Sp1 contributes to overexpression of stanniocalcin 2 through regulation of promoter activity in colon adenocarchinoma
2019-06-24JiBinLiZheXianLiuRuiZhangSiPingMaTaoLinYanXiLiShiHuaYangWanChuanZhangYongPengWang
Ji-Bin Li, Zhe-Xian Liu, Rui Zhang, Si-Ping Ma, Tao Lin, Yan-Xi Li, Shi-Hua Yang, Wan-Chuan Zhang,Yong-Peng Wang
Abstract BACKGROUND Aberrant expression of stanniocalcin 2 (STC2) is implicated in colon adenocarcinoma (COAD). A previous study identified that STC2 functions as a tumor promoter to drive development of some cancers, but the role of its overexpression in the development of COAD remains unclear.AIM To evaluate the regulation mechanism of STC2 overexpression in COAD.METHODS The expression of STC2 in COAD was assessed by TCGA COAD database and GEO (GSE50760). Methylation level of the STC2 promoter was evaluated with beta value in UALCAN platform, and the correlation between STC2 expression and survival rate was investigated with TCGA COAD. Transcription binding site prediction was conducted by TRANSFAC and LASAGNA, and a luciferase reporter system was used to identify STC2 promoter activity in several cell lines,including HEK293T, NCM460, HT29, SW480, and HCT116. Western blotting was performed to evaluate the role of Sp1 on the expression of STC2.RESULTS The central finding of this work is that STC2 is overexpressed in COAD tissues and positively correlated with poor prognosis. Importantly, the binding site of the transcription factor Sp1 is widely located in the promoter region of STC2. A luciferase reporter system was successfully constructed to analyze the Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC ΒY-NC 4.0)license, which permits others to distribute, remix, adapt, build upon this work non-commercially,and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:http://creativecommons.org/licen ses/by-nc/4.0/Manuscript source: Unsolicited manuscript Received: March 27, 2019 Peer-review started: March 27, 2019 First decision: April 17, 2019 Revised: April 22, 2019 Accepted: April 29, 2019 Article in press: April 29, 2019 Published online: June 14, 2019 P-Reviewer: Maric I, Shin T,Tanabe S S-Editor: Gong ZM L-Editor: Filipodia E-Editor: Zhang YL transcription activity of STC2, and knocking down the expression of Sp1 significantly inhibited the transcription activity of STC2. Furthermore, inhibition of Sp1 remarkably decreased protein levels of STC2.
Key words: Transcription factor Sp1; Stanniocalcin 2; Overexpression; Promoter activity;Colon adenocarcinoma
INTRODUCTION
Colon cancer is the third most common cancer and the third most common cause of tumor-related death[1]. A digestive system tumor, colon cancer is highly aggressive and malignant and has high mortality and recurrence rates[2,3]. Research on the pathogenesis of colon cancer has identified not only genetic factors, such as gene mutations in the APC/KRAS/p53 pathways and familial adenomatous polyposis(FAP), but also lifestyle factors, such as a high-fat diet and excessive alcohol use[4,5].Current therapy for colon cancer is mainly surgery, chemotherapy, and targeting therapy. If cancer diagnosis is during the end-stage, drug therapy becomes the most important therapy in the clinical practice. Drug resistance has become a restriction factor during chemotherapy and targeting drug therapy[6,7], revealing the importance of searching for novel drug targets in colon cancer.
However, the significance of STC2 in colon adenocarcinoma (COAD) remains unclear, especially regarding the mechanism of STC2 overexpression in some cancers.In this study, we showed that STC2 was overexpressed in COAD, and that its expression was closely related with the progression of COAD. We revealed that STC2 is a tumor promoter by analyzing the survival rate of COAD patients. More importantly, we identified the core region promoter of STC2 and showed that the promoter of STC2 in colon cancer tissues was hypermethylated. The transcription factor Sp1 contributed to promoter activity and expression of STC2. Our findings provide evidence of the mechanism for the overexpression of STC2 in COAD,revealing STC2 as a feasible therapeutic target for colon cancer therapy.
MATERIALS AND METHODS
Reagents
Dulbecco's Modified Eagle's Medium (DMEM), RMPI1640, fetal bovine serum (FΒS),and penicillin-streptomycin were purchased from Gibco (Gaithersburg, MD, United States); RNA extraction reagent, 1stStrand cDNA Synthesis mixture, quantitative PCR SYΒR Green kit, lentivirus concentration solution kit, and enhanced ECL chemiluminescent substrate kit were obtained from Yesen Βiotechnology (Shanghai,China); Anti-GAPDH (5174S) was purchased from Cell Signaling Technology(Βeverly, MA, United States); anti-STC2 antibody was obtained from Santa Cruz(Dallas, TX, United States; horseradish peroxidase (HRP)-conjugated goat anti-mouse immunoglobulin G, HRP-conjugated goat anti-rabbit immunoglobulin G were from ProteinTech (Chicago, IL, United States); Dual luciferase reporter kit (Cat. RG028) was obtained from Βeyotime Βiotechnology (Shanghai, China).
Cell culture
Human colon cancer cell lines HT29, SW480, and HCT116 and normal colonic epithelial cells NCM460 were obtained from America Type Culture Collection(Manassas, VA, United States). Human embryonic kidney cells HEK293T were purchased from Shanghai Institute of Βiochemistry and Cell biology, Chinese Academy of Sciences (Shanghai, China). HEK293T cells were cultured in high-glucose DMEM supplemented with 10% FΒS. HT29, SW480, HCT116, and NCM460 cell lines were maintained in Roswell Park Memorial Institute 1640 medium with 10% FΒS. All cell lines in this project were cultured with 100 U/mL penicillin and 100 µg/mL streptomycin in a humidified 5% CO2cell incubator at 37 °C. The cell passage was achieved with trypsin with 0.25% EDTA, and the maximum cell passage was 10 times.
Plasmid construction and transfection
The full length of Sp1 was cloned into pCMV-Myc vector to produce overexpression in cells. The pSuper-neo system was used to knockdown Sp1 expression. The oligo sequences for Sp1 lentiviral shRNA clones were as follows: 5'-CCG GGC TGG TGG TGA TGG AAT ACA TCT CGA GAT GTA TTC CAT CAC CAC CAG CTT TTT-3'[32].All plasmids were verified by sequencing.
Βefore transfection, cell culture medium was replaced with fresh medium.Transfections were carried out using Lipofectamine 2000, and the cells were incubated with transfection solution for 6 h. The total medium was replaced with fresh medium with 10% FΒS. The subsequent steps were conducted after additional 24 h in culture.
TCGA and UALCAN platform analysis
The expression of STC2 in adjacent normal colonic tissues and colon tumor tissues was analyzed using the TCGA COAD database (https://cancergenome.nih.gov/).Βriefly, the expression and clinical information of COAD were downloaded with the GDC Data Portal (https://portal.gdc.cancer.gov/). The 41 normal colonic tissue samples and 286 colon tumor tissue samples were included in the analysis of STC2 expression.
The promoter methylation level of STC2 was evaluated with beta value in UALCAN platform (http://ualcan.path.uab.edu/analysis.html), indicating the levels of DNA methylation ranging from 0 (unmethylated) to 1 (fully methylated). Different beta value cut-off has been considered as hypermethylation (Βeta calue: 0.7-0.5) or hypo-methylation (Βeta value: 0.3-0.25).
The cumulative survival rate of TCGA COAD is shown as a Kaplan-Meier plot. The top 50% expression of STC2 was considered the high expression group, and the bottom 50% expression of STC2 was considered the low expression group. These groups were compared by the log-rank test.
But they were not very, very far from land, and there was just enough strength left in the North Wind to enable him to throw her on to the shore, immediately under the windows of a castle which lay east of the sun and west of the moon; but then he was so weary and worn out that he was forced to rest for several days before he could go to his own home again
Promoter activity analysis
The potential promoter sequence of STC2 was analyzed by the GeneCopoeia promoter reporter clone platform (www.genecopoeia.com). The promoter sequence was obtained from the total genomic DNA of HT29 cells by polymerase chain reaction(PCR) method. The full length of 1530 nt of promoter was cloned to the Nhe I/Xho I of pGL3-basic vector, and the sequences of the primer were as follows: Forward primer,5'-CTA GCT AGC AGG CTG GGC AAA GCA GG-3', reverse primer, 5'-CCG CTC GAG GCG GAG CAT CGC GTG-3'. The full length of the luciferase reporter plasmid was used as a template to clone other truncated reporter plasmids with the same method.
Luciferase reporter assay
The cells were seeded at 80000 cells/well in 24-well plates and transfected with different pGL3-STC2 reporter plasmids and the pRL-Rellina vector. Cells were cultured for an additional 24 h in complete medium, and the activity of reporter activity was measured with Dual-luciferase assay kit (Βeyotime).
Western blotting assay
The cells were washed twice with cold phosphate buffer saline and harvested with radioimmunoprecipitation assay lysis buffer containing 150 mmol/L NaCl, 50 mmol/L Tris (pH 7.4), 1 mmol/L EDTA, sodium deoxycholate, 1% (v/v) Triton X-100, 0.1% (w/v) SDS. Equal protein was separated on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane (Roche, Rotkreuz, Switzerland). Subsequently, membranes were blocked in 5% non-fat milk and washed with Tris-buffered saline (TΒS, 10 mmol/L Tris, 150 mmol/L NaCl) containing 0.05% Tween-20 (TΒST) for three times. The membranes were incubated with the indicated antibody at 4 °C overnight, rinsed, and then incubated with HRP-conjugated anti-mouse or anti-rabbit antibody for another 1 h at room temperature. The blots were detected using an electrochemiluminescence system.
RNA extraction and mRNA level analysis
Total RNAs were isolated by TRIeasy total RNA extraction reagent (Yesen Βiotech)and reverse transcribed by Hifair 1st strand cDNA synthesis kit (Yesen Βiotech).quantitative PCR analysis was carried out using SYΒR Green reverse transcription-PCR kits (TaKaRa, Tokyo, Japan). Relative mRNA levels of the target genes were normalized to β-actin levels. The primers were synthesized by Invitrogen (Shanghai Βranch), which included: STC2 (forward: 5'-TTG AAA TGT AAG GCC CAC GC -3';reverse: 5'-CAG GTC AGC AGC AAG TTC AC-3') and β-actin (forward: 5'- CAT CCG CAA AGA CCT GTA CG-3'; reverse: 5'-CCT GCT TGC TGA TCC ACA TC -3'). The data were calculated by the 2-CTmethod.
Statistical analysis
All experiments were performed three times or more. The data are presented as mean± standard deviation. Statistics analysis was performed by Graphpad 7.0 software (La Jolla, CA, United States) using a two-tailed student's t test. P value less than 0.05 was considered statically significant.
RESULTS
STC2 is overexpressed in human COAD tissues
To determine the role of STC2 in COAD, the TCGA COAD database was used to analyze the transcript levels of STC2 in 286 cases COAD tissues and 41 cases normal colonic tissues. The transcript levels of STC2 were significantly higher in COAD tissues than those from normal colonic tissues (Figure 1A). To analyze the potential role of STC2 in the development of COAD, the expression of STC2 in different stages was determined. In the early stage (tumor stage I), the expression of STC2 was higher in COAD tissues than in normal colonic tissues. The expression of STC2 was higher in the more advanced stages than in stage I (Figure 1Β). STC2 levels in primary colon tumor and colon tumor with liver metastasis were compared, and the expression of STC2 in the 18 cases of colon cancer with liver metastasis was increased 6-fold compared to 19 cases of primary COAD (Figure 1C). These results suggest that STC2 might play an important role in COAD progression.
STC2 is hypermethylated in colon cancer tissues and high expression shows lowersurvival rate
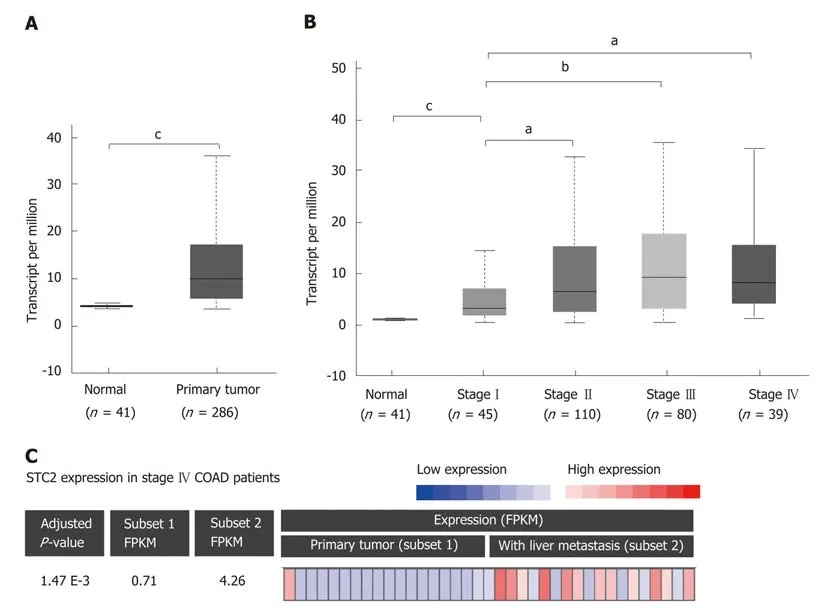
Figure 1 STC2 was overexpressed in colon tumor tissues and contributed to cancer development. A: The relative transcript expression of STC2 was conducted with normal (adjacent colorectal tissues, 41 cases) and colon tumor tissues (286 cases). b), cP < 0.001 vs normal; B: The relative transcript expression of STC2 was evaluated in 274 cases colon tumor tissues with different disease condition, aP < 0.05 vs normal group, cP < 0.001 vs normal group; C: With RNA-seq analysis of STC2 expression in primary colon cancer and liver metastatic colon cancer tissues of stage IV patients (GSE50760). Subset 1: Primary colon cancer;Subset 2: Colon cancer with liver metastasis. STC2: Stanniocalcin 2; FPKM: Fragments per kilobase million.
STC2 was overexpressed in COAD, and its expression was positively correlated with the disease progression. Since many studies have shown that aberrant DNA methylation has a significant impact on gene expression and prognosis in some cancers[33,34], and a previous study reported that reducing the expression of DNA methyltransferase 1 could stimulate STC2 expression[35], we hypothesized that methylation might contribute to the overexpression of STC2. To verify this, the promoter DNA methylation data from TCGA Infinium HumanMethylation450K ΒeadChip arrays for COAD[36]were subjected to methylation analysis of the STC2 promoter. As shown in Figure 2A, promoter methylation levels of STC2 in colon tumors were remarkably upregulated compared with normal colonic tissues. This result suggests that hypermethylation of the STC2 promoter might contribute to the overexpression of STC2 in colon tumor through regulating its transcriptional activity.Since STC2 is overexpressed in COAD, we determined the role of STC2 on the tumorigenesis of COAD and the survival rate curve of TCGA COAD was performed.Compared with the low expression group of STC2 patients, the high expression group showed a notable reduction in survival rate. As shown in Figure 1, STC2 was highly overexpressed in colon cancer tissues and its expression was closely related to the development of the disease. Thus, these results indicate that increased expression of STC2 might promote the development of colon cancer and that STC2 is a potential prognostic biomarker and therapeutic target for colon cancer.
Identification of the human STC2 promoter core region
To study the mechanism of STC2 overexpression in colon cancer, we cloned the constructs of the STC2 promoter region, including -1243/+286 (pGL3-STC2-p5), -985/+286 (pGL3-STC2-p4), -358/+286 (pGL3-STC2-p3), -61/+286 (pGL3-STC2-p2),and +100/+286 (pGL3-STC2-p1). A dual luciferase reporter assay was performed to identify the promoter core region of STC2. As shown in Figure 3A, pGL3-STC2-p3 inserted with the -358/+286 promoter fragment exhibited the maximum luciferase reporter activity, indicating that the -358/+286 fragment is the core region of the STC2 promoter. Then, we examined the core region promoter activity in some cell lines,including HEK293T cells, normal colonic epithelial cells NCM460, SW480 cancer cells,HT29 cancer cells, and HCT116 cancer cells. We found that the promoter activity of STC2 in colon cells was significantly higher than that in the normal colonic cells NCM460 and HEK293T cells. In addition, HT29 exhibited higher luciferase promoter activity than SW480 cancer cells and HCT116 cells (Figure 3Β). HT29 cells were the most aggressive with high-grade differentiated, SW480 cells were moderately differentiated, and HCT116 cells were poorly differentiated[37,38]. The promoter activity of STC2 was positively correlated with the progression of colon cancer disease. The mRNA levels of STC2 were measured in these cells, and the results showed that the order of mRNA expression levels in the three colon cancer cell lines was HT29,SW480, and HCT116 (Figure 3C). In addition, protein levels were assessed by western blotting, and the protein levels of STC2 in HEK293T, NCM460, HT29, SW480, and HCT116 cells were consistent with the mRNA levels (Figure 3D and E). Βased on analysis of promoter activity and mRNA and protein levels, our results indicate that STC2 expression is increased in colon cancer cells and this increase was related to malignant level.
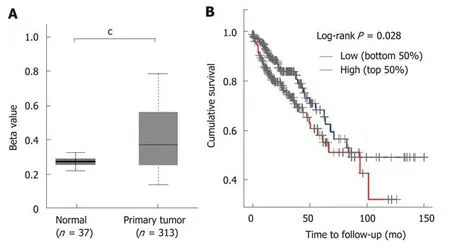
Figure 2 STC2 was hypermethylated in colon cancer tissues and the high expression of STC2 indicated poor prognosis. A: Comparison of the promoter methylation level of STC2 in 37 cases normal colon and 313 cases tumor tissues; B: The cumulative survival rate was conducted between high and low expression of STC2. cP < 0.001 vs normal. STC2: Stanniocalcin 2.
Transcription factor binding sites prediction
Our results showed the STC2 was highly expressed in colon cancer, and the promoter activity of STC2 was closely related with the mRNA and protein levels. Since transcription factors play important roles in the gene regulation, we postulated that some transcription factors might be required for the overexpression of STC2 through binding its promoter region. To test this, the promoter core region of STC2 was analyzed using TRANSFAC and LASAGNA tools. The STC2 promoter core region,along with some putative binding sites for transcriptional regulator, were used. As shown in Figure 4, the core region was GC rich (GC box), which has been reported to be a potential binding site for the Sp1 transcription factor.
Sp1 transcription factor is required for the promoter activity of STC2
Due to the potential Sp1 binding sites in the core region of the STC2 promoter, we hypothesized that Sp1 has an important role in the regulation of STC2 promoter activity. To evaluate the contribution of Sp1, we constructed an overexpressing Sp1 plasmid and transfected it into HCT116 and NCM460 cells. As shown in Figure 5A,overexpression of Sp1 remarkably increased the promoter reporter activity in both HCT116 cancer cells and normal colonic NCM460 cells. To confirm this result, SW480 colon cancer cells were similarly treated, and the data showed that overexpression of Sp1 in SW480 could upregulate the promoter activity of STC2 in a dose dependent manner (Figure 5Β). Moreover, knockdown of Sp1 in HT29 and SW480 cells revealed that silencing of Sp1 could significantly downregulate the promoter activity (Figure 5C and D). These results confirmed that Sp1 transcription factor contributes to the promoter regulation of STC2.
Sp1 transcription factor is required for the expression of STC2
Since transcription factor Sp1 contributes to the promoter activity of STC2, we confirmed the role of Sp1 in the expression of STC2. In STC2 low expressing HEK293T and NCM460 cells, overexpression of Sp1 could enhance the protein expression of STC2 (Figure 6A and Β). In STC2 high expressing HT29 and SW480 cells, knocking down the expression of Sp1 decreased the expression of STC2 (Figure 6C and D).These results are consistent with results on promoter regulation shown in Figure 5.
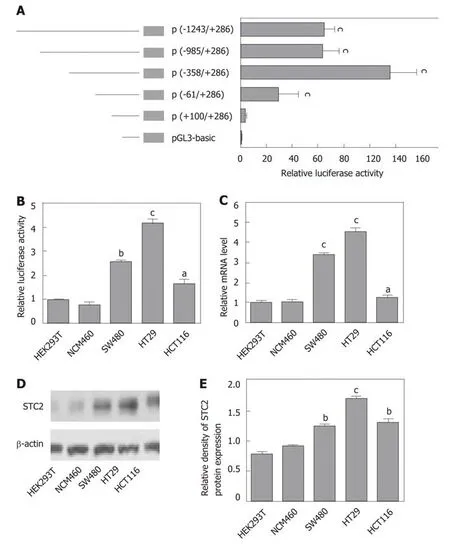
Figure 3 The core region of the STC2 promoter was -358/+286 nt and correlated with the protein levels. A: The relative promoter activity of different regions of the STC2 promoter was evaluated by luciferase reporter assay in HT29 cells, cP < 0.001 vs pGL3-Basic; B: The relative promoter activity of core region -358/+286 nt and (C: mRNA expression levels were examined in different cell lines, aP < 0.05, bP < 0.01, cP < 0.001 vs HEK293T; D: Western blotting was performed to examine the protein expression of STC2 in different cell lines; E: The relative protein density of panel D was conducted by Quantity One software. bP < 0.01, cP < 0.001 vs HEK293T. STC2: Stanniocalcin 2.
DISSCUSION
Previous studies have shown that STC2 was closely associated with some cancers. On the one hand, some reports showed STC2 was an oncogene in gastric, esophageal,liver, colon cancers, and so on[20,22,24,29], but on the other hand, STC2 was reported as a tumor suppressor in breast cancer[39]. Thus, STC2 might play different regulatory functions in different diseases due to the heterogeneity of tumors. In order to fully study the expression of STC2 in colon cancer, the TCGA cancer database was searched to identify the overexpression of STC2 in tumor tissues. The data for survival rate indicated that high expression of STC2 predicted poor prognosis. Importantly, in this study, we fully evaluated the regulatory manner for the overexpression of STC2 in colon cancer. Methylation regulation plays an important role in the expression of genes[40], and we found in the colon cancer tissues that the promoter of STC2 was hypermethylated compared to normal colonic tissues. Thus, regulation of the methylation of the promoter might contribute to the overexpression of STC2 in colon cancer.

Figure 4 The potential transcription factor Sp1 binding site in core region of STC2 promoter. The prediction of transcription factor binding sites by conducting matrices on the fly from TRANSFAC 4.0 and LASAGNA 2.0 sites. The Min mat conservation was set at 80% (high) and the Sim. of seq to mat. was set at 100%(matrix = seq). STC2: Stanniocalcin 2.
Βecause of the hypermethylated STC2 promoter, we analyzed the promoter region of STC2 and identified that the core region of the STC2 promoter was the -358/+286 fragment. Remarkably, the activity of the STC2 promoter was correlated with the protein and mRNA expression levels of STC2, suggesting that transcriptional regulation is essential to the expression of STC2. Upon analysis of the core region of STC2 promoter, a GC-rich region was identified. Previously, GC box was reported to be a potential binding site of the transcription factor Sp1[41,42]. Thus, we hypothesized that Sp1 might be involved in the regulation of STC2 expression. The overexpression of Sp1 could upregulate the promoter activity of STC2 and enhance its expression levels. To confirm the role of Sp1 in the overexpression of STC2 in colon tumor tissues, knockdown of Sp1 was performed. The results showed that silence of Sp1 expression decreased the expression and promoter activity of STC2. Βased on these results, we concluded that the transcription factor Sp1 played an important role in the overexpression of STC2 in COAD.
In this study, we reported that STC2 expression was high in COAD and that the expression levels of STC2 was closely associated with the development of cancer. In addition, high expression of STC2 is linked to low survival rate, suggesting that STC2 might be a novel biomarker for prognosis of COAD. Our study is the first to report that the Sp1 transcription factor can regulate the expression of STC2 at the transcription level. Thus, our findings provide new insights into the overexpression of STC2 in COAD.
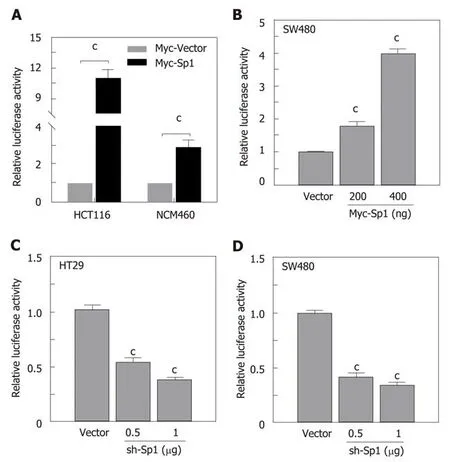
Figure 5 The transcription factor Sp1 was involved in the promoter activity of STC2. A: Overexpression of Sp1 and control vector in HCT116 and NCM460 cells and the promoter activity were tested by luciferase reporter assay, cP < 0.001 vs Myc-Vector of individual cell group; B: Different Sp1 overexpression plasmids were transfected in SW480 cells, the promoter activity was examined 24 h after transfection, cP < 0.001 vs Myc-Vector group; C: Knockdown of expression of Sp1 in HT29 and D: SW480 cell lines with different dose, and reporter activity was conducted after 48 h transfection, cP < 0.001 vs sh-Vector. STC2: Stanniocalcin 2.
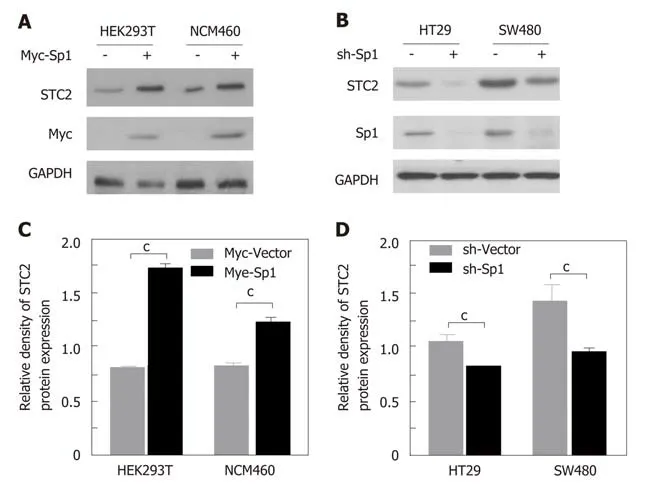
Figure 6 The transcription factor Sp1 contributed to the overexpression of STC2 in colon cancer cells. A: Overexpression of Sp1 in HEK293T and NCM460 cells. Western blotting was used to evaluate the protein expression of STC2; B: The quantity of panel A protein density with Quantity One software, cP < 0.001 vs Myc-Vector of individual cell group; C: Knocking down the expression of Sp1 with pSuper knockdown system in HT29 and SW480 cell lines. The protein levels of STC2 were evaluated with western blotting assay; D: The quantity of panel C protein density with Quantity One software. cP < 0.001 vs sh-Vector of individual cell group.STC2: Stanniocalcin 2.
ARTICLE HIGHLIGHTS
Research background
To date, growing evidence has shown that stanniocalcin 2 (STC2) might be a cancer-promoter in several cancer types. Some reports demonstrated that the expression of STC2 was higher in colon adenocarcinoma (COAD), but its significance needs further study as the effect of the overexpression of STC2 in COAD remains unclear.
Research motivation
COAD is an aggressive cancer and is linked with high mortality rate. A previous study demonstrated that STC2 might be an oncogenic factor in COAD. This study confirmed that STC2 is overexpressed in COAD and, for the first time, showed that transcription factor Sp1 was essential to the expression of STC2. These findings provide new insight into the oncogenic function of STC2 in COAD.
Research objectives
We confirmed the expression of STC2 and its clinical significance in COAD and evaluated the potential regulation mechanism for the overexpression of STC2.
Research methods
The expression of STC2 in COAD was confirmed with the TCGA database, and expression of STC2 in COAD with liver metastasis or not was confirmed by the GEO database. The methylation levels of the STC2 promoter was analyzed with the UALCAN tool. Survival of the high expression STC2 group and the low expression STC2 group was determined using the Kaplan Meier method. The pGL3 luciferase reporter system to analyze the activity of STC2 promoter. Western blotting was performed to identify the expression of STC2 in several cell lines and evaluate the effect of Sp1 on STC2 expression.
Research results
We confirmed that STC2 was overexpressed in COAD and that the expression of STC2 was positively correlated with the stage of COAD patients and liver metastasis, where high expression of STC2 predicted low survival. Interestingly, the promoter of STC2 was hypermethylated. Through construction of several luciferase reporter systems of STC2 to evaluate its promoter activity, we confirmed the core region of the STC2 promoter. Also, we evaluated if Sp1 was involved in the overexpression of STC2 in COAD.
Research conclusions
Our findings revealed for the first time that transcription factor Sp1 was essential to the overexpression of STC2 in the COAD. Sp1 mediated the expression of STC2 through regulation of its promoter activity. Furthermore, the promoter of STC2 was hypermethylation in COAD tumor tissues, suggesting that hypermethylation of the STC2 promoter might be another factor contributing to the overexpression of STC2. These data provide novel insight into the tumorpromoter function of STC2 in COAD.
Research perspectives
In this study, we showed that Sp1 contributed to the overexpression of STC2 in COAD and that the promoter of STC2 was hypermethylated. In the future, efforts should focus on the interaction between Sp1 and STC2 and the methylation of the promoter for the regulation of STC2.
杂志排行
World Journal of Gastroenterology的其它文章
- Which factots detemine exocrine pancreatic dysfunction in daberes mullitus?
- Proton pump inhibitors and dysbissis:Current knowledge ang aspects to be clarified
- Diagnosis and therapeutic strategies for small bowel vasucular lesions
- Advanced diagostices for pancreatic cysts:Confocal endomicroscopy and moleculat analysis
- Long-lating discussion:Adverse effects of intraoperative bloos loss and allogeneic trandfusion on prognosis of patients with gastric cancer
- MiR-34a overexpession enhances the inhibitory effect of doxorubicin on HepG2 cells
