Age-related changes in resting-state functional connectivity in older adults
2019-07-17LaiaFarrPermanyerriaManchoForaMarcMontalFlaquerDavidBartrFazdiaVaquAlczarMaribelPerCebolleroJoanGurdiaOlmos
Laia Farràs-Permanyer , Núria Mancho-Fora , Marc Montalà-Flaquer David Bartrés-Faz , Lídia Vaqué-AlcázarMaribel Peró-Cebollero , Joan Guàrdia-Olmos
1 Quantitative Psychology Section, Faculty of Psychology, University of Barcelona, Barcelona, Spain
2 Department of Medicine, Faculty of Medicine and Healthy Sciences, University of Barcelona, Barcelona, Spain
3 Institute of Neuroscience, UB Institute of Complex Systems, University of Barcelona, Barcelona, Spain
4 Institut d'Investigacions Biomèdiques August Pi i Sunyer, Barcelona, Spain
Abstract Age-related changes in the brain connectivity of healthy older adults have been widely studied in recent years, with some differences in the obtained results. Most of these studies showed decreases in general functional connectivity, but they also found increases in some particular regions and areas. Frequently, these studies compared young individuals with older subjects, but few studies compared different age groups only in older populations. The purpose of this study is to analyze whole-brain functional connectivity in healthy older adult groups and its network characteristics through functional segregation. A total of 114 individuals, 48 to 89 years old, were scanned using resting-state functional magnetic resonance imaging in a resting state paradigm and were divided into six different age groups (< 60, 60-64, 65-69, 70-74, 75-79,≥ 80 years old). A partial correlation analysis, a pooled correlation analysis and a study of 3-cycle regions with prominent connectivity were conducted. Our results showed progressive diminution in the functional connectivity among different age groups and this was particularly pronounced between 75 and 79 years old.The oldest group (≥ 80 years old) showed a slight increase in functional connectivity compared to the other groups. This occurred possibly because of compensatory mechanism in brain functioning. This study provides information on the brain functional characteristics of every age group, with more specific information on the functional progressive decline, and supplies methodological tools to study functional connectivity characteristics. Approval for the study was obtained from the ethics committee of the Comisión de Bioética de la Universidad de Barcelona (approval No. PSI2012-38257) on June 5, 2012, and from the ethics committee of the Barcelona's Hospital Clínic (approval No. 2009-5306 and 2011-6604) on October 22, 2009 and April 7, 2011 respectively.
Key Words: brain connectivity; resting state; default mode network; aging; healthy; functional connectivity;resting state network; age groups
Introduction
Societal aging is a worldwide phenomenon with implications in many aspects of life, among which we can identify social implications and individual consequences. Regarding individual implications, various studies have confirmed that aging is linked to a decline in cognitive functioning (Damoiseaux et al., 2008; Onoda et al., 2012), even if we consider the population of healthy older individuals whose activities of daily living are within the normal range. Studies on cognitive decline confirm the shrinkage of global grey matter,the breakage of anatomical connections (Raz and Rodrigue,2006), alterations in the anatomical connectivity between regions (O'Sullivan et al., 2001), and a generalized decrease in functional connectivity (Damoiseaux et al., 2008; Onoda et al., 2012; Huang et al., 2015).
Age-related changes in brain connectivity (both anatomical and functional) have recently been studied in several articles, especially using functional magnetic resonance imaging (fMRI) in resting-state paradigms. Spontaneous blood oxygen level-dependent (BOLD) signals have been used to characterize regional functional connectivity and investigate changes in a variety of neurological and psychiatric disorders (Chen et al., 2011). This is a non-invasive method that provides an indirect proxy for the “ongoing” brain's hemodynamics (Gorges et al., 2014) and has become a powerful tool to investigate functional coordination between brain areas(Ystad et al., 2011). In particular, studies involving subjects in rest (in the absence of external stimuli) can reflect an intrinsic property of brain functional organization that serves to stabilize brain ensembles, consolidate the past, and prepare us for future events (Raichle and Snyder, 2007).
The most consistently identified resting state network(RSN) is the default mode network (DMN), which is a set of different brain regions with decreased activity during several tasks but increased activity during resting (Raichle et al., 2001). The main regions of the DMN are the medial posterior frontal cortex, the posterior cingulate cortex, the inferior parietal lobe and the hippocampus. Changes in DMN activity have been detected in studies related to different types of cognitive decline pathologies (e.g., mild cognitive impairment or Alzheimer's disease) and other disorders such as major depression (Li et al., 2002, 2012; Rombouts et al., 2005; Zhou et al., 2008; Binnewijzend et al., 2012). For example, Vemuri et al. (2012) found increased functional connectivity in DMN and other RSNs in Alzheimer's disease.In addition, recent studies have found that DMN is also affected in cognitively preserved older adults, as well as other RSNs (Onoda et al., 2012; Huang et al., 2015; Damoiseaux,2017).
Several studies that compared the connectivity between healthy young adults and healthy older adults found several changes in RSNs. For example, previous studies found increased inter-network connections and decreased intra-network connections, probably related to age-related cognitive decline (Geerligs et al., 2015; Huang et al., 2015; Grady et al., 2016). This increased number of connections in RSNs that occurs in healthy elderly is described as a compensatory mechanism to explain overactivation in connectivity (Seidler et al., 2010), but is also common in neurodegenerative pathologies (Vemuri et al., 2012). Age seems to be related with functional connectivity reductions, especially in integrated networks and affecting significantly anterior and posterior components of the DMN (Andrews-Hanna et al., 2007; Ng et al., 2016). The hippocampus is one of the most affected regions by this disruption, particularly between the posterior hippocampus to other hippocampal and DMN regions(Damoiseaux et al., 2016). Another study explains that aging disrupts not only the DMN but also other RSNs such as the sensorimotor (SM) or the visual (V), and these relationships appeared to be independent from grey matter changes (Onoda et al., 2012). Other regions, such as the right frontoinsular cortex, show decreased functional connectivity within the DMN in healthy older adults compared to young adults (He et al., 2013). Also, the Dorsal Attention Network exhibited age-related decreases, especially for long-range functional connectivity density (Tomasi and Volkow, 2012). Recently,Siman-Tov et al. (2017) compared the functional connectivity in different age groups (young, middle-aged, and old)and found that connectivity decline was already evident in the middle-aged group, suggesting that aging-related neural changes can start early in adulthood. Additionally, Ystad et al. (2011) found that age-related decline in cognitive processing speed was correlated to the fiber integrity between the subcortical nuclei, suggesting that widely distributed cortical connections from the participants provide an important hub for the distributed resting-state network connectivity.Related with brain segregation, one study found a decrease in segregation of brain systems defined by their patterns of resting-state correlations (Chan et al., 2014). In terms of functional cohesion, Betzel et al. (2014) found that RSNs corresponding to cognitive and sensorimotor functions undergo significant age-related refinement, in general becoming less functionally cohesive with age. Then, with age, stronger functional connections are mediated by less efficient and multi-step anatomical paths (Betzel et al., 2014). Longitudinal studies showed similar results, demonstrating changes in functional connectivity related with age, both increased and decreased connectivity depending on brain areas and networks, including putamen-occipital and frontal-occipital connectivity (Fjell et al., 2016, 2017). For a recent review of aging effects in structural and functional brain connectivity,see Damoiseaux (2017). In summary, the author concludes that changes, especially functional ones, seem to particularly affect the DMN, determining that older adults would have less connectivity between networks. In addition, the increase in age-related connectivity seems to indicate cognitive impairment, either present or in the near future. The results from whole brain data, and not focused on specific regions,suggest that those areas involved in DMN play a central role in global connectivity. There is also a lower functional segregation in advanced ages. Finally, the author concludes that the existence of changes in functional connectivity patterns in relation to age can be reaffirmed.
Although age is markedly related to changes in functional and anatomical connectivity, more studies are needed to expand upon the characteristics of brain functional connectivity in healthy older adults since some results from different investigations are still inconsistent. Most show decreased functional connectivity, but in other cases, increased connectivity was observed (especially between-networks functional connectivity) (Damoiseaux, 2008, 2017; Onoda et al., 2012;Geerligs et al., 2015; Huang et al., 2015). Additionally, Hirsiger et al. (2016) found no association between functional connectivity strength and age. These differences could be caused by the study characteristics and the approach chosen by the authors (such as cross-sectional versus longitudinal or whole-brain versus specific brain systems), therefore more research is needed to address these issues. Moreover, to the best of our knowledge, a comparison of brain connectivity amongst different age groups only in older adult populations has rarely been approached in the existing literature.
The main hypothesis in our work states that age affects the degree of cognitive decline differently, so we intend to compare connectivity patterns and characteristics in different age groups from middle to advanced age. Therefore, we contemplated two different objectives. First, we intended to analyze the whole-brain functional connectivity of each age group using a partial correlation analysis and a pooled correlation analysis. Second, we studied the network characteristics of the DMN through functional segregation.
Participants and Methods
Participants
The data used in this study included resting-state sequences from 114 healthy individuals aged 48-89 (68.93 ± 7.97) years(50% females) from three different studies conducted at the Department of Medicine, Faculty of Medicine and Health Sciences, University of Barcelona. All participants were independent in daily life activity.
The exclusion criteria included illiteracy or an inability to understand the protocol or undergo neuropsychological tests mentioned in the next section, prior cerebrovascular accident, any relevant psychiatric illness, advanced cognitive deterioration, dementia, or other neurodegenerative diseases(e.g., Parkinson's disease), any chronic illness expected to shorten survival (grave diseases such as heart failure, chronic liver disease, kidney failure, blood disease or cancer) and any MRI-related incompatibility (the presence of metallic objects within the body, pacemaker or claustrophobia). Inclusion criteria were related to the neuropsychological assessment,so they are described in Instruments section.
Written informed consent (Additional files 1-3) was obtained from each participant prior to taking part in the study in accordance with the Declaration of Helsinki and approved by the institutional ethics committees. Details of ethics committee agreement and affiliation are included in the section MR Image Acquisition.Although the original sample included 122 participants, in the present study, individuals with severe movement artifacts or incomplete rs-fMRI time series were excluded from the analysis. More specifically, three individuals were discarded because their absolute root mean square movement was above half a voxel (Power et al., 2012)and five participants had incomplete fMRI recordings. Therefore, the remaining sample of 114 participants was analyzed.
The participants were split into six age groups (< 60,60-64, 65-69, 70-74, 75-79, ≥ 80 years) to compare functional connectivity states in a healthy aging process (with the following group sizes: n1 = 12; n2 = 21; n3 = 29; n4 = 22; n5= 21 and n6 = 9). There were only two participants younger than 55 years old in the first group, and only four participants older than 85 years old. These ranges were selected to detect slight differences in relatively short aging intervals,and we considered that including these participants would not produce a noticeable impact in the results.
Instruments
With regard to the neuropsychological assessment, normal cognitive functioning according to age standardized norms was determined through a neuropsychological evaluation,including the administration of the Mini-Mental State Examination (MMSE), the Boston Naming Test (BNT), the National Adult Reading Test (NART), and the Vocabulary Scale in the Wechsler Adult Intelligence Scale (WAIS-Voc). Participants from two out of the three protocols were also evaluated with the Rey Auditory Verbal Learning Test (RAVLT) and the rest of the individuals were evaluated with the Grober and Buschke Test (BUSCHKE). These tests were used as exclusion/inclusion criteria to confirm that participants did not have any cognitive decline in terms of behavioral performance. Therefore, the performances were similar among all participants, because all of them must fit the classification criteria about absence of cognitive impairment. Consequently, the results in these tests have not been used as explanatory variables in any further statistical analysis.
The MMSE is a 30-item screening tool designed to evaluate orientations to time and place, immediate and delayed recall,attention and calculation, and language and visual construction (Folstein et al., 1975; Tombaugh and McIntyre, 1992).
Language ability and verbal IQ were assessed with the Boston Naming Test (BNT) (Goodglass et al., 1983), the Vocabulary Subtest of the Wechsler Adult Intelligence Scale 3rd Edition and the National Adult Reading Test (NART) (Nelson, 1982). The WAIS-Voc and the NART can also reflect information about premorbid IQ (Lezak et al., 2004), which is considered essential in healthy and cognitively impaired older adults (Solé-Padullés et al., 2009; Parra et al., 2013).
Verbal memory was assessed with the Rey Auditory Verbal Learning Test (RAVLT) (Rey, 1964) or the Grober and Buschke Free and Cued Selective Reminding Test (Grober and Buschke, 1987) depending on the protocol. These tests evaluate memory-related functions such as short-term auditory-verbal memory, the retention of information, and differences between free recall and delayed recall.
Level of education was also registered in every participant with the following categories: primary school (less than 8 years of education), secondary school (between 8 and 15 years of education) and university level (more than 15 years of education). Every age group of the proposed classification had participants from every level of education.
MR imaging MR image acquisition
All participants were scanned with a Siemens Magnetom Trio Tim syngo 3-T system at the Centre de Diagnòstic per la Imatge of Hospital Clínic (Barcelona, Spain). A high-resolution T2 and T1-weighted structural image was obtained for each subject with a magnetization-prepared rapid acquisition gradient-echo (MPRAGE) 3-dimensional protocol(repetition time [TR] = 2300 ms, echo time [TE] = 2.98 ms,240 slices, slice thickness = 1 mm and field of view [FOV]= 256 mm). A resting-state fMRI dataset was acquired with the subjects instructed to lay down with their eyes closed.The length of this acquisition was different depending on the sample:
· Protocol 1: n = 32 participants, TR = 2000 ms, TE = 16 ms, slice thickness = 3 mm, interslice gap = 25%, FOV= 220 mm, total: 5 minutes. The Ethics Committee from Comisión de Bioética de la Universidad de Barcelona approved this study on June 5, 2012 with approval number:PSI2012-38257 (Additional file 4).
· Protocol 2: n = 59 participants, TR = 2000 ms, TE = 16 ms, slice thickness = 3 mm, interslice gap = 25%, FOV =220 mm, total: 10 minutes. The Ethics Committee from Barcelona's Hospital Clínic approved this study on October 22, 2009 with approval number: 2009-5306 (Additional file 5).
· Protocol 3: n = 23 participants, TR = 2000 ms, TE = 19 ms,slice thickness = 3 mm, interslice gap = 25%, FOV = 220 mm, total: 5 minutes. The Ethics Committee from Barcelona's Hospital Clínic approved this study on April 7, 2011 with approval number: 2011-6604 (Additional file 6).
It is easy to see that protocols 1 and 3 recorded 150 dynamic points while protocol 2 recorded a total of 300 dynamics. This difference between protocols complicates the statistical processing of the data but there are two viable ways to remediate this setback. The first one consists in truncating the temporal registry of protocol 2 and only using the first 150 points so that all the input data has the same dynamics. The other solution to the aforementioned problem is to introduce a statistical weight to all the dynamic points and then globally average all the data (Hunter and Schmidt,2004). By doing this weighting, despite the protocol 2 has more dynamic points, they will have the same impact as the ones from protocols 1 and 3 on the overall count. In the present study, we implemented the latter solution and the data was treated so all the dynamic points had the same statistical weight in the overall sum (see Statistical Analysis)so no impact was expected to the functional connectivity as this studies involve only a resting state analysis and no other time dependent task was performed. Also a difference in the TE on protocol 3 is reported but it is so slight that no further effect was seen on the sample data between protocols.
The structural T2 images of every participant were revised to identify any possible abnormality before including it into the statistical analysis. No structural abnormalities or alterations were found in any participant.
Image preprocessing
The structural image data were analyzed using a FSL (FMRIB Software Library v5.0, Oxford, UK) preprocessing pipeline adapted under authorization from Diez et al. (2015), with its parameters adjusted to fit our experimental data. T1 images were reoriented to match the same axes as the templates and a resampled Anterior Commissure-Posterior Commissure aligned image with 6 degrees of freedom (df) was created.Then, all non-brain tissue was removed to obtain an anatomic brain mask that would be used to parcel and segment the T1 data images. The final step involved registering our structural data images to the normalized space using the Montreal Neurological Institute reference brain (Ashburner and Friston, 1999).
For the purpose of obtaining the functional connectivity matrices, the fMRI images were preprocessed using FSL as follows. First, a slice time correction based on the TR of the image acquisition was carried out to obtain thirty contiguous slices in the AC-PC plane. The input images were reoriented to match the template axes and motion correction was computed to co-register all of the volumes with the central one so that all of the voxels of the different volumes belonged to the same brain point. Then, all non-brain tissue was removed and, to get a better signal to noise ratio, the volumes were smoothed with a 6-mm Full Width at Half Maximum isotropic Gaussian kernel. Also, intensity correction and band pass filtering between 0.01 and 0.08 Hz were applied to the data.The resulting functional data images were registered and normalized to the standard MNI space. Finally, the white matter and the cerebrospinal fluid effects were removed so that no other interference was added to the fMRI signal.
To determine whether the subjects were able to stay still during the session, an extra step was added at the pipeline in which motion statistics such as DVARS (defined as the spatial standard deviation of successive difference images),the Framewise Displacement and Jenkinson's Framewise Displacement (Power et al., 2012) were calculated using FSL.This step allows for the computation of the movement of the subject during the MRI acquisition and permits discarding of the data if there has been substantial displacement. The entire preprocessing pipeline was implemented with the FSL software.
Regions of interest
The regions of interest (ROI) were defined by the Automatic Anatomical Labeling atlas (Tzourio-Mazoyer et al., 2002).This atlas contains 90 cortical and subcortical areas, 45 on each hemisphere, that are alternatively interspersed (i.e.,starting with the left precentral, the right precentral, the leftfrontal sup, the right frontal_sup, etc.). To acquire the full signal of a given ROI, it is necessary to compute an average over the entire time-series of all of the voxels of a given brain area following the Automatic Anatomical Labeling atlas(available by request).
Statistical analysis
One of the most recurrent analyses in functional connectivity is the correlation coefficient matrices computed from the fluctuations of the blood oxygen level-dependent (BOLD)signal. Two methods were used to obtain the functional connectivity: the partial correlation and the pooled correlation.
Neuropsychological assessment
One-way ANOVA was conducted to determine the existence of differences in test scores of neuropsychological evaluations among age groups using R version 3.5.2 (R Core Team,Vienna, Austria).
Partial correlation
The partial correlation is a matrix of N × N dimensions,where N = 90 is the number of ROIs that we intend to study and each element of the matrix represents the correlation coefficient between two given ROIs. Moreover, the truly important feature of this particular method is the fact that the partial correlation does not consider the contribution by the common neighbors to the correlation coefficient between any two ROIs.
The partial correlation can be computed from a standard correlation matrix defined as positive and invertible and it is given by:

where p = C-1is the inverse of the correlation matrix. Additionally, the partial correlation for all of the participants was computed using the partialcorr function implemented in MATLAB (R2016b, The MathWorks Inc., Natick, MA,USA).
Pooled correlation matrix
Since that the purpose of this work is to compare the connectivity structures between ROIs between age groups, the matrices of partial correlations obtained in each participant presented some differences between them, especially due to the fact that in some cases the duration of the sessions of registration were slightly different. This implied that some of the matrices of partial correlations showed slight changes in the final degrees of freedom. To avoid the minimum bias in the estimation of an average correlation matrix by age group,instead of using a direct estimation, a correlation matrix weighted by the number of registered brain volumes was obtained for each age group. The best option to estimate a unique correlation matrix by age group was to use the Hunter and Schmidt (2004) pooled correlation. The pooled correlation averages the simple Pearson correlation coefficients of the subjects within any of the aforementioned age groups over all of their time-points, including the effect of number of observations. Obviously, the pooled correlation matrix has an N × N dimension, where N = 90 represents the same ROIs as the ones evaluated in the partial correlation. The general expression is:

where n represents the time-points, r is the correlation coefficient between ROIs i, j and g is the number of subjects in this particular case. i and j are the row and column indicators of the correlation matrix. For instance, r34refers to the correlation between ROIs 3 and 4. k is the participant's indicator. It takes values form 1 to g, where g is the total number of individuals in the sample.
To compute the pooled correlation matrix from the subjects within a given age group, their simple correlation matrices must be homogeneous. This can be tested by computing the Q-test (Cheung and Chan, 2005), which assesses the uniformity of the variances among the correlation coefficients within an age group. The Q statistic follows a χ2distribution with N(N-1)/2 degrees of freedom and is expressed as:

where N is the total number of ROIs under study and is the mean of the correlation coefficients of each i, j pair of ROIs across the individuals of a given age group given by the letter g, i and j are the row and column indicators of the correlation matrix. For instance, r34refers to the correlation between ROIs 3 and 4. Moreover, the variance matrix was upper-triangularized, and the main diagonal was not taken into account. k is the participant's indicator. It takes values form 1 to g, where g is the total number of individuals in the sample. S stands for the variance among the correlation coefficients within an age group.
The hypothesis of homogeneity is rejected when any of the probability values obtained by testing individual correlation coefficients of the matrix are smaller than a significance level corrected for multiple comparisons, i.e., Bonferroni (Cheung and Chan, 2005). This condition is given by:
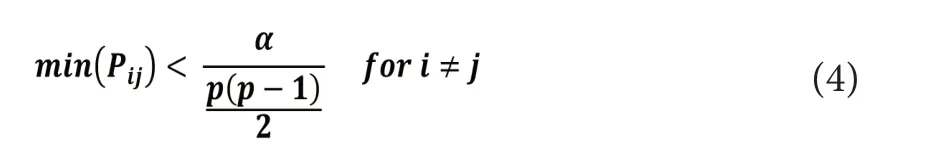
where Pijrepresents the P-value of the ROIs i and j, α is the significance level (0.05) and P represents the number of comparisons to be done.
Density of connections within the RSN regions
For each group, structures of synchronous activation were detected in the pooled correlation analysis. This subset of ROIs comprising DMN regions, visual areas, and sensorimotor areas was further analyzed through networks analysis,using pooled correlation coefficient as network links. Coefficient values greater than 0.2 were drawn to illustrate graph density, while a threshold of r ≥ 0.5 was used to highlight links with higher intensity of functional connectivity.
Network characteristics of the DMN: functional segregation
The functional segregation assumes that brain regions have the ability to develop specialized processing tasks by themselves and then integrate all of the information into more complex processing stages (Friston, 2011). This is possible because these brain regions are interconnected, forming groups and clusters in the already known functional networks. A simple measure of segregation is the clustering coefficient, which can be calculated by computing the fraction of triangles around a given node of the network. These triangles, or 3-cycles, represent the nearest functional neighbors of a ROI that are functional neighbors of each other (Rubinov and Sporns, 2010).
Since we have no anatomical information from tractography on the subjects under study, we cannot know whether their brain areas are strongly connected, but the pooled correlation matrices can be used to infer the intensity of the ROIs' signal correlation. Moreover, if we imagine the correlation coefficients between two brain regions as the definition of the edges of the 3-cycles, we can compute the areas of these triangles between neighboring ROIs. The areas of the cycles can be calculated using Heron's expression, which is:
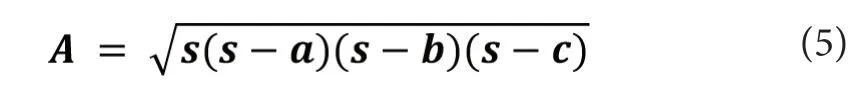
where a, b and c are the side lengths of the triangle and s is the semiperimeter, which is:

A threshold was applied to the 3-cycles to obtain only those areas whose sides were above 0.6 to get the most representative figure.
Results
Neuropsychological results
All of the individuals in our sample had scores higher than 24 in the Mini-Mental State Examination. In addition, no statistically significant differences were observed between the groups as determined by one-way ANOVA in either the National Adult Reading Test (F(5, 106) = 1.893, P = 0.102) or in the WAIS-Voc (F(5, 101) = 1.092, P = 0.369). Statistically lower scores in the BNT were detected in the oldest group compared to all of the others (F(5, 107) = 3.556, P = 0.005,Tukey's HSD adjusted P < 0.001) (Table 1).

Table 1 Statistical description of neuropsychological measures between age groups and significance of Q statistic
Functional connectivity
Partial correlation
After deleting the autocorrelations and the anticorrelations,no pattern was detected through the partial correlation analysis in any of the groups (it can be seen by request).Furthermore, the range (r) and median (Md) values for each age group were calculated: < 60 years old, r = 0.0026, 0.6792,Md = 0.1522; from 60 to 64 years old, r = 0.0100, 0.5840, Md= 0.1388; from 65 to 69 years old, r = 0.0198, 0.6201, Md =0.1428; from 70 to 74 years old, r = 0.0118, 0.5760, Md =0.1368; from 75 to 79 years old = 0.0113, 0.5725, Md = 0.1530;80 years old or older, r = 0.0003, 0.6767, Md = 0.1463. Thereby, the correlation between the majority of the regions was proven weak and non-significant, except for some sparse spikes along the matrix's main diagonal.
Pooled correlation matrices
As for the homogeneity hypothesis of the correlation coefficients, all P-values associated with the Q statistic were approximately 1 (Table 1), which implies that the values of each variance matrix were equivalent. However, given the number of sum terms in the calculation of the Q statistic(df = 4005) may be considered as a more descriptive than inferential approach to estimation of correlation homogeneity. Altogether, the Q statistic took small values for each age group, which indicates that the variances were small and leads us to conclude that the correlation coefficients were homogeneous.
Additionally, Figure 1 shows the intensity of the pooled positive correlations between Automatic Anatomical Labeling brain areas for each age group, where two main structures of synchronous activation can be detected in all groups. Namely, these structures comprise areas from 1 to 30 on the one hand and from 43 to 70 on the other hand, which include several DMN regions. Table 2 shows a detailed classification of the ROIs examined and the RSN they belong to.
The analysis of the pooled correlation across the six age groups reveals a decreasing pattern in the functional connectivity of the brain areas associated with aging. More specifically, this decrease is accentuated in individuals aged between 65 and 79 years. Interestingly, compared to this age group, the group of individuals ≥ 80 years old showed slightly higher correlation values between brain areas.
Figure 2 shows the decreasing pattern described above in relation to the number of statistically significant correlations in every age group. This pattern is maintained in different threshold values, from P = 0.05 to P = 0.00001.
Density of connections within the RSN regions
To further analyze the density of the functional connectivitynetworks across age groups, we studied the structures that arose in the whole-brain analysis, including the DMN areas and other sensorimotor (SM) and visual (V) regions involved in the resting state (Table 2). The distinction between the ventral DMN (DMNv) and the anterior DMN (DMNa)was determined by previous work with healthy older adults by Huang et al. (2015). The non-ventral or the non-anterior DMN regions were also considered and included in the DMN group classification. Graph plots were built through the qgraph (version 1.5) package for R version 3.5.2 (R Core Team, Vienna, Austria) (Epskamp et al., 2012).

Table 2 ROIs extracted from the AAL atlas and the RSN they belong to in all age groups

Figure 1 Intensity of pooled positive correlations between Automatic Anatomical Labeling brain areas for each age group.The color bar of the legend indicates the intensity of the correlations found between the brain regions, with 1 (yellow) representing a full positive correlation and 0 (deep blue) the lack of correlation between the regions analyzed.
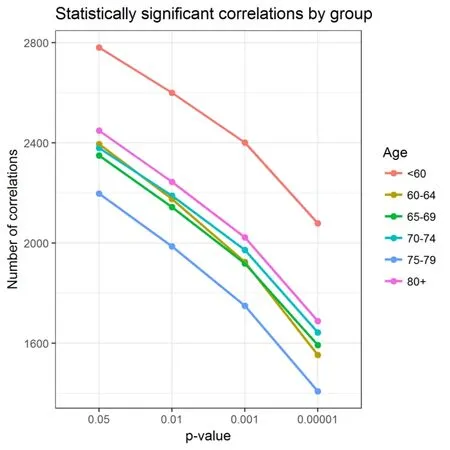
Figure 2 Statistically significant pooled correlations between group and threshold.There were 12 participants in the < 60-year-old group, 21 participants in the 60-64-year-old group, 29 participants in the 65-69-year-old group, 22 participants in the 70-74-year-old group, 21 participants in the 75-79-year-old group, and 9 participants in the ≥ 80-year-old group.
Figure 3 shows a network representation of the functional connectivity in each age group, where nodes are RSN brain regions and links are Pearson correlations between ROIs.Only correlations higher than 0.2 were drawn and correlations equal or higher than 0.5 were drawn in thicker lines.The first group of individuals, up to 60 years old, showed the highest density of functional connectivity compared to the other groups, especially in the visual and sensorimotor regions. Links between the visual region and the DMNv and between the sensorimotor region and the DMN showed high intensity in most cases. We also found high intensity in the regions of the DMN between them.
Participants between 60 and 64 years old and between 65 and 69 years old showed similar connectivity patterns related to the density and the intensity of the connections. A slight density decrease was observed in all regions, particularly in the sensorimotor region, the DMNv and the DMNa. Less intensity was also found in the connections of these structures,except in the visual system, which has a very similar pattern of density and intensity in those groups.
Individuals between 70 and 74 years old exhibited less intensity in their resting-state networks relative to the previous groups but not prominently in the density of the functional connections.
The group of participants between 75 and 79 years old showed the most significant changes in the density of the links and in the intensity, as Figure 3 shows. A remarkable reduction was observed in both cases and this group seemed to be the most affected relative to the loss of connectivity intensity and density compared to all of the other groups,including the oldest group.
In the oldest group including individuals aged ≥ 80 years,we found more functional connectivity compared to the other groups. The DMNv showed a density in its connections similar to that of the 60-64-year-old age group and an increase in the sensorimotor areas and the DMN regions compared to the previous groups. However, no regions reached the connectivity intensity and density shown by the younger group, which included participants aged < 60 years.
Network characteristics of the DMN: functional segregation
The 3-cycle regions whose edges, i.e., correlation coefficients,were over the 0.6 threshold were plotted (Figure 4) to obtain their frequency distribution and to highlight any difference among the groups. The Kruskal-Wallis sum rank test highlight differences in distribution among the age groups (χ2=38.97, df = 5, P < 0.001). At first glance, all groups showed the same tendency in their area distributions, i.e., more triangles of small areas and fewer cycles with larger surfaces.The number of triangles was not homogeneous across all groups (χ2= 147.39, df = 5, P < 0.001), with the group of individuals between 75 and 79 years old exhibiting a remarkable decrease in the overall counts, with evidence to be further discussed. It is important to notice that the older group presents fewer cycles of small areas but more cycles of medium and large areas.
Furthermore, for the purpose of obtaining a more precise description of the functional connectivity in each age group,we examined the statistical estimators of the triangles between the ROIs (Table 3). Although the main descriptors remain invariant across the groups, two parameters present variability: the number of triangles and the skewness.
The first value confirms what we have already stated: there is a non-negligible density variation of functional connectivity in the DMN regions. On the other hand, it is easy to observe that groups three and six show an increase and a decrease, respectively, in their distribution asymmetry.

Figure 3 Density of functional connectivity in age groups (Pearsoncorrelation rxy > 0.2, line width threshold: rxy ≥ 0.5).G1: Group 1 (< 60 years old, n = 12); G2: group 2 (60-64 years old, n= 21); G3: group 3 (65-69 years old, n = 29); G4: group 4 (70-74 years old, n = 22); G5: group 5 (75-79 years old, n = 21); G6: group 6 (≥ 80 years old, n = 9). DMN: Default Mode Network; DMNa: anterior Default Mode Network; DMNv: ventral Default Mode Network; SM: sensorimotor; V: visual. Each color shows a different resting state network included in the study.
Discussion
This study has two objectives. First, we intended to analyze the whole-brain functional connectivity of different older age groups through a pooled correlation analysis. Second,we studied the network characteristics of the DMN through functional segregation.
Regarding the first objective, the pooled correlation analysis showed evidence of a decrease in aging-related functional connectivity. Specifically, the groups including participants between 65 and 79 years showed this decrease more markedly as the age group advanced. However, the group of 80-yearolds and older showed increased functional connectivity compared to the younger age groups. Partial correlation results did not show any pattern in any of the groups.
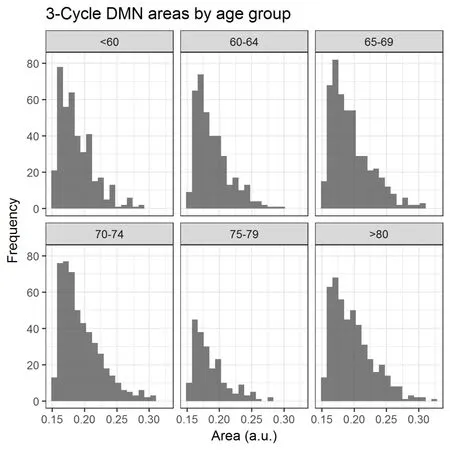
Figure 4 Histogram of the 3-cycle Default Mode Network areas by age group.The interpretation of brain regions as polygonal vertices makes possible to define the area comprehended between theses DMN brain regions,which were calculated with Matlab 2016b using Heron's expression. A histogram of the area values was performed to highlight the differences both in counts and distribution between age groups. The unit for Y axis is count.

Table 3 Statistical estimators of the triangles between ROIs in each age group
With regard to the second objective, we can discuss different results. The study on the density in the connections inside of the DMN showed a progressive decrease in density relative to aging, except for participants of aged 80 years or more, who showed denser functional connectivity in their RSNs. The study of functional segregation through triangles showed a similar number of triangles in the different groups, except for participants between 75 and 79 years old,who manifested an abrupt reduction in the number of triangles. Apart from the frequency of the triangles, their distributions resembled one another except for the participants aged 80 years or more, which showed less asymmetry than the others.
These findings confirm the results that were observed previously in other studies. Increased between-networks connectivity in older adults had already been identified (Betzel et al., 2014; Geerligs et al., 2015; Huang et al., 2015; Fjell et al., 2016) but not as noticeably in a particular age group including subjects older than 79 years old. This could be explained as a compensatory mechanism or a compensatory reaction, found in healthy older adults, as well as in mild cognitive impairment and in the early stages of Alzheimer's disease.
Before the age of 79 years, our results showed that every age group showed progressive but marked connectivity decline, particularly in intra-networks connectivity. These results are consistent with previous studies that described a disruption in the RSNs related with aging (Andrews-Hanna et al., 2007; Onoda et al., 2012; He et al., 2013; Ng et al.,2016) and are partially consistent with other previous studies(Tomasi and Volkow, 2012; Huang et al., 2015; Siman-Tov et al., 2017). Specifically, Huang et al. (2015) found a diminution in functional connectivity in the ventral DMN and in the sensorimotor and visual systems. Our results showed a marked decrease in the DMNv but not in the sensorimotor or visual systems. Especially in the visual system, we found a very similar functional connectivity pattern across the different age groups and a subtle decrease in the sensorimotor network, particularly in individuals between 60 and 69 years old. We also found a slight increase in functional connectivity in the sensorimotor system and the DMN after 80 years old, which is consistent with the results of Geerlings et al.(2015). In addition, Siman-Tov et al. (2017) found early significant reductions in the functional connectivity between the DMN, the visual system and other networks. They also found increased connectivity between specific regions of different networks that involved some DMN regions. These results potentially match ours, with marked functional connectivity decreases that appear early, but with specific increases in particular regions. We did not notice the described decrease in the visual network, but we found it in the other described regions. Therefore, the results of the present study did not match with that reported by Hirsiger et al.(2016), evincing an association between functional connectivity and age. Finally, in terms of functional segregation, our results are hardly consistent with those reported by Chan et al. (2014), showing an age-related decrease in segregation in different brain systems.
The present research provides new details on the effects of aging in the estimation of brain functional connectivity. Age has often been used as a continuum variable in correlation or regression in previous documents, so the use of age groups is new in this area. Studying age-related alterations in connectivity with age-group classifications also contributed to the clarification of which age shows more noticeable functional decreases and when a compensatory mechanism of this connectivity dysfunction could appear. Therefore, our results showed a progressive diminution in functional connectivity relative to the number of connections and their intensities up to 74 years old. Between 75 and 79 years of age, the most noticeable decrease appeared, with important reductions in intra- and inter-network connectivity and a lower intensity of these connections. Finally, from 80 years old onward,more connectivity than in previous groups was observed in our sample. These differences in functional connectivity comparing the number of correlations are statistically significant across different P-values. Therefore, noticeable changes related with the number of connections seem to occur while age draw on.
The presence of more functional connectivity in participants over 80 years old is probably related to compensatory mechanisms that have been thoroughly described, but also could be explained because of a high degree of resilience of these participants. Since they have shown a correct performance in neuropsychological assessment and they have surpassed their life expectancy, another valid reason could be just survivability. More specifically, participants older than 80 years old who are cognitively preserved could reflect better functional connectivity as our results evidenced. More studies regarding this population could help to clarify the observed differences in functional connectivity.
The study of functional connectivity and, especially, results visualization has become a challenge. Studying the whole brain implies working with a large amount of data and analysis techniques should be carefully selected. Therefore, we decided to use a 90-ROI atlas to simplify this information to study the most important brain regions related to resting-state networks.
Correlation matrices are commonly used in functional connectivity research and density maps are being used progressively because of their clarity in the identification of connectivity inter- and intra-networks and their intensities (Farràs-Permanyer et al., 2015). Therefore, using 3-cycle regions,we complemented the information from previous results.These analyses allowed for more details on the functional connectivity characteristics of our participants, thus complementing all of the results with more accuracy, specifically in the DMN network.
As it was mentioned before, the use of age groups is relatively new in this area. Studies usually distinguish between young and older individuals, or compare young, middle aged and older adults, as in Siman-Tov et al. (2017), but we did not find any article that studied one population, as older adults, dividing age groups. The criterion of group classification, separating every group by 5 years, was selected for different reasons. One reason was the intention to define groups with a similar number of participants, trying to form them as homogeneous as possible. Another reason was related to the aim of finding differences between groups: we had to establish a criterion that permitted to spot these differences and it had to be not so narrow (for example, 2 or 3 years)and not so wide (8 or 10 years). We did not expect to find many differences between groups with differences of 2 or 3 years, and we expected to find greater differences between participants differenced by 10 years. For these reasons, and having no examples of previous research with this kind of classification, we chose a 5-year distinction between groups.
There are some limitations that merit consideration in this study. First, resting-state fMRI includes the possible confounding of automatic bodily activity (for example, cardiac pulsations and respiratory rhythm) (Birn et al., 2006). It is important to keep in mind that this is an indirect measure of brain activity, which could involve inaccuracies, and it also provides much valuable information on brain functioning.
Second, the participants in our study came from three different protocols that used almost the same neuropsychological tests for assessment, except in the case of the memory examination. Two different tests were used for this evaluation depending on the protocol, namely the RAVLT and the BUSCHKE. Even though memory results could differ,these tests are admissible as exclusion criteria for individuals below the thresholds. For this reason, these tests were not taken into account in the later analyses.
Another limitation is the sample size, which is not homogeneous across the groups under study. More specifically, the first group (below 60 years old) and the last group (above 80 years old) include fewer individuals than the other groups.
Finally, we can identify two limitations related to the statistical procedure. On one hand, the threshold applied to the 3-cycle segregation analysis was selected to highlight the regions with a Pearson's correlation value above 0.6 and to avoid selecting those with links that had weaker intensities.However, further research should be conducted on the optimization of the intensity threshold. On the other hand, it is important to remember that the Q statistic used in the variance homogeneity test has limited statistical power. Nonetheless, it is more important to remark that it was not used for the purpose of hypothesis testing but rather to quantify the variances of correlation coefficients in each group.
It should also be noted that this study has some strong points to consider. The use of different analysis strategies reinforces all of the results and conclusions obtained, therefore increasing the results' consistency. In particular, 3-cycle areas allow for the confirmation of correlation matrices results and provide strong information about the characteristics of the functional connectivity in our sample.
Regarding the sample, it is important to emphasize that this study has a large sample of healthy older individuals.This large sample permitted the creation of different age groups from many age ranks, which allowed for the contemplation of the analysis. Usually, in older populations, participants present with symptoms of different diseases (physical and mental) that impede their participation in fMRI research. Therefore, an exhaustive evaluation has been conducted in this study to include only healthy older individuals of different ages.
In conclusion, we demonstrated that the characteristics of brain functional connectivity are related to aging. The age groups showed differences in the number of activated regions during resting state and differences in intra- and inter-network connectivity related to the number and intensity of the connections. We showed an aging-related progressive decrease in functional connectivity that was particularly apparent in individuals between 75 and 79 years old, with slight increased connectivity after 80 years. These results were consistent with previous studies that found age-related connectivity diminution and the existence of compensatory mechanisms in older adults (Andrews-Hanna et al., 2007;Onoda et al., 2012; Geerligs et al., 2015; Huang et al., 2015;Ng et al., 2016; Damoiseaux, 2017; Siman-Tov et al., 2017).All of the different estimators showed the same pattern. Finally, the present research could expand the understanding of brain functioning in various older age populations, and future studies in this area may provide more details about the associated characteristics.
Author contributions:Concept of study: JGO; design of study: JGO;literature search: LFP, NMF, MMF; definition of intellectual content:LFP, MMF, MPC, JGO; data analysis: NMF; data acquisition: DBF, LVA;statistical analysis: LFP, NMF, MMF; manuscript preparation, editing, review: LFP, NMF, MMF, MPC; clinical studies: DBF; guarantor: LFP, JGO;approval of final manuscript for publication: all authors.
Conflicts of interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Financial support:This study was supported by the Grup de Recerca en Tècniques Estadístiques Avançades Aplicades a la Psicologia (GTEAAP)members of the Generalitat de Catalunya's 2014 SGR 326 Consolidated Research Group (GRC) and was made possible by the PSI2013-41400-P project of Ministerio de Economia y Competitividad of the Spanish Government.
Institutional review board statement:Approval for the study was obtained from the ethics committee of the Comisión de Bioética de la Universidad de Barcelona (approval No. PSI2012-38257) on June 5, 2012,and from the ethics committee of the Barcelona's Hospital Clínic (approval No. 2009-5306 and 2011-6604) on October 22, 2009 and April 7, 2011 respectively. All participants provided written informed consent for their inclusion in the present study.
Declaration of patient consent: The authors certify that they have obtained all appropriate participant consent forms. In the forms, the participants have given their consent for their images and other clinical information to be reported in the journal. The participants understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Reporting statement: This study followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.
Biostatistics statement: The statistical methods of this study were reviewed by the professors in biostatistics and the author of this article, Dr.Joan Guàrdia Olmos.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: For data sharing, individual participant data,study protocol or informed consent will not be available. However, if there is any question about the details of statistical analysis plan or other information in the manuscript that need further explanation, researchers can contact the research group via e-mail to laiafarras@ub.edu.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Gabriele Siciliano, Universita degli Studi di Pisa,Neurological Clinic, Clinical and Experimental Medicine, Italy.
Additional files:
Additional files1-3: Model consent forms 1-3.
Additional files4-6: Ethical approval documents 1-3.
Additional file 7:Open peer review report 1.
杂志排行
中国神经再生研究(英文版)的其它文章
- Novel miRNA, miR-sc14, promotes Schwann cell proliferation and migration
- Role of behavioral training in reducing functional impairments after stroke
- Remodeling dendritic spines for treatment of traumatic brain injury
- Acute drivers of neuroinflammation in traumatic brain injury
- More than anti-malarial agents: therapeutic potential of artemisinins in neurodegeneration
- Why microglia kill neurons after neural disorders?The friendly fire hypothesis
