Predictive values of multidetector-row computed tomography combined with serum tumor biomarkers in preoperative lymph node metastasis of gastric cancer
2019-07-13HuihuiBaiJingyuDengNannanZhangHuifangLiuWentingHeJinyuanLiuHanLiang
Huihui Bai, Jingyu Deng, Nannan Zhang, Huifang Liu, Wenting He, Jinyuan Liu, Han Liang
Department of Gastric Cancer, Tianjin Medical University Cancer Institute & Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin’s Clinical Research Center for Cancer, Tianjin 300060, China
Abstract Objective: Multidetector-row computed tomography (MDCT) and serum tumor biomarkers are commonly used to evaluate the preoperative lymph node metastasis and the clinical staging of gastric cancer (GC). This study intends to evaluate the clinical predictive value of MDCT and serum tumor biomarkers in lymph node metastasis of GC.Methods: The clinicopathologic data of 445 GC patients who underwent radical gastrectomy were retrospectively analyzed to evaluate the diagnostic value of MDCT and serum tumor biomarkers in lymph node metastatic staging of GC before surgery.Results: With the multinomial logistic regression analysis, the independent relative factors of lymph node metastasis of GC were identified as tumor size, depth of tumor invasion, vessel invasion, vascular embolus, and soft tissue invasion. The optimal critical value of the short diameter of lymph nodes detected by MDCT scanning for evaluation of preoperative lymph node metastasis was 6.0 mm, with 75.7% as predictive accuracy of lymph node metastasis compared to the postoperative pathological results of GC patients. In addition, the critical value of the short diameter of lymph nodes combined with serum tumor biomarkers [including carbohydrate antigen (CA)-724 and CA-199] could show an enhancement of predictive sensitivity of lymph node metastasis (up to 89.3%) before surgery.Conclusions: MDCT combined with serum tumor biomarkers should be adopted to improve preoperative sensitivity and accuracy of lymph node metastasis for GC patients.
Keywords: Gastric cancer; MDCT; serum tumor biomarkers; preoperative
Introduction
The incidence of gastric cancer (GC) has decreased in recent decades; however, it remains one of the most common fatal disease in the world (1). Lymph node metastasis is one of the most important independent risk factors that can negatively affects the prognosis of patients with GC (2,3). Accurate preoperative diagnosis of lymph node metastasis is the key to giving important assistance for the preliminary assessment of the optimal therapy mode of tumors to improve the prognosis of GC patients. Thus far,multidetector-row computed tomography (MDCT) is one of the most practical methods for accurately detecting the extent of lymph node involvement in GC before surgery.
MDCT is superior to other physical examination methods for the initial assessment of lymph node metastatic staging of GC before surgery due to its higher sensitivity and specificity (4,5). Several studies have been demonstrated that serum tumor biomarkers provide some key markers to lymph node metastasis in GC (6-8).
In light of the abovementioned considerations, we designed this study to analyze the clinical diagnostic values of MDCT in combination with serum tumor biomarkers for accurately predicting lymph node metastasis in GC before surgery.
Materials and methods
Patients
We recruited 701 patients who were diagnosed with GC and underwent the curative gastrectomy plus D2 lymphadenectomy in Tianjin Medical University Cancer Hospital in 2017. The eligibility criteria for inclusion in this study were as follows: 1) gastric adenocarcinoma identified using the histopathological examination; 2) both MDCT and serum tumor biomarkers were examined within a week before surgery; and 3) complete follow-up data collected. The exclusion criteria in this study were as follows: 1) neoadjuvant therapy performed before surgery;2) concurrent with other cancers; or 3) residual stomach cancer. Ultimately, 445 GC patients were included in this study. The diameters of the lymph nodes in the preoperative MDCT scanning were measured by the researchers themselves in all patients and blinded to the pathological results. All patients’ informed consent was obtained.
Clinicopathologic variables
The medical records of the patients’ clinicopathologic data were reviewed, including the age at surgery, gender,primary tumor location, tumor size, depth of tumor invasion, tumor histopathological type, Lauren classification, type of gastrectomy, extent of lymphadenectomy, examined lymph node count, disease stage,vessel invasion, perineuronal invasion, soft tissue invasion,and vascular thrombus.
Surgical management
All patients underwent curative gastrectomy plus D2 lymphadenectomy. Primary tumors were resected en bloc using lymphadenectomy according to the guidelines of the Japanese Gastric Cancer Association (9). The surgical procedures were mainly based on the Japanese Gastric Cancer Treatment Guidelines (10).
Diagnostic criteria
Lymph nodes with the maximum short diameters<10.0 mm (or according to ROC of cut-off values <6.0 mm in our research) were considered as the negative nodes,whereas those with maximum short diameters ≥10.0 mm(or according to ROC of cut-off values ≥6.0 mm in our research) were diagnosed as positive nodes from the MDCT scanning results. The upper limitations of the normal value of diagnostic criteria for the four serum tumor biomarkers were as follows: 6.9 U/mL for carbohydrate antigen (CA)-724, 37 U/mL for CA-199,20 U/mL for CA-242, and 5 μg/L for carcinoembryonic antigen (CEA).
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics (Version 24.0; IBM Corp., New York, USA).Continuous variables were expressed as mean and range.The univariate correlation analysis was performed using the χ2test, and the multinomial logistic regression was performed for multivariate correlation analysis of various clinicopathologic characteristics. Statistical significance was defined as P<0.05. The receiver operating characteristic curve (ROC curve) and the area under the curve (AUC)were used to assess the independent and combined diagnostic values of MDCT and serum tumor markers for the lymph node metastasis of GC.
Results
All included 445 GC patients (306 males and 139 females)who had undergone the curative gastrectomy plus D2 lymphadenectomy in Tianjin Medical University Cancer Hospital between January 2017 and December 2017 were retrospectively analyzed. The mean age was 57.8 (range,17.0-82.0) years. The total number of examined lymph nodes from all 445 GC patients was 18,132, and 2,294 positive lymph nodes were detected from the 244 GC patients. The disease pathologic (pN) staging of all included patients was according to the eighth edition TNM classification for GC proposed by the American Joint Committee on Cancer (11), and a total of 201 pN0 stage cases, 53 pN1 stage cases, 75 pN2 stage cases, and 116 pN3 stage cases were detected. The maximum short diameter of lymph nodes in MDCT scanning ranged from 0 to 27 mm,with a mean of 5.4 mm. The mean levels of CA-724, CA-199, CA-242, and CEA were 14.70 (range, 0.20-301.10)U/mL, 44.45 (range, 0.60-1,000.00) U/mL, 19.33 (range,0.10-702.29) U/mL, and 4.35 (range, 0.20-123.40) μg/L,respectively.
Univariate and multivariate analyses of risk factors of lymph node metastasis in GC patients
Eight factors showed significant statistical associations with lymph node metastasis of GC using the univariate correlation analysis: tumor size, depth of tumor invasion,tumor histopathological type, disease stage, type of gastrectomy, perineuronal invasion, soft tissue invasion,and vascular thrombus (Table 1). All eight characteristics were included in a multivariate logistic regression analysis,where, tumor size, depth of tumor invasion, perineuronal invasion, vascular thrombus, and soft tissue invasion were demonstrated to be independently correlated factors to lymph node metastasis of GC (Table 1).
Clinical values of MDCT scanning in diagnosis of lymph node metastasis of GC patients before surgery
Usually, a maximum short diameter of a lymph node detected ≥10.0 mm by the MDCT scan is considered as a clinical metastatic node for GC patients (10). Initially, we adopted the criterion of a positive lymph node of GC as the maximum short diameter ≥10.0 mm of lymph nodes in MDCT. All GC patients included in the study were divided into two groups on the basis of lymph node metastasis status (N- for negative nodes, and N+ for positive nodes)assessed using both MDCT scanning before surgery and pathological examination after surgery, respectively.Compared with the postoperative pathological results of all included GC patients, the sensitivity, specificity and accuracy of MDCT scanning results of lymph node metastasis of GC were 48.0%, 86.6%, and 65.4%,respectively (P<0.001) (Table 2).
A large area under the ROC curve signified a high diagnostic accuracy. In the present study, AUC was 0.807 when the status of lymph node metastasis of GC was
diagnosed using MDCT scanning before surgery (Table 3;Figure 1A). However, the optimal diagnostic maximum short diameter of lymph nodes using MDCT scanning for lymph node metastasis of GC was 6.0 mm, with various cut-off values of the maximum short diameter of lymph nodes included in the ROC curve analysis. The sensitivity and specificity values of the clinical lymph node metastatic staging diagnosed using MDCT scanning were shown in Table 4. Results show that when the critical value for the diagnosis of lymph node metastasis is 6.0 mm, its sensitivity(75.8%) and accuracy (75.7%) increased, although the specificity (75.6%) was decreased slightly. There was a significant difference in the diagnostic value of lymph node metastasis between the critical value of 10 mm and 6 mm(χ2=59.112, P<0.001). In other words, the maximum short diameter of lymph node ≥6.0 mm in MDCT scanning shows excellent diagnostic value.
Comparison of clinical values of serum tumor biomarkers in lymph node metastasis group and non-lymph node metastasis group in diagnosis of GC before surgery
The clinical values of four serum tumor biomarkers(including CA-724, CA-199, and CA-242 and CEA) in diagnosing GC lymph node metastasis were elucidated. We found that the median and interquartile range of CA-724[1.69 (2.27) vs. 5.71 (21.22)], CA-199 [9.31 (12.81) vs. 20.39(38.95)], and CA-242 [3.91 (3.89) vs. 4.43 (7.74)] in nonlymph node metastasis group were significantly lower than those in lymph node metastasis group (P<0.05) (Table 5).Using the ROC curve analysis, the AUC of both CA-242 and CEA curves was found to be less than 0.7, whereas those of CA-724 and CA-199 were greater than 0.7 (Figure 1B). Furthermore, CA-724 and CA-199 showed excellent diagnostic values for lymph node metastasis of GC compared with CA-242 and CEA. Therefore, we removed CA-242 and CEA from the study. According to the ROC curve analysis, the optimal critical value of the diagnosis using CA-724 and CA-199 for lymph node metastasis of GC was 2.69 U/mL and 25.10 U/mL, respectively, with various cut-off values of the critical value of the diagnosis included in the ROC curve analysis.
Combination serum tumor biomarkers (CA-724 and CA-199) with MDCT scanning in diagnosis of lymph node metastasis in GC
In order to improve the sensitivity of the diagnosis, we determined positive for lymph node metastasis when any of
MDCT scanning, CA-724 or CA-199 was diagnosed positive. The sensitivity, specificity and accuracy of the lymph node metastasis diagnosed using CA-724 (the positive diagnostic criteria were higher than 2.69 U/mL)combined with CA-199 (the positive diagnostic criteria were higher than 25.10 U/mL), were 65.9%, 78.6%, and 71.7%, respectively. While, the sensitivity, specificity and accuracy of the lymph node metastasis diagnosed using
MDCT scanning combined with CA-724 and CA-199 in diagnosing lymph node metastasis of GC were 89.3%,61.2%, and 77.6%, respectively (Table 2), which were significantly different from the combined diagnosis of CA-724 and CA-199 (χ2=54.018, P<0.001). In addition, the AUC of MDCT scanning combined with CA-724 and CA-199 was 0.849 (Table 3; Figure 1B). Thus, MDCT scanning combined with CA-724 and CA-199 can improve the preoperative sensitivity and accuracy of lymph node metastasis for GC patients.
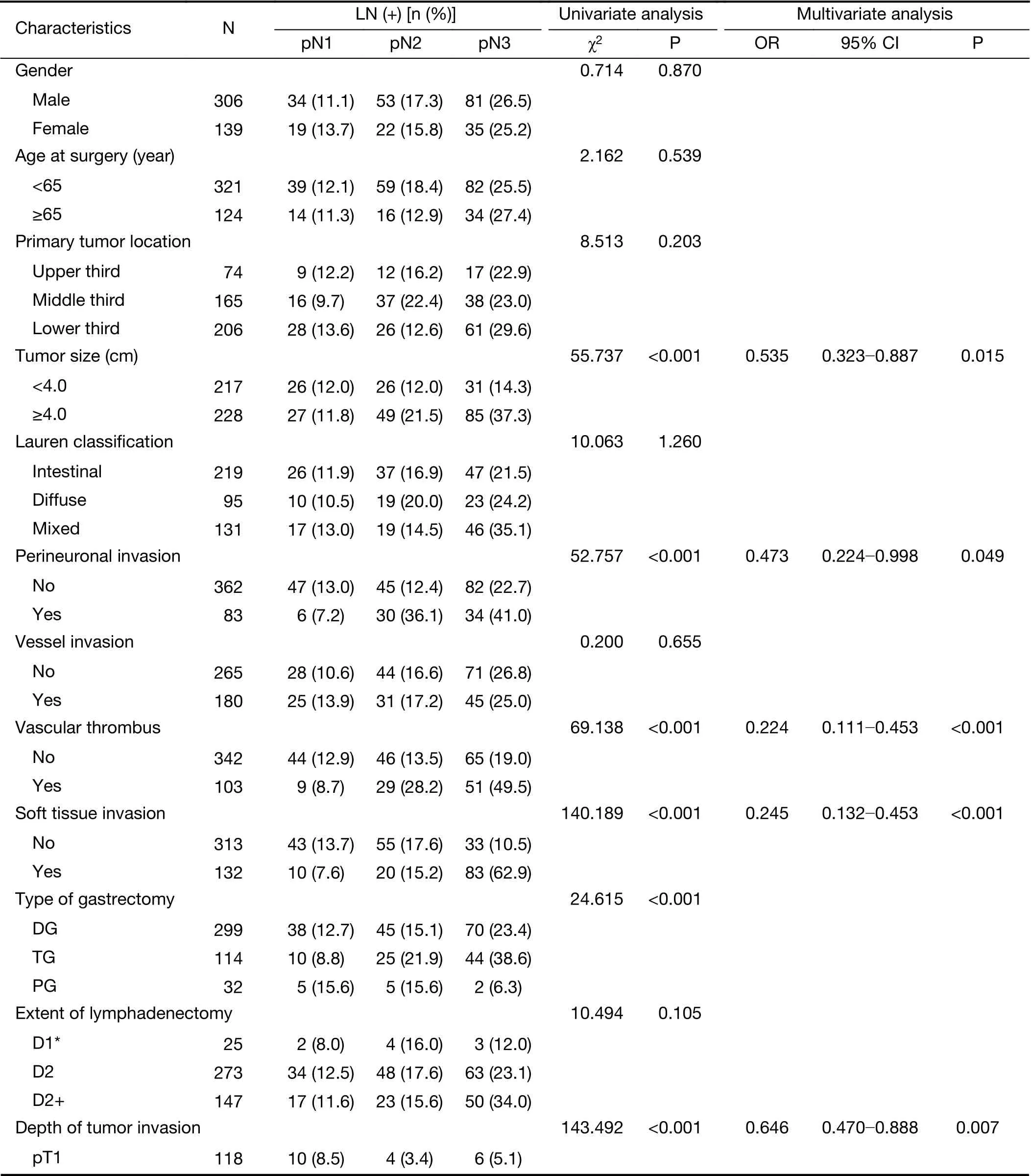
Table 1 Univariate and multivariate analysis of risk factors of 445 GC patient cohort

Table 1 (continued)

Table 2 Comparison of lymph node metastatic stages between clinical stage and pathological stage

Table 3 Optimal cut-off values of various variables by using ROC curve
Discussion
Lymph node metastasis, tumor invasion depth and distant metastasis are important factors affecting the prognosis of GC patients. Assessing the lymph node metastasis before surgery is essential. In this study, tumor size, depth of tumor invasion, perineuronal invasion, vascular thrombus,and soft tissue invasion were independent risk factors for lymph node metastasis of GC. In particular, the tumor size and depth of tumor invasion can be well evaluated by endoscopic ultrasonography before surgery (12). Patients with tumors larger than 4 cm in diameter or the depth of invasion was deeper should be highly vigilant for lymph node metastasis. Nevertheless, no gold standard exists for the preoperative diagnosis of lymph node metastasis in GC patients. Moreover, the National Comprehensive Cancer Network (NCCN) does not have a recommended specific imaging examination to diagnose lymph node metastasis.MDCT scanning is the most commonly used imaging examination to evaluate the preoperative lymph node metastasis of GC. Previous studies have reported that the sensitivity and specificity of the diagnosis of lymph node metastasis of GC range from 27% to 90% and 60% to 91%, respectively (13-15). Furthermore, the ideal diagnostic criteria should be highly specific and sensitive.
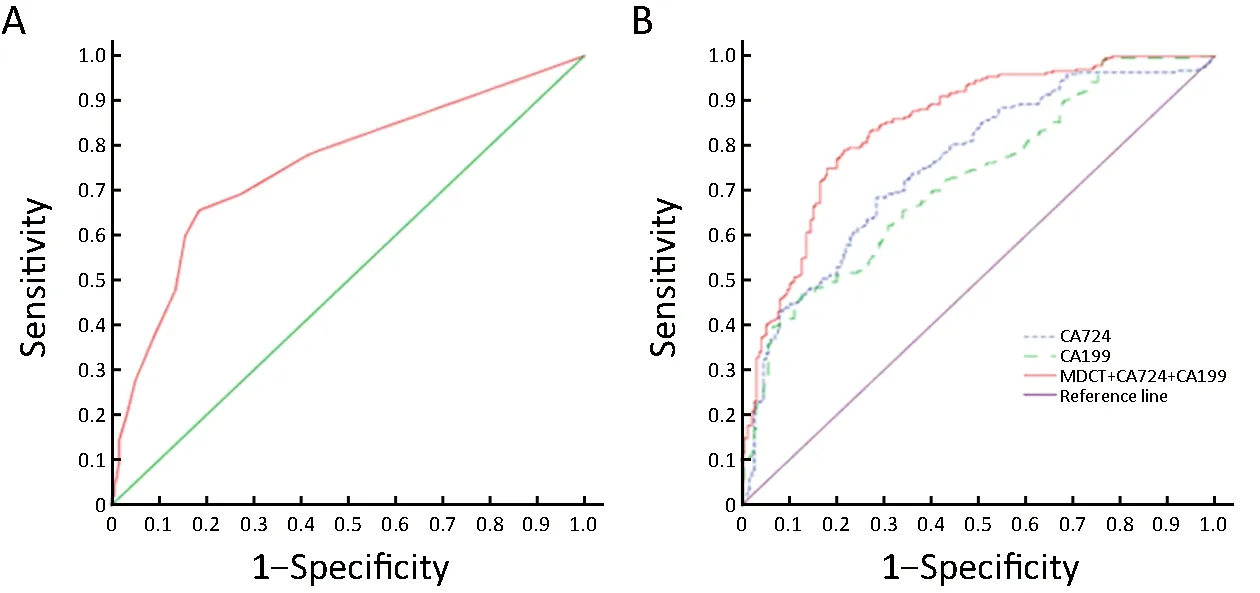
Figure 1 Receiver operating characteristic (ROC) curve of each variable in predicting lymph node metastasis in gastric cancer. (A) ROC curve of multidetector-row computed tomography (MDCT) scanning; (B) ROC curve of single and combined variables. CA, carbohydrate antigen.
The criteria for the diagnosis of lymph node metastasis of GC using MDCT scanning are extremely controversial(14,16-18). The size of the lymph nodes is the most commonly used standard to diagnose lymph node metastasis, where 10.0 mm is generally considered to signal potentially positive lymph node metastasis (19,20).However, the diagnostic criteria for lymph node metastasis have been different in previous studies. Yan et al. (21)considered that the detection rate of lymph node metastasis is related to the location of the lymph nodes. A short diameter of perigastric lymph node ≥6.0 mm, or a short diameter of extraperigastric lymph node ≥8.0 mm is the diagnostic criteria for lymph node metastasis. Kawaguchi et al.(22) took the short diameter of lymph nodes ≥8.0 mm, an MDCT value of the enhancement portal phase was greater than 100 HU, and round lymph nodes as bases for a positive diagnosis of lymph node metastasis. In this study,when the maximum short diameter of lymph nodes ≥10 mm in MDCT scanning was used as the diagnostic criteria for lymph node metastasis, although the specificity in the diagnosis of lymph node metastasis was up to 86.6%, the sensitivity was only 48.0%, and the accuracy was poor.Similar results were obtained by Kubota et al. (20).According to the cut-off value, when 6.0 mm was used as the diagnostic critical value of positive lymph nodes,sensitivity, specificity and accuracy reached 75.6%, 77.8%,and 75.7%, respectively. Sensitivity decreased slightly, but specificity and accuracy increased compared with 10.0 mm as the diagnostic criteria. Results were similar to those of Morgagni et al. (13). In addition, our study believes that when there is no lymph node metastasis or numerous lymph node metastases are present, the preoperative cN staging is easier to distinguish, while the number of lymph node metastasis in the cN1 and cN2 stages ranges from 1 to 6, the preoperative staging is more difficult than that in cN0 and cN3 stages. Thus, MDCT scanning has certain limitations for preoperative N staging.

Table 5 Comparison of level of serum tumor markers between lymph node metastasis group and non-lymph node metastasis group in GC patients
The detection of serum tumor biomarkers is simple and reproducible. However, most of the serum tumor biomarkers are non-specific antigens with low sensitivity and specificity. CA-724, CA-242, CA-199 and CEA are widely used for screening gastrointestinal tumors. Previous studies suggested that combining these four serum tumor biomarkers in detecting GC could improve the sensitivity and specificity of the diagnosis (23-25). However, the diagnostic value of serum tumor biomarkers for lymph node metastasis of GC is rarely studied. Among the four serum tumor biomarkers, CA-724, CA-199, and CEA are considered as reliable biomarkers in terms of their diagnostic value in lymph node metastasis of GC in most previous studies (6,26-30). For example, Kim et al. (26) and Duraker et al. (27) deemed that CEA has a satisfactory diagnostic value in the diagnosis of GC and lymph node metastasis. Ucar et al. (28) showed that CA-724 and CA-199 in the lymph node metastasis group were significantly higher than in the non-lymph node metastasis group. Chen et al. (29) reported that CA-724 is the most relevant tumor marker for GC, and the combined detection of CA-724,CA-199 and CEA can improve diagnostic sensitivity without affecting specificity. Li et al. (6) found that the level of serum tumor biomarkers was significantly higher in lymph node metastasis group than in non-lymph node metastasis group, and the positive rate of serum tumor biomarkers was also increased with the increase of N staging. CA-242 is mainly used for preoperative screening of gastrointestinal tumors such as colorectal cancer and pancreatic cancer (31,32), but there are few studies on the diagnostic value of lymph node metastasis in gastric cancer.
In the present study, we found that the levels of CA-724,CA-199 and CA-242 were significantly different between the lymph node metastasis group and the non-lymph node metastasis group, whereas the CEA level was not. The ROC curve showed that the AUC of CA-724 and CA-199 was greater than 0.7, indicating a satisfactory diagnostic accuracy for lymph node metastasis of GC, whereas the AUC of both CA-242 and CEA was less than 0.7,indicating a poor diagnostic accuracy for the lymph node metastasis of GC.
Although the level of serum tumor biomarkers in the lymph node metastasis group was higher than that of the non-lymph node metastasis group, it cannot independently used to evaluate the presence of lymph node metastasis in GC patients. NCCN guidelines also did not recommend serum tumor biomarkers as indicators for preoperative evaluation of lymph node metastasis in GC patients (7). In this study, we have reason to believe that CA-724 and CA-199 can show excellent sensitivity and specificity in the codiagnosis of lymph node metastasis of GC as an auxiliary of MDCT scanning. In other words, the level of serum tumor biomarkers and MDCT scanning can be combined to diagnose the presence of lymph node metastasis before surgery in GC patients, to some extent, CA-724 and CA-199 could enhance the sensitivity of the lymph nodes metastasis of GC patients.
This study has several limitations. First, other factors,such as the location and morphology of lymph nodes, were not considered except the size of lymph nodes when lymph node metastasis in GC was diagnosed. Second, imaging cannot clearly determine the location and the number of lymph nodes before operation. In the areas where the vascular shape is clear, such as in the abdominal aorta and splenic artery, the metastatic lymph nodes are relatively clear, and the diagnostic rate of positive lymph nodes using MDCT scanning is relatively high. The diagnostic rate of positive lymph nodes was extremely low in the area with unclear vascular shape. Finally, inflammation can also cause lymph node enlargement. Therefore, lymph node enlargement caused by inflammation or metastasis of cancer is sometimes hard to distinguish (33), which affects the diagnosis of lymph node metastasis.
Acknowledgements
This study was supported in part by grants from the Programs of National Natural Science Foundation of China (No. 81572372), National Key Research and Development Program “major chronic non-infectious disease research” (No. 2016YFC1303202), and National Key Research and Development Program “precision medicine research” (No. 2017YFC0908304).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
杂志排行
Chinese Journal of Cancer Research的其它文章
- Cancer IgG, a potential prognostic marker, promotes colorectal cancer progression
- Correlation of radiotherapy with prognosis of elderly patients with hormone receptor-positive breast cancer according to immunohistochemical subtyping
- Impact of crizotinib on long-term survival of ALK-positive advanced non-small-cell lung cancer: A Chinese multicenter cohort study
- Burden of colorectal cancer in China, 1990-2017: Findings from the Global Burden of Disease Study 2017
- Hexokinase II promotes the Warburg effect by phosphorylating alpha subunit of pyruvate dehydrogenase
- Mutant p53 increases exosome-mediated transfer of miR-21-3p and miR-769-3p to promote pulmonary metastasis
