Adipose-derived stem cell conditioned medium for the treatment of amyotrophic lateral sclerosis:pre-clinical evidence and potential for clinical application
2019-07-17ChandlerL.Walker
Amyotrophic lateral sclerosis (ALS) is a devastating progressive neurodegenerative disease that causes death of upper and lower motor neurons (MNs) in the central nervous system (CNS). The disease afflicts most people in prime periods of productivity in life,and it is estimated approximately 200,000 individuals in the United States live with ALS and any given time. Though a significant percentage of individuals with ALS have a genetic or hereditary form of the disease, the majority are sporadic cases with unknown etiologies. Regardless of cause, onset and progression of the disease is similar in all ALS patients, with minor initial outward symptoms followed by rapid deterioration of motor function leading to widespread paralysis, respiratory dysfunction and death. Despite the distressing and debilitating nature of ALS, no cures and limited potential treatment options exist. Therefore, identifying targets for effective therapy leading to delayed disease progression, increased quality of life, and extended lifespan are critical areas of investigation. In addition, identifying biomarkers of disease progression is incredibly important as diagnosis often occurs once the disease is in late stages and lifespan is only an average of 3-5 years after diagnosis. As the cellular and physiological processes known to influence or be involved in ALS are numerous, the complexity of the disease is a major detriment in developing effective therapies.Aside from the ubiquitous death of MNs, inflammatory and immunologic response in the spinal cord, brain and target muscles, and signal pathway changes that precede or are induced by MN death have been identified at multiple stages of disease progression. As with most neural disorders and diseases, a multifactorial approach to therapy is likely the most appropriate.
Stem cells are promising potential treatments for multiple conditions, and researchers have demonstrated these effects broadly.Of these, adipose-derived stem cells (ASCs) are unique and have many benefits for the treatment of neural injury and disease. ASCs are multipotent mesenchymal stem cells that are easily obtained from adipose tissue. As such, isolating and purifying ASCs for autologous transplantation or treatment is much more accessible than for bone marrow-derived stem cells or other mesenchymal stem cells. Location and method of isolation influence the differentiation potential and other phenotypic characteristics of ASCs, but most are readily obtained from subcutaneous fat via lipoaspiration.In general, isolated ASCs tend to express similar surface markers to pericytes, favor differentiation toward vascular pericyte lineage,and evidence suggests these cells may be isolated from perivascular regions within the adipose tissue (Traktuev et al., 2008). In spite of this seemingly vascular-centric nature of ASCs, transplantation of these cells into neural tissue for studying their therapeutic effects has yielded variable responses of ASCs to the local microenvironment that effect differentiation and cell fate.
In neural therapy, recent studies have shown benefits of CNS transplantation of ASCs in stroke and spinal cord injury animal models. When transplanted into the spinal cord, ASCs have demonstrated the potential to differentiate toward a neuronal phenotype and promote remodeling of the microenvironment of the injured cord including promoting serotonergic axonal regeneration (Kolar et al., 2014). In a stroke model, transplanted ASCs reduced cell death, infarct volume, and reduced inflammatory cytokine expression in injured brain tissue when combined with mild hypothermia (Zhao et al., 2018). Though the microenvironment affects the function and fate of ASCs following transplantation, their beneficial properties arise from the paracrine effects of trophic and neurotrophic factor secretion by ASCs. This has promoted exploration of the use of conditioned medium from cultured ASCs as a potentially less invasive, yet still effective treatment paradigm.
Neuroprotective and regenerative effects of ASC conditioned medium(ASC-CM): ASC-CM has been shown to be effective in the experimental treatment of a myriad of neurologic pathologies,injuries, and conditions. Recent studies have demonstrated neuroprotective and regenerative effects of ASC-CM in animal models of Parkinson's disease, hypoxic-ischemic brain injury, and peripheral nerve injury. Our research and studies by our collaborators have demonstrated that administration of human ASC-CM maintains neuromuscular innervation when given prior to onset of this early pathology (Walker et al., 2018), and late stage treatment (at symptom onset) has proven effective at reducing MN death, delaying symptom progression, and extending lifespan in the classic mutant superoxide dismutase 1 (mSOD1)G93A transgenic mouse model of ALS (Fontanilla et al., 2015). ASC-CM is a complex combination of paracrine factors that could influence the CNS and peripheral neuromuscular interaction separately or simultaneously in imparting such outcomes in this ALS mouse model.
ASC-CM is a cocktail of protective and growth-inducing proteins:Hundreds of proteins are secreted by ASCs, and the benefits observed from ASC-CM therapy in neurological diseases have been attributed or confirmed to be due in large part to the various trophic factors released from the cells into the medium. Neuroprotective effects of ASC-CM are known to be highly influenced by the presence of brain-derived neurotrophic factor (BDNF) (Wei et al., 2009),nerve growth factor (Fontanilla et al., 2015), insulin-like growth factor-1 (IGF-1) (Wei et al., 2009), and glial cell line-derived neurotrophic factor (GDNF) (Palomares et al., 2018). These findings are unsurprising, as individual trophic factors have long been associated with protective or reparative effects in neurologic injury and disease,mostly in animal models. Unfortunately, systemic and intrathecal administration of individual growth factors has shown limited or conflicting therapeutic efficacy in ALS patients.
The most evidence related to clinical study of trophic factor delivery appears to be centered on BDNF and IGF-1. Different types of administration and doses of IGF-1 and BDNF have been tried,though findings have been difficult to interpret in the context of safety and treatment benefit. Ultimately, dosing issues and side effects, such as paresthesia, have been a recurring problem with no therapeutic efficacy observed or with modest influence on certain outcomes. In general, the overall view of such treatments is that any single factor is likely not going to be therapeutically effective,especially in relation to the risks for optimizing such approaches.A promising therapeutic aspect of ASC-CM, however, is the ability to delivery several beneficial neurotrophic factors in one systemic treatment approach. In showing neuroprotective, symptomatic and survival benefits in a mouse model of ALS, it is possible that many different individual factors exhibited benefits, with nerve growth factor a noted contributor to these effects (Fontanilla et al., 2015).As mentioned animal research showing evidence of therapeutic benefits of individual trophic factors is common, though similar benefits are not replicated in clinical application. In our recent work, we demonstrated a clear peripheral influence of ASC-CM on the preservation of innervated neuromuscular junctions (NMJs)when administered before and through a period of initial neuromuscular disconnection in the mSOD1G93A mouse model of ALS(Walker et al., 2018), and any or all of the listed trophic factors could have played a role in imparting this benefit. However, it is most likely a synergistic collective effect dependent on the cocktail of factors, which is promising for overcoming limitations of previous clinical trophic factor treatment paradigms.
Central versus peripheral effects of systemic ASC-CM:An important question in the treatment of ALS with ASC-CM is whether peripheral benefits (e.g. NMJ innervation) involves only the treatment effects on the target itself, or whether a central effect (e.g. neurotrophic stimulation of spinal cord motor neuron innervating the muscle) may be involved. The effects of ASC-CM in the described studies resulted from localized and systemic administration, which highlights the consideration of the state of the blood-brain and blood-spinal cord barrier (BSCB) for practical application of ASCCM from a clinical perspective. Therefore, location and timing of treatment and pathological characteristics of disease progression influence the efficacy of therapeutic ASC-CM administration.
Based on our observed effects of early treatment and NMJ preservation (Walker et al., 2018) and the evidence of post-symptomatic treatment-induced MN survival and extended lifespan (Fontanilla et al., 2015), these findings suggest systemic ASC-CM delivery could act on both central and peripheral components of the nervous and neuromuscular systems. This idea is supported by previous research into the disruption of the BSCB in the SOD1G93A mouse model of ALS. Zhong et al. (2008) demonstrated that the BSCB is disrupted in the ALS mouse model as early as 60 days of age, a pre-symptomatic time period before the observation of MN loss. As such, if the BSCB remains “leaky” from this period throughout disease progression,large molecules like neurotrophic factors can reach typically restricted intraparenchymal areas of the CNS microenvironment containing MN cell bodies and other cells. To our knowledge, no earlier time points have been reported concerning the integrity of the BSCB in the mSOD1G93A mouse model of ALS. Our preliminary evidence suggests that the BSCB may be disrupted as early as 35 days of age, which is the date we began administration of ASC-CM in our investigation of NMJ preservation in this mouse model (data not shown). As such, the effects we observed in the periphery at the NMJ may have involved a central influence of ASC-CM. Recent research showed that BSCB barrier repair could slow progression of motor neuron degeneration in the mSOD1G93A ALS mouse (Winkler et al., 2014). Since Ad-MSCs are known to have pro-angiogenic and vasculature-associated properties, it cannot be discounted that ASCCM could help seal the leaky BSCB, contributing positively to the disease in this way, as well.
Trophic regulation in the ventral horn of the spinal cord of ALS patients is known to be altered compared to healthy individuals(Duberley et al., 1997). Part of this dysregulation in individuals with ALS involves alterations in spinal ventral horn and motor neuron trophic factor receptor expression. In the ventral horn of post-mortem ALS patient spinal cord, IGF-1 receptor expression was upregulated especially in the ventral horn of cervical and sacral levels of the cord. This indicates that surviving neurons may be responsive to IGF-1 in systemic ASC-CM treatment. Likewise,mRNA for trkB receptor, the receptor for BDNF, is also upregulated in ventral horn spinal MNs in ALS patients (Seeburger et al., 1993). As ASC-CM is known to contain these factors, systemic treatment may allow for a neuroprotective central effect on the spinal motor neuron soma, in addition to possible peripheral influence on the muscle and NMJ.
As upper MNs, including corticospinal neurons, are also affected in disease progression and the blood brain barrier disruption could span the entire CNS, it is possible ASC-CM may be influencing upper MN health and survival as part of its mechanism of action.Concerning cerebral motor cortex upper MNs, information concerning trophic factor and receptor expression is limited, as most studies have focused on the lower MN. However, some research has provided potential insight into upper MN expression and modulation of trophic factors in ALS and the neuronal response of MNs to these factors. In a study of BDNF and neurotrophin-3 treatment,no difference was observed between localization of these factors in motor cortex neurons and those of healthy controls, indicating that the cortical influence of specific trophic factors may not be as robust as observed in spinal MNs. This is supported by findings suggesting neurotrophin-3 and BDNF treatment does not provide a neuroprotective response in cortical MNs (Van Westerlaak et al.,2001); however this was an in vitro study, which restricts the extent of potential interpretation. Information related to trophic factor receptor expression is even more limited; therefore, it remains unclear how ASC-CM trophic factors may influence morphology and survival of upper MNs in ALS. Though morphologic changes associated with neurodegeneration occur in cortical MNs in ALS,further study is necessary concerning trophic factor production and response in these cells. This information is required for more thorough investigation of the potential trophic influence of systemic ASC-CM therapy on cortical upper MNs.
Future directions for ASC-CM therapy in ALS:ASC-CM is already in clinical trials for other conditions, so it is quite conceivable based on benefits observed in animal models of ALS that it could be effectively tested in ALS patients. Key issues of consideration are when to start treatment, length of treatment and safety. As patients that are diagnosed first visit the clinic with outward symptoms,it is generally considered that the disease is in such an advanced stage that treatments beginning at this point in time will be of little benefit. This is reflected in the marginal and debatable benefits of currently approved clinical therapies. In addition, if it is desirable to utilize one's own ASCs for conditioned medium collection, this requires additional time before treatment can begin. Despite these limitations, this is currently the most feasible and clinically relevant approach for developing and testing treatments for ALS. Our colleagues' demonstration that systemic ASC-CM treatment after symptom onset imparts therapeutic benefits is encouraging in this direction of application.
Research into identifying biomarkers for ALS has greatly increased in recent years, and if identified, perhaps the disease can be detected and treatment started before progression into such advanced stages. As ALS is primarily sporadic in nature, a biomarker-based screening approach will work best for those with a genetic pre-disposition, such as a known hereditary SOD1 mutation. If the disease is diagnosed early enough before advanced stages of disease progression, systemic ASC-CM may be even more beneficial providing central neuroprotective or regenerative benefits and observable improvement in peripheral innervation for delayed symptom progression and prolonged survival.
Concerning safety, in a broad view, little outward evidence suggests long-term administration of ASC-CM is unsafe or has undesirable side effects. From an immunologic perspective, it is also a concern that continued systemic treatment with ASC-CM could induce an immune response against factors in the conditioned medium, decreasing the efficacy of treatment over time.Our initial investigation into the immune system response to longterm ASC-CM therapy showed that there were no differences in immunoglobulin isotype levels between ASC-CM-treated and vehicle-treated mSOD1G93A mice and ASC-CM-treated non-transgenic mice (data not shown). This suggests some measure of safety and possible continued efficacy of treatment over long periods of time; however, further studies are required to obtain a broader understanding of the systemic influence of continued exposure to systemic ASC-CM. How specific immune and glial cells may respond to long-term ASC-CM treatment also requires additional study.In addition, much research has focused on the spinal lower MN,even though upper MNs are also affected in ALS. As such, more general research into trophic function in cortical MNs and in the microenvironment of the motor cortex is required. Though general research into the morphological and survival benefits of ASC-CM on cortical upper MNs is possible, specific mechanistic information of how these benefits are obtained will require a more established foundation of pathologic trophic factor and receptor alteration in the motor cortex.
Conclusion:Systemic ASC-CM delivery is both effective in ameliorating disease progression in late-stage ALS mice and beneficial in protecting NMJ denervation in early pre-symptomatic stages of disease, however, research into ASC-CM as a therapy for ALS is in its infancy (Figure 1). Early pre-symptomatic treatment affects initial neuromuscular denervation, but it is not known whether this benefit can contribute to long-term functional benefits. Post-symptomatic treatment is neuroprotective and extends lifespan in mSOD1G93A mice, and reduced glial fibrillary acid protein expression implicates reduced inflammation as part of the treatment effects (Fontanilla et al, 2015). As both peripheral and central effects are observed, it is also unclear if the CNS or the neuromuscular interface is the primary site of therapeutic ASC-CM influence. Perhaps a combination of treating both of these components is necessary for optimal therapeutic efficacy. Lastly, how well the observed responses in the mouse model of ALS will translate to human therapy remains to be investigated. We believe our research sets the foundation for further investigations into the long-term benefits of early continuous ASC-CM treatment, as well as studies into therapeutic effects when administered at intermediate stages of disease in the mSOD1G93A mouse model of ALS. With a more thorough understanding of the different treatment effects, both positive and negative, in animal models of disease, we anticipate that future clinical application and testing of ASC-CM treatment is possible.
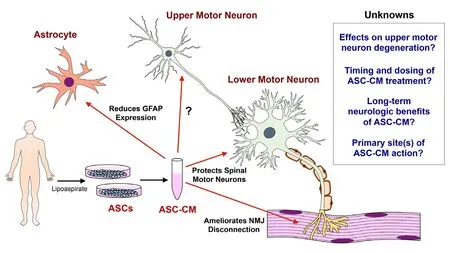
Figure 1 What we know and do not know about ASC-CM therapy.Post-symptomatic treatment with ASC-CM reduces intraspinal GFAP expression, increases motor neuron survival, and prolongs lifespan, while early pre-symptomatic administration ameliorates NMJ denervation.The effects of ASC-CM on upper motor neurons, how different durations of treatment influence long-term neurological and survival outcomes and the primary sites of ASC-CM effects require further investigation. ASC-CM:Adipose-derived stem cell conditioned medium; GFAP: glial fibrillary acidic protein; NMJ:neuromuscular junction; ASCs:adipose-derived stem cells.
The foundational research performed by the labs of Dr. Keith March and colleagues is valuable to this article. I thank Drs. March and Kathryn J. Jones for guidance and collaboration in the execution,evaluation and continuation of the research directions proposed in this manuscript.
This work was supported in part by funding from the United States Department of Veterans Affairs (IK2 RX002688-01A2, to CLW).
Chandler L. Walker*
Department of Biomedical and Applied Sciences, Indiana
University School of Dentistry, Indianapolis, IN, USA
*Correspondence to: Chandler L. Walker, PhD, chalwalk@iu.edu.orcid: 0000-0002-8616-8263 (Chandler L. Walker)
Received:December 6, 2018
Accepted:February 27, 2019
doi: 10.4103/1673-5374.253514
Copyright license agreement:The Copyright License Agreement has been signed by the author before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Novel miRNA, miR-sc14, promotes Schwann cell proliferation and migration
- Role of behavioral training in reducing functional impairments after stroke
- Remodeling dendritic spines for treatment of traumatic brain injury
- Acute drivers of neuroinflammation in traumatic brain injury
- More than anti-malarial agents: therapeutic potential of artemisinins in neurodegeneration
- Why microglia kill neurons after neural disorders?The friendly fire hypothesis
