550D)and a two-electrode configuration.A larger Ti plate (geometric area 2 cm2)substrate and a platinum foil (geometric area 1 cm2)were employed as the working and counter electrode,respectively.Prior to use,the Ti plates were polished with 2 000 grit carbimet paper,etched in 18%(w/w)HCl at 85 ℃ for 15 min to remove the surface oxide,and washed with Milli-Q water.Similar foam films can also be fabricated by electrodeposition under a constant voltage of 7 V for 200 s in different precursor solutions.
1.3 Determ ination of real surface areas
The real surface areas of these bimetallic and monometallic foam films were estimated from the double layer capacity measurement and the details of experiment and calculation were described in the Supplementary Information (Fig.S1,S2 and S3).
1.4 Characterization
The morphology images of the electrodeposited foam films were taken with a JEOL JSM-6360 scanning electron microscope (SEM)operating at 25 kV.TEM measurements were performed with JEOL-1230 electron microscope operating at a voltage 200 kV.Their bulk compositions were analyzed with an energy-dispersive X-rayspectrometer(EDS)using Tecnai 20.X-ray diffraction(XRD)analysis of the resulting products was carried out on a Dmax Rapid IIR diffractometer (Cu Kα radiation(λ=0.154 2 nm))in a scan range of 20°~120°,and the exposure time was 15 min at 40 kV and 40 mA.
测算末级渠系水价应在严格控制管护人员、约束成本以及清理、取消不合理收费的基础上,按照补偿末级渠系运行管理和维护费用的原则核定,末级渠系水价的计算公式为:
1.5 Catalytic decomposition of form ic acid and product analysis
The catalytic reactions of formic acid by the deposited foam films were performed at room temperature(~25 ℃)either in a test tube or in a beaker containing a solution of 6.64 mol·L-1HCOOH and 3.32 mol·L-1HCOONa.For anlalysis of gas chromatography(GC 5890F)[44],the produced gases were collected by the draining water method using a glass funnel and a gascollecting bottle (35 mL)that were placed upside down.In addition,a graduated gas-collecting tube was used to record the gas volume released during 2 h.The deposited foam catalysts can be activated repeatedly after being washed with Milli-Q water and dried under an infrared lamp,or treated by potential cycling in 1 mol·L-1H2SO4within the range of-0.65 to 1 V at a scan rate of 100 mV·s-1.
2 Results and discussion
2.1 Characterization of the electrodeposited AuPd foam film s
The SEM images in Fig.1 show the porous structures of the prepared AuPd foam films with different compositions by electrodeposition on Au disk electrodes (1 mm diameter)under a constant potential of-4 V for 300 s.The hierarchical pores in the forms were highly interconnected and look similar to each other(columns 1~2),resembling those of pure Au and pure Pd foams (Fig.S4).The pore size increased from bottom to top up to about 20μm due to the coalescenceof hydrogen gas bubbles during evolution from the substrate.The foams consisted of dense nanodendrites (column 3).Such dendritic AuPd alloy foams have larger surface area and provide more active sites like steps,corners,kinks and edges,which are beneficial to catalytic reactions[30].Similar foam structures were obtained using either Ti disks (2 mm diameter)or Ti plates (geometric area 2 cm2)under violent hydrogen evolution,which are given in Fig.S5 and S6.However,such foam structure was not observed (Fig.S7) while lessened the cathodic polarization extent from-4 to-0.8 V,where no severe hydrogen evolution occurred during electrodeposition.
The detailed dendritic structures of Au1Pd1foams were further characterized by TEM and high-resolution TEM (HRTEM).As seen in Fig.2a and 2b,the dendrites were comprised of abundant nanocrystals sizing from 10 to 30 nm.Such morphology indicated that the formation of dendrites underwent orientated attachment of deposited nanocrystals[45-47].The HRTEM image in Fig.2c reveals the atom lattice.The lattice fringe spacings of 0.234,0.224 and 0.229 nm corresponded to the interplanar distance of Au (111),Pd (111)and AuPd (111)planes[48-49],respectively.In our experimental conditions,the Au1Pd1foam film was rapidly electrodeposited in short duration.
The atomic compositions of AuPd foams were analyzed by EDS (Fig.3),which were roughly consistent with those of their corresponding precursor solutions(Table S1).It indicates that the foam composition could be controlled by the ratio of precursors.

Fig.1 SEM images of 3D porous AuPd alloys electrodeposited on Au disk electrodes (1 mm diameter)at-4 V for 300 s in 2 mol·L-1 H2SO4 containing different Au/Pd ratios of precursor concentrations

Fig.2 (a,b)TEM and (c)HRTEM images of the dendritic structure in the Au1Pd1 alloy foam

Fig.3 EDS spectra for the foam films of(a)Au100Pd29,(b)Au1Pd1 and (c)Au1Pd3
In order to further determine the feature of bulk phase,the AuPd foam films were characterized by XRD.Fig.4 shows the XRD patterns of the deposited AuPd alloy foams as well as pure Pd and Au foams.The diffraction peaks at 38.23°,44.34°,64.63°,77.65°and 81.89°were assigned to Au (111), (200), (220),(311)and (222)planes,respectively,according to PDF No.04-0784 of Au;and the diffraction peaks at 40.09°,46.47°,68.26°,82.33°and 86.88°were assigned to Pd (111),(200),(220),(311)and (222)planes,respectively,according to PDF No.46-1043 of Pd.The diffraction peaks of Au100Pd29,Au1Pd1and Au1Pd3samples fall well between those of pure Au and Pd,presenting a smooth transition from an Au-like pattern to a Pd-like pattern with the increase of Pd content.It suggests that the AuPd porous films are single-phase alloy nanomaterials.The detailed 2θvalues of all samples are presented in Table S2.

Fig.4 XRD patterns for foam films of pure Au,pure Pd and AuPd alloys
2.2 Hydrogen production from form ic aciddecom position on the AuPd foam film s
We found that the foam films of Au,Pd and AuPd alloys deposited on Au disk electrodes exhibited high catalytic activity in hydrogen generation from decomposition of formic acid at room temperature (~25 ℃).As demonstrated in Fig.5,vigorous gas released as soon as the foam film electrodes were immersed in the solution of 3.32 mol·L-1HCOONa+6.64 mol·L-1HCOOH.Among them,the Au1Pd1foam film catalyst showed the highest reactive activity for the decomposition of formic acid.Similar phenomena were also observed on the foam films deposited on Ti disk electrodes (2 mm diameter)and on the Ti plate (geometric area 2 cm2)for formic acid decomposition as shown in Fig.S8 and S9,respectively.

Fig.5 Photos for the hydrogen production at room temperature in 3.32 mol·L-1 HCOONa+6.64 mol·L-1 HCOOH from the decomposition of formic acid on the foam films of(a)Au,(b)Au100Pd29,(c)Au1Pd1,(d)Au1Pd3,and (e)Pd deposited on gold disk electrodes (1 mm diameter)

Fig.6 (a)Output volume of reforming gas (H 2+CO2)during 120 min on the porous film catalysts of Au,Au100Pd29,Au1Pd1,Au1Pd3,and Pd in 6.64 mol·L-1 HCOOH+3.32 mol·L-1 HCOONa at room temperature (~25 ℃);(b)Cycling performance of the Au1Pd1 foam film
The cumulation of CO at the foam film surface might be the reason for the decreased in decomposition rate of formic acid after 20 min.We found that the activity of the catalysts could be easily renewed by cleaning them in pure water and followed by drying in air under an infrared lamp for 2 h.During this treatment,the adsorbed CO was removed by oxygen species on the film surface resulted from the water and/or air.Fig.6b shows the results for repeated performances of the Au1Pd1foam film catalyst in formic acid decomposition.After the cleaning and drying treatment,the catalytic activity of Au1Pd1foam film was restored completely and the released gas volumes were almost the same for the two repeated performances,especially in the first 20 min.
The presence and removal of poisonous CO adsorbed on the AuPd foam films were confirmed by cyclic voltammograms in H2SO4solution (Fig.S11).On the Au1Pd1foam film without treatment by cleaning and drying there was a big sharp oxidation peak at 0.49 V in the first positive potential sweep,which is ascribed to the oxidation of adsorbed CO formed previously from the dissociative adsorption of formic acid.Meanwhile,hydrogen desorption peak vanished in the first positive potential sweep because the adsorbed CO inhibited the hydrogen adsorption.However,no such CO oxidative peak was observed for the Au1Pd1foam film after cleaning and drying.It can also be seen that one potential cycle in 1 mol·L-1H2SO4solution could remove the adsorbed CO because hydrogen desorption peak around-0.5 V reappeared.This is another convenient way to reactivate the catalysts.The repeated performance of the Au1Pd1foam film reactivated by potential cycles is shown in Fig.S12.
We also prepared Pd-covered Au (Pd/Au)foam films by galvanic displacement between Au foams and PdCl2solution because the Au nanodendrites were highly reactive.According to Chung and his co-workers,galvanic displacement of Au by Pt or Pd can only occur at places of active Au atoms[52],so the deposited Pd layer would be very thin.The Pd/Au foam film in Fig.S13 displayed similar morphology with that of AuPd alloy foam films (Fig.1).EDS analysis (Fig.S14)of the Pd/Au foam film showed that the average surface atomic ratio of Au to Pd was about 55.73∶44.27.However,the atomic ratio of Au to Pd varied from place to place because the active Au atoms on the dendritic Au foam film were not distributed uniformly.
A photo for the hydrogen production at room temperature from decomposition of formic acid on the Au/Pd foam film deposited on Ti plate was shown in Fig.S9d.The Pd/Au foam film catalyst possessed much higher activity for the formic acid decomposition (Fig.7)than pure foam films of Au and Pd (Fig.6a).101.6,7.89 and 61.40 mL·m-2reforming gas were obtained in 2 h on the foam films of Pd/Au,pure Au and pure Pd,respectively.However,the performance of Pd-covered Au foam film towards formic acid decomposition was a little poor than that of Au1Pd1alloy foam film (Fig.6a).

Fig.7 Output volume of reforming gas (H2+CO2)during 120 min with the Pd/Au foam film catalyst in 6.64 mol·L-1 HCOOH+3.32 mol·L-1 HCOONa at room temperature (~25 ℃)
2.3 Hydrogen production from form ic aciddecom position on bimetallic AgPd foam film s
We further deposited Ag1Pd1foam film and investigated its catalytic activity for formic acid decomposition at room temperature (~25 ℃).Fig.8 shows typical SEM images of the deposited Ag1Pd1foam film.As can be clearly seen,the foam film have similar hierarchical porous structures,resembling that of AuPd foam films.Nevertheless,the wall of Ag1Pd1foam(Fig.8c)consisted of much smaller and thinner dendtrites than those of AuPd foams in Fig.1.
The representative TEM images in Fig.9 (a,b)demonstrated that the prepared nanodendritic AgPd foam film comprise of samll nanocrystals sizing from 10 to 20 nm.Such morphology also indicated that the formation of nanodendrites underwent orientated attachment of deposited nanocrystals[45-47].Fig.9(c)shows the HRTEM image of an AgPd sprout.The lattice fringe spacings of 0.224 and 0.232 nm corresponded to the interplanar distance of Pd (111)(PDF No.46-1043)and Ag (111)(PDF No.65-2871)planes,respectively.The orientations of the crystal planes suggested that the growth direction was along the [111]axis.On the other hand,we found that most of the lattice fringe spacings were assigned to Pd (111)plane on the surface of AgPd nanoparticles.The reason would be that galvanic replacement reaction took place between the freshly deposited Ag and Pd2+ions in solution.This explantion was supported by EDS analysis shown in Fig.S15,where the atomic percentage of Pd in the AgPd foam was somewhat higher than that in the corresponding feeding solution.
The bulk phase of the AgPd foam film was characterized by XRD (Fig.10).The standard card of cubic Ag (PDF No.65-2871)and the XRD pattern of deposited pure Pd foam film are also shown in Fig.10 for comparison.The diffraction peaks of (111), (200), (220)and (311)planes both from pure Ag and from AgPd alloy appeared,suggesting that the Ag1Pd1foam film was a mixture of metallic Ag and AgPd alloy.

Fig.8 SEM images of 3D porous AgPd film electrodeposited on Au disk electrodes (1 mm diameter)at-4 V for 300 s in 2 mol·L-1 H2SO4 containing 1 mmol·L-1 AgNO3+1 mmol·L-1 Pd(CH3COO)2

Fig.9 (a,b)TEM,and (c)HRTEM images of the dendritic structure in the Ag1Pd1 foam film electrodeposited in the solution of 1 mmol·L-1 AgNO3+1 mmol·L-1 Pd(CH3COO)2+2 mol·L-1 H2SO4

Fig.10 XRD patterns of foam films of Ag1Pd1 and pure Pd
The Ag1Pd1foam film also showed high catalytic activity toward the decomposition of formic acid at room temperature,which would be observed by naked eyes(Fig.S16).Fig.11 gives the relationship between released gas volume and collection time.The original reaction rate on the Ag1Pd1foam was rather fast and about 128.2 mL·m-2reforming gas was released in the first 8 min and gave a maximum output of 279.5 mL·m-2reforming gas in 2 h,which was more than that on the Au1Pd1foam film catalyst due to the electronic effect[2].Comparison of catalytic activities of AuPd,AgPd foams and different nano-power catalysts for hydrogen generation from formic acid were showed in Table 1.Among all the catalysts tested,Ag1Pd1foam exhibited the high catalytic activity with the TOF value of 308 h-1at room temperature toward hydrogen generation from formic acid,which is higher than of most previously reported values.

Fig.11 Output volume of reforming gas (H2+CO2)during 120 min for the foam film catalyst of Ag1Pd1 in 6.64 mol·L-1 HCOOH+3.32 mol·L-1 HCOONa solution at room temperature (~25 ℃)
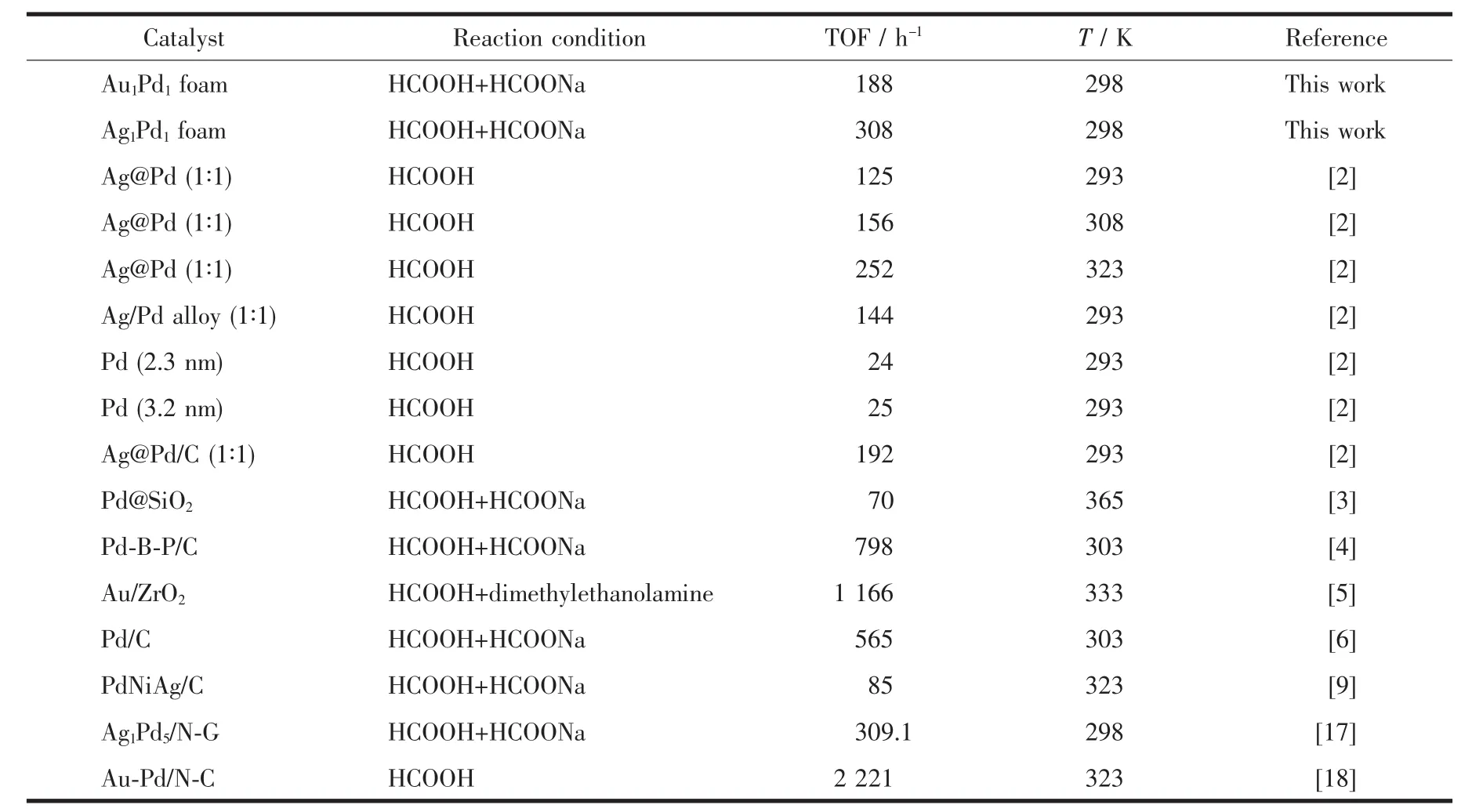
Table 1 Com parison of activities of different catalysts for hydrogen generation from form ic acid
3 Conclusions
To conclude,we have provided a facile and green way to prepare AuPd and AgPd foam film catalysts composed of special porous structures with nanodendritic walls.Among the prepared AuPd dendritic foam film catalysts,the foam of Au1Pd1displayed the highest catalytic activity for the decomposition of formic acid at room temperature,and the foam of Ag1Pd1was better than Au1Pd1.The high catalytic activity of the dendritic foam films of Au1Pd1and Ag1Pd1is attributed to the presence of abundant active sites like steps,corners,kinksand edges in thenanodendrites,and to theelectronic effect.Such supported thin films of nanocatalysts are easier to operate than that of dispersed nanoparticles and homogeneous catalysts because they are convenient for controlling,seperating and recycling.We hope that this work opens a new avenue to develop supported solid film nanocatalysts for hydrogen production from formic acid decomposition in aqueous solution.
Supporting information isavailable at http://www.wjhxxb.cn
