What does “Disruptive” mean?Thoughts on the NIH SCI 2020 meeting
2019-07-17VanceP.Lemmon
On September 12 and 13, 2019, the National Institute of Neurological Disorders and Stroke (NINDS), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), as well as other federal agencies and several private foundations sponsored a stake holder meeting at the National Institutes of Health (NIH) Bethesda campus with a provocative title: “SCI 2020: Launching a decade for disruption in spinal cord injury research”. Over the past decade,“disruptive” has become a cool buzz word for entrepreneurs to use to market their technology. In this context, disruptive means “innovative,ingenious, and unconventional” (typically definition #2 in online dictionaries). The hope is their new technology or business model is so powerful it will upend current technologies and come to dominate the market place. Uber's destruction of taxi companies worldwide is a leading example of being “disruptive”.
Over the past decade or so there are at least four examples of disruptive research impacting spinal cord injury (SCI) research: Park and his colleagues discovered that PTEN KO makes retinal ganglion cell axons regenerate (Park et al., 2008); Harkema, Edgerton and colleagues' studies suggest that epidural stimulation can enable walking in SCI patients(Harkema et al., 2011); Lu, Tuszynski and colleagues' demonstration that neural stem cell transplants produce dramatic axon growth and behavioral recovery (Lu et al., 2012). Kigerl and Popovich's microbiome studies uncovered a surprisingly strong interaction between the gut and SCI (Kigerl et al., 2016). These disruptive results have spurred a large amount of SCI research but translation to the clinic lags. Nonetheless,their impact shows that new ideas and approaches are critical for large steps forward. A meeting about disruption in SCI research over the next decade could not be ignored.
However, disruption has another and, indeed, primary definition:“unruly, rowdy, disorderly and attention-seeking”. It was hard to imagine that the SCI 2020 meeting would be about this. A review of the agenda, however, did not highlight new disruptive technologies or ideas that would help SCI researchers leap forward or quickly bring new therapies to the clinic. Perhaps the meeting organizers had this second definition in mind.
The meeting was kicked off by Michael Boninger, MD, from the University of Pittsburgh. He told a long and interesting fantasy version about SCI treatment in the year 2040. It involved lots of high-tech sensors used by first responders and physical therapists. There was a rapid infusion of therapeutics during the decompression surgery, followed two days later by the insertion of sophisticated electrodes into the cord and brain to provide functional electrical stimulation (FES). Intense rehabilitation was followed by another surgery involving biomaterials, stem cells,growth factors and chemoattractants. An undertone in the story was the neuromodulations will become standard of care, whereas stem cell therapies might only be used in special circumstances. This was, of course,followed by intense rehabilitation over many weeks. Dr. Boninger argued that to achieve this vision substantial changes in research strategies will be needed, including enhanced collaboration across institutions, research in higher species, as well as advocating for rehabilitation and psychological counseling. He was the first of many to argue that overly optimistic projections and press releases are counterproductive.
Next up was an SCI patient perspective by Robert Wudlick, who described his accident that occurred diving into shallow water and the subsequent treatments. This was followed by Lyn Jakeman, The Director of the NINDS Division of Neuroscience, who presented the organizers' vision for the meeting. She talked about the state-of-the-art in the field, such as advances in understanding the injury response and the interaction between the injured cord and the rest of the body. She also highlighted the NIH's perception that SCI researchers and other stakeholders are in silos that are limiting advances. She also pointed out that the pharmaceutical/biotech industry was poorly represented at the meeting. I observed that neurosurgery/spine surgery community was also largely missing. It was my take-away that industry and surgeons think the SCI field in particular, and central nervous system (CNS)injury in general, is not ripe for moving major therapeutic advances into the clinic in the next few years. She presented her vision for the meeting in two critical slides at 1:55 in the Day 1 video (Figure 1). Can the SCI community, scientists, clinicians and funding agencies agree on goals that are desirable and achievable. At some later time, stakeholders could decide how to achieve those goals.
The first panel, chaired by Linda Noble-Haeusslein, focused on the acute phase of SCI. Major barriers that need to be overcome include ensuring potential SCI patients are sent immediately to a level 1 trauma center. William Whetstone suggested we adopt the stroke model of treating within the first hour. Otherwise, appropriate interventions will never be delivered. Many SCI treatments that work in animals, fail in humans. A plausible explanation is that the animals are given the treatments in minutes to hours after the injury while humans rarely receive treatments this quickly. James Guest explained that a new strategy for informed consent to participate in an acute SCI clinical trial is needed. Many people argue that informed consent from an SCI patient is impossible in the first 24 hours, yet this is needed to participate in a clinical trial. Panel members, like Kim Anderson, said the SCI community could be a surrogate for a basic informed consent and then that could be used to get consent from the family. This kind of Community“consent” is intriguing, but an NIH representative corrected the panel by mentioning that this is a community consultation, and not consent.James Guest pointed out the litigious nature of surgery in the U.S. and this would need to be accounted for as well. Sasha Rabcjevsky was disruptive, saying that the focus on acute therapies is misguided; 95% of animal studies use acute models, yet > 95% of SCI patients are in the subacute or chronic state! An obvious counter argument is that 100%of chronic SCI patients were once acute patients. But his point is an important one.
The next panel, chaired by Michael Sofroniew, concerned strategies for repair focused on plasticity and regeneration. He presented his work on combining biomaterials with altering intrinsic mechanism in neurons to enable behavioral recovery. Other panelists discussed the variety of genes, molecular mechanisms and cell therapies that had proven effective in animal models. James Guest discussed the need for alternative trial methods, such as Bayesian or recursive partition, to speed human trials where small numbers of patients and very long treatment regimens are needed to assess effectiveness. A question was raised by a member of the audience but not answered: How can we decide which combination strategies are most likely to translate to effective therapies in the clinic? A couple of my colleagues and I wondered if machine learning strategies could somehow be brought to bear on this issue.
The final panel of the day had a provocative title: With Us, Not For Us: Community Activity and Priorities. It was composed of important SCI stakeholders; Matthew Rodreick, Robert Wudlick, Jennifer French,Kim Anderson-Erisman, Sasha Rabchevsky, John Chernesky and Barry Munro. All the individuals are leading representatives of the SCI community, have SCI or a family member with SCI. Prior to the meeting they launched a survey of the SCI community that had 1800 respondents and they reported important results. Of course, the SCI community wants breakthroughs that lead to significant improvements. But history shows this is difficult. They also want small improvements that impact aspects of daily living; bladder, bowel and sexual functions are extremely important but are not being addressed with sufficient urgency. Data show that life expectancy of people with SCI has not improved the last forty years, and in fact might be getting worse. They made a human rights argument that as individuals who have suffered SCI, they need to be involved throughout the SCI research cycle, helping to shape priorities as well as experimental strategies and tactics. They argued that less effort should be devoted to understanding Molecular Mechanisms Of Action and more effort should be devoted to testing undefined “low hanging fruit”. They raised the question about the need for endless safety trials. To help address that question, clearly input from the FDA is needed. At the end of the session, Barry Munro made a very emotional plea; “We are dying! People are dying! People are suffering!”(6 hours and 30 minutes in the Day 1 video) (Figure 2). He said that the SCI research community lacks urgency. He described the burden SCI individuals have, rising very early to go through their bowel and bladder programs to be ready for 8:30 meetings. However, many of the scientists in the audience were aware because they also get up early and stay up late 7 days a week for months at a time to care for scores of animals with bladder and bowel issues. He argued that the best way to fix the ineffective strategies being used is for the SCI community to seize control of the funding systems and reorder the priorities to focus on therapeutic targets that can have more immediate impact in the clinic.
This was clearly the main goal of the meeting. This was the “disruption in SCI research”. The effect on the audience was strong. Many of the researchers I spoke to were surprised and actually offended. Several leftthe meeting early, realizing that they had little to learn that could shape their research over the next few years.
An issue not addressed at this meeting that impacts SCI research is the ongoing threats scientists endure from animal rights terrorists at their work places and at the front doors of their homes.
I was struck that the complaints and new strategy being argued for by the SCI community ignored basic facts about how the drug/therapeutic development process worldwide works. Unless the “low hanging fruit” involves an FDA-approved drug or device, it will be unlikely that the fruit becomes a standard care in the SCI clinic in the next 10 years.For example, there are many studies on the time and money it takes to develop a new drug (for examples see DiMasi et al., 2016; McNamee et al., 2017) (Figure 3). Once scientists identify a disease process it takes, on average, 25 years to find a drug target, 4 or more years to find a compound that perturbs the target (1 year), modify the compound to give it drug like properties (2-4 years), and then 7 years to prepare the product for Phase 1 trials, run a Phase 1 trial, Phase II trials, Phase 3 Trails, submit a Regulatory Filing and obtain a decision. SCI clinical trails take much longer. This timeline may not incorporate the decades the scientists need to understand the biology of the system, develop in vitro and in vivo assays in animals that robustly mimic the human condition and then identify and validate the drug targets!
This new disruptive strategy also does not address the “elephant absent from the room” problem. The absence of pharma/biotech representatives from the meeting. To move therapeutics from the research lab to the clinic, massive resources are needed. In 2019, the average cost to bring a new drug to the market is about $2 Billion. This requires investment from public and private resources. Lately venture capitalists(VCs) are involved in early stage drug development and Big Pharma has become more risk aversive. The typical time that VCs want to earn a 5-10X return on their investment is 3-5 years! This is a big mismatch with the 20-30 year timeline for drug development.

Figure 2 Barry Munro appealing for help.SCI patients and advocates think funding agencies and scientists are working on the wrong problems and are concerned that SCI scientists lack a sense of urgency. (6 hours and 30 minutes in the Day 1 video).
Session 3 on disruption was followed by a poster session and social,giving time for the participants to digest and discuss what they heard during the day.
The next morning, the NINDS Director, Walter Koroshetz talked about research and the $80,000,000 per year spent by NIH on SCI research. He also noted that there are about 400 other neurological diseases and conditions that compete with SCI for research dollars and that you do not want to have any of those conditions. He also mentioned that the cure for SCI is likely to come from another field. It sounded like he was arguing for definition two of “disruption”, i.e. that innovative ideas from other areas will be needed.
Session 4, led by Edelle Field-Fote, focused on neuromodulation.Neuromodulation was defined as “inhibition, stimulation, modification,or therapeutic alteration of activity in the central, peripheral, or autonomic nervous system.” (Keller and Krames, 2009). A major theme was the importance of intense rehabilitation during recovery, based on both animal and human studies. But Michele Basso showed data that timing of training is critical to get the best outcomes. Some exciting FES studies were reviewed, showing that important daily living outcomes can be improved. But effective devices are not showing sustained availability in the market.
Session 5, led by Richard Shields, focused on secondary health problems of chronic SCI. Major points echoed Session 4, emphasizing the critical importance and large impact of rehabilitation and the emerging view that neuromodulation via electrical stimulation or transcranial magnetic stimulation has persistent beneficial effects. Importantly, the positive effects of physical therapy (PT) do not plateau within one year(Morrison et al., 2018). An alarming piece of data was that in the clinic over the past 40 years, the amount of PT SCI patients receive after injury has been cut in half and the rate of secondary complications has doubled (National SCI Statistical Center “Facts and Figures at a glance 2016”) (Figure 4). Of course, these are correlations and it is not possible to infer cause and effect from this data alone. But it needs urgent clarification and brought back emotional memories of Barry Munro's speech from the day before.
Session 6 was chaired by Jose Contreras-Vidal and discussed the revolution in neuroengineering and robotics and how it is influencing individuals with different degrees of SCI. Ann Spungen discussed exoskeleton devises, and mentioned that not all patients want one, and not all patients who want it can get one (due to complications such as severe bone loss). Importantly, the fact that a second person is needed for safety and support severely limits independence. Gregoire Courtine discussed epidural stimulation and emphasized that we must tailor combinations of biological repair and engineering strategies based on their specific mechanisms and their interactions. We will not be able to blindly combine stem cells with epidural stimulation and expect it to work (he said he has tried already).

Figure 3 Drug development is expensive,inefficient, and fraught with failures.Due to the very high failure rates resulting from safety or efficacy concerns, investors have very rigorous requirements concerning safety before they will pay for clinical trails. This figure is based on McMamee et al. (2017) and shows median times for 138 new drugs and biologics.
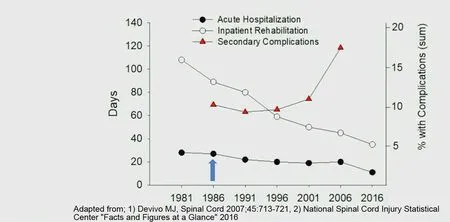
Figure 4 Secondary complications for spinal cord injury patients are increasing! 1:53 in Day 2 video.
The meeting ended with breakout sessions on research priorities for the next decade. There were sessions for each of the major topics of the meeting. The summaries of those sessions are:
1) Acute SCI a. Develop improved strategies for treatment of very acute SCI in the ER b. Identify biomarkers that can predict prognosis and treatment c. Develop improved pre-clinical animal models that include studies on therapeutic window d. Develop SCI centers of clinical excellence that can provide state of the art therapies and also conduct effective research
2) Plasticity and Regeneration a. What is needed to promote axonal growth, correct synapse formation and reconstruction of circuits b. What kinds of cells are in injury sites and what do they do?c. Develop technologies that can transform human and animal-based research to provide reliable outcome measures to quickly assess the effectiveness of treatments
3) Chronic SCI a. Encourage longitudinal clinical studies of outcomes important to people with SCI b. Expand studies on the use of neuromodulation (plasticity, rehabilitation, devices, pharmacology)c. Exploit big data of outcomes of clinal care to show cost effectiveness of interventions to influence insurance providers and regulatory agencies
4) More chronic SCI a. Develop decent common data elements to permit collection of data from multiple sites (this is not as easy as it sounds - VL)b. Identify lifestyle factors that reduce morbidity and mortality c. Improve therapies to provide safe and effective bowel, bladder and sexual function d. Improve our understanding of the interaction between SCI and systemic biology, especially the immune system, inflammation and the gut microbiome.
5) Robotics and Neuromodulation a. Expand research on devices that hold promise to improve functional recovery b. Make devices more user friendly and capable of use in the home c. Make devices more robust and fault tolerant
Summary: The SCI 2020 meeting is a wakeup call for the SCI research community. The very large worldwide community of individuals with SCI and their family members are justifiably concerned about the pace of progress. But mismatches between research system realities (“where is the innovation”, grant durations versus the time it takes to do meaningful chronic studies in animals and people, etc.), clinical trial funding mechanisms, FDA approval processes, and patient needs and expectations will require dramatic changes in strategies and tactics. Those were not addressed at this meeting. If the SCI patient community wants to use its frustration to accelerate the development of specific therapies, it may want to look at an aggressive top-down approach using a contract research organization model and have all studies done in parallel from the beginning to find robust therapies. Angel investors or foundations in it for the long haul will be needed. Alternatively, the National Center for Advancing Translational Sciences (NCATS) model of funding translational research might work, but rock solid therapeutic targets will be required. This strategy could lead to “disruptions” that could dramatically accelerate the pace of development of novel therapeutics.
Meeting Link: https://meetings.ninds.nih.gov/Home/Agenda/21041
Speaker List: https://meetings.ninds.nih.gov/Home/Speakers/21041
Meeting videos available on demand from NIH
SCI 2020 Day 1: https://goo.gl/rWGpw2
SCI 2020 Day 2: https://goo.gl/zbwVuf
Acknowledgments: Vance Lemmon is the holder of the Walter G. Ross Chair in Developmental Neuroscience. Feedback from anonymous colleagues helped shape this commentary. However, the opinions expressed here are his own and not those of the any funding agency or the University of Miami.
Financial support: Vance Lemmon is listed as an inventor on patents held by the University of Miami relevant to nerve regeneration. He is the co-founder of a drug discovery start-up working in early stage drug discovery relevant to cancer.
Vance P. Lemmon*
The Miami Project to Cure Paralysis, Department of Neurological Surgery; Center for Computational Science, Miller School of Medicine, University of Miami, Miami, FL, USA
*Correspondence to: Vance P. Lemmon, PhD,
VLemmon@med.miami.edu.
orcid: 0000-0003-3550-7576 (Vance P. Lemmon)
Received:February 28, 2019
Accepted:March 5, 2019
doi: 10.4103/1673-5374.255969
Copyright license agreement:The Copyright License Agreement has been signed by the author before publication.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCom-mercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Novel miRNA, miR-sc14, promotes Schwann cell proliferation and migration
- Role of behavioral training in reducing functional impairments after stroke
- Remodeling dendritic spines for treatment of traumatic brain injury
- Acute drivers of neuroinflammation in traumatic brain injury
- More than anti-malarial agents: therapeutic potential of artemisinins in neurodegeneration
- Why microglia kill neurons after neural disorders?The friendly fire hypothesis
