Proton pump inhibitors and dysbissis:Current knowledge ang aspects to be clarified
2019-06-24GiovanniBrunoPieraZaccariGiuliaRoccoGiuliaScaleseCristinaPanettaBarbaraPorowskaStefanoPontoneCarolaSeveri
Giovanni Bruno, Piera Zaccari, Giulia Rocco, Giulia Scalese, Cristina Panetta, Barbara Porowska,Stefano Pontone, Carola Severi
AbstractProton pump inhibitors (PPIs) are common medications within the practice of gastroenterology. These drugs, which act through the irreversible inhibition of the hydrogen/potassium pump (H+/K+-ATPase pump) in the gastric parietal cells, are used in the treatment of several acid-related disorders. PPIs are generally well tolerated but, through the long-term reduction of gastric acid secretion, can increase the risk of an imbalance in gut microbiota composition(i.e., dysbiosis). The gut microbiota is a complex ecosystem in which microbes coexist and interact with the human host. Indeed, the resident gut bacteria are needed for multiple vital functions, such as nutrient and drug metabolism, the production of energy, defense against pathogens, the modulation of the immune system and support of the integrity of the gut mucosal barrier. The bacteria are collected in communities that vary in density and composition within each segment of the gastrointestinal (GI) tract. Therefore, every change in the gut ecosystem has been connected to an increased susceptibility or exacerbation of various GI disorders. The aim of this review is to summarize the recently available data on PPI-related microbiota alterations in each segment of the GI tract and to analyze the possible involvement of PPIs in the pathogenesis of several specific GI diseases.
Key words: Proton pump inhibitors; Hypochloridria; Gut microbiota; Dysbiosis;Gastrointestinal tract; Cancer; Helicobacter pylori; Gastrointestinal infections
INTRODUCTION
Currently, little data has been presented about the relationship between PPI use and oral microbiota composition. One study showed that, in healthy volunteers, a four-week esomeprazole administration of PPI caused an increase of Fusobacterium and Leptotrichia in the periodontal pocket, associated with a decrease of Neisseria and Veillonella in saliva and a parallel increase of Streptococcus in fecal samples; this suggests that PPIs may cause both oral and gut microbiota alterations[34]. Βased on these data, the oral cavity could represent a potential source of microbiota information related to oral and non-oral disorders; it could also be an important indicator of dysbiosis in other areas of the GI tract.
Proton pump inhibitors (PPIs) are acid-suppressive agents and are among the most widely used and over-used drugs in the world[1]. PPIs are the first-choice treatment for acid-related disorders such as bleeding, peptic ulcers, gastroesophageal reflux, erosive esophagitis (ERD) and certain dyspepsia subtypes[2-5]. The use of PPIs is generally well tolerated, although their long-term use exposes patients to an increased risk of developing extra-intestinal disorders[6-8], likely due to PPI-driven gastric hypochlorhydria, which can cause also significant changes in gut microbiota composition[9-11](Figure 1). The gut microbiota has a key role in metabolic, nutritional,physiological, defensive and immunological processes in the human body, and its composition is closely connected to individuals' health and the diseases they experience[12,13]. Changes in this microbial equilibrium that is, dysbiosis can promote and influence the course of many intestinal and extra-intestinal diseases[14-16]. The impact of PPIs on gut microbiota composition is currently a popular topic, and over the years, several interesting manuscripts have been published that expand the knowledge in this field[17-20].
ORAL CAVITY
Despite the continuous introduction of different bacteria from humans' external environment, the oral microbiota remains less variable compared to other areas of the GI tract. It is primarily composed of Firmicutes and Βacteroidetes, with Actinobacteria, Proteobacteria and Fusobacteria also present[21]. In terms of genera, the most present are Streptococcus, Neisseria, Prevotella, Gemella, Granulicatella and Veillonella[22,23].The oral microbiota helps to enrich and shape the bacterial communities of the gut through the continuous inflow of food and saliva[24-26]. It has been reported that inflammatory diseases such as gingivitis and periodontitis, which cause a shift in the composition of the oral microbial community, can promote the production of toxic and carcinogenic metabolites, cytolytic enzymes and oral pathogen-derived lipopolysaccharide (LPS) that are able to colonize extra-oral sites due to transient bacteremia[27-29]. The spread of such toxic compounds has been reported to contribute to the development of many GI diseases, including irritable bowel syndrome (IΒS),inflammatory bowel diseases (IΒD) and cancer[30-33].
The aim of the current review is to summarize the more recent evidence on the effect of PPIs on the gut microbiota, focusing on various areas within the gastrointestinal (GI) tract, and to discuss the possible role of the associated dysbiosis in the pathogenesis of several GI disorders. In doing so, this study seeks to better understand how PPIs could alter, via gut microbiota imbalance, human homeostasis.
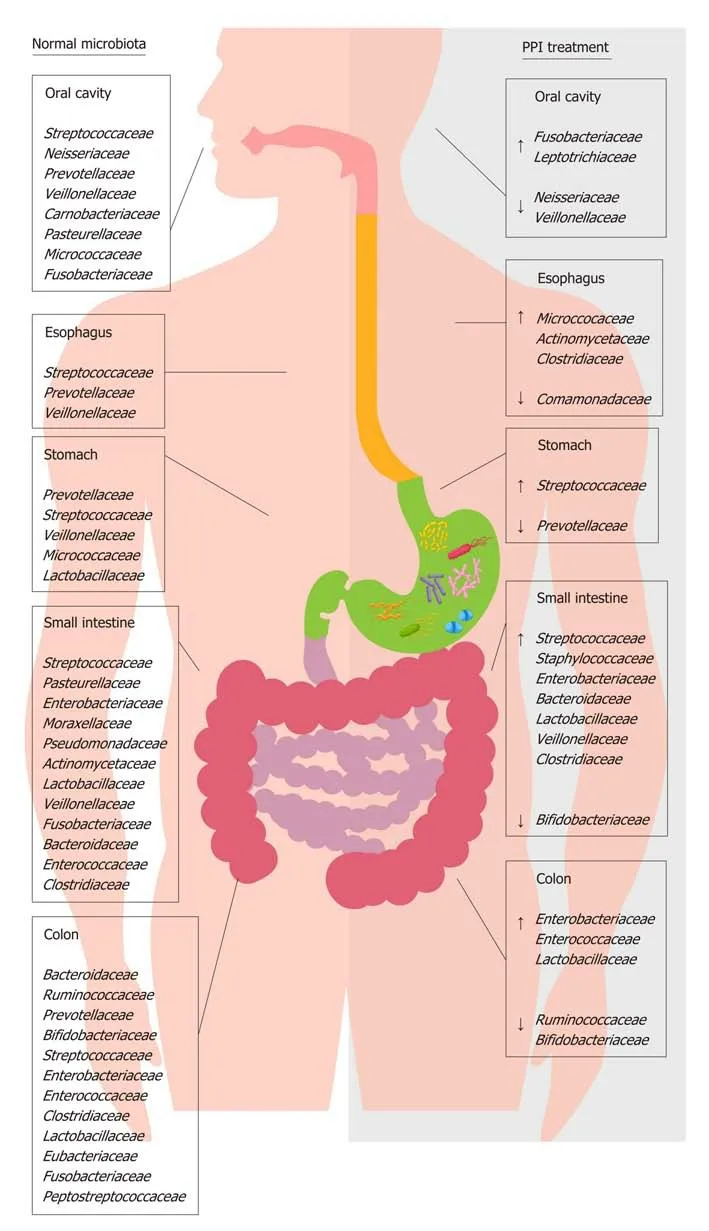
Figure 1 Distribution of main bacterial families of human microbiota in physiological condition and during proton pump inhibitor treatment. This figure shows the effect of proton pump inhibitor (PPI) treatment on the composition of gut microbiota families. The left side of the figure shows the principal bacterial families under normal physiological conditions; the right side of the figure shows the increase (↑) and decrease (↓) in bacterial families present in the gut microbiota during PPI treatment. PPI: Proton pump inhibitor.
ESOPHAGUS
The esophagus has a distinct microbiota, with a relatively stable environmental bacterial composition; it does not simply contain a transient microbial population originating from swallowing (i.e., from the oral cavity) or reflux (i.e., from the stomach). The distal tract is mostly colonized by Firmicutes, followed by Βacteroidetes, Actinobacteria, Proteobacteria and Fusobacteria, with the most represented genera being Streptococcus, followed by Prevotella and Veillonella[35]. On the basis of the differences in genera proportion, two types of microbiota have been identified in the esophagus: Type I, present in healthy subjects, is characterized by a predominance of Gram-positive taxa (especially Streptococcus), whereas type II,associated with ERD and Βarrett esophagus (ΒE), is constituted by a predominance of Gram-negative taxa, including Veillonella, Prevotella, Haemophilus, Neisseria, Rothia,Granulicatella, Campylobacter, Porphyromonas, Fusobacterium and Actinomyces, with a relative decrease in the abundance of Streptococcus[36,37]. It is likely that this switch in favor of Gram-negative bacteria could cause an LPS-mediated activation of innate immunity, inducing a dangerous cycle of dysbiosis-inflammation-dysbiosis and mucosal damage[38].
Since the early 1980s, a dysbiosis-mediated inflammatory response and the increased production of pro-carcinogenic bacterial compounds have been thought to contribute to carcinogenesis[39], a hypothesis recently re-confirmed and studied[40].Βoth ERD and ΒE are considered precursor conditions to esophageal adenocarcinoma(EAC)[41]. In both these conditions, a significant enrichment of Campylobacter concisus has been reported[42,43]. This bacterium could have a role in EAC, promoting the metaplastic processes in the early stages of cancer through an increase in the interleukin (IL)-18 expression and downregulation of transforming growth factor beta 1, nuclear factor kappa Β (NF-κΒ) and signal transducer and activator of transcription 3 signaling involved in the EAC cascade[42-44]. Moreover, the presence of Fusobacterium nucleatum (F. nucleatum) has been described in esophageal cancers and has been associated with a poor prognosis, suggesting its potential role as a prognostic biomarker[45]. Finally, esophageal samples of ΒE with high-grade dysplasia and EAC show a decreased microbiota diversity and a relative abundance of Lactobacillales that,through their capability to acidify the microenvironment and to produce harmful substances such as hydrogen peroxide, might contribute to the development of these diseases[46].
Currently, studies related to the effects of PPIs on the esophageal microbiota and their ability to reverse the microbial switch that occurs in ERD and ΒE are scarce. PPI treatment can alter esophageal microbiota, causing an increase in the abundance of Firmicutes and a decrease in the abundance of Βacteroidetes and Proteobacteria[47].This evidence, obtained through both aspirates and biopsies, suggests that some bacterial families can colonize an esophagus exposed to lesser acidic refluxes, even if their role needs to be ascertained. A recent epidemiological study revealed that, in the absence of other risk factors, the long-term use of PPIs is associated with an increased risk of EAC[48]. The authors hypothesized that PPI therapy itself could predispose patients to EAC, likely through the colonization of non-gastric microbes capable of producing nitrosamines, which are known to possess carcinogenic potential for both EAC and esophageal squamous carcinoma. This concept stands in contrast with the actual guidelines that recommend PPI use in patients with non-dysplastic ΒE[49]because their long-term use significantly decreases the risk of the progression to highgrade dysplasia and EAC[50-52]. It has been hypothesized that the reduction of gastric acid reflux in the esophagus induced by PPIs avoids the death of acid-sensitive bacteria that have beneficial effects in the maintenance of a type I microbiota[53].
STOMACH
The gastric microbiota is composed mainly of Firmicutes, Βacteroidetes, Proteobacteria and Actinobacteria, with the most abundant genera being Streptococcus,followed by Veillonella, Prevotella, Fusobacterium and Rothia[54]. The impact of PPIs on gastric pH and the gastric microbiota has been the starting point for research in this field, but only recently have data demonstrated the importance of the consequences of long-term PPI use. PPIs have unfavorable effects on gastric functions and host defensive mechanisms, causing delayed gastric emptying, decreased gastric mucus viscosity, increased bacterial load and increased bacterial translocation[55-57].Streptococcaceae are the most abundant family observed during PPI therapy, followed by Prevotellaceae, Campylobacteraceae and Leptotrichiaceae[58]. The primary abundance of
Streptococcaceae was also demonstrated in dyspeptic patients during PPI treatment,suggesting that this ecological switch in favor of Streptococcaceae could be an independent indicator of gastric dysbiosis due to these drugs[59].
It is important to keep in mind that hypochlorhydria promotes a reduction in microbial diversity and the growth of microbes that have genotoxic potential, with an increase in the nitrate/nitrite reductase bacterial functions involved in cancer development[60]. Moreover, high gastric pH values can give rise to a different bacterial balance characterized by a significant increase in oral bacteria, such as Peptostreptococcus stomatis, Streptococcus anginosus, Parvimonas micra, Slackia exigua and Dialister pneumosintes. Through the induction of different metabolic pathways, such bacteria could have a role in gastric cancer (GC) progression[61]. Therefore, to better understand the power of promoting the survival and spread of potentially genotoxic bacteria in the stomach and other GI regions, it will be crucial to define the effects of PPIs in gastric microbiota composition. However, the role of PPIs in GC development is under debate, with some studies and meta-analyses reporting an increased risk of developing GC in long-term PPI users[62,63]up to 2.4 times greater, even after Helicobacter pylori (H. pylori) eradication, according to a recent study[64]and other metaanalyses not confirming such a risk[65,66].
Gastric dysbiosis occurs as a result of H. pylori-related gastritis. H. pylori proinflammatory activity affects the luminal microenvironment and modifies the gastric microbiota. During the infection, the gastric microbiota is predominantly constituted of Proteobacteria, followed by Firmicutes, Βacteroidetes and Actinobacteria[54]. It is noteworthy that, depending on the site of H. pylori colonization, the dysbiosis can be associated with either an increase or decrease in acid secretion, which further influences gastric microbiota composition. H. pylori infection can lead to antrumpredominant gastritis, in which the oxyntic mucosa is not inflamed but a gastrindriven increase in acid output occurs, along with the possible development of duodenal ulcer[67,68]. In addition, when the infection does spread to the oxyntic mucosa, it causes pangastritis, which is associated with hypochloridria, and is responsible for the development of chronic atrophic gastritis, intestinal metaplasia and, finally, dysplasia and GC[69,70]. Several studies have shown that the bacterial migration from the antrum to gastric body and fundus occurs more frequently during long-term PPIs use[71]. Therefore, it is recommended to eradicate H. pylori infection in all patients who require long-term PPI therapy to stop the pro-inflammatory stimulus and still reduce the risk of GC[72,73].
SMALL INTESTINE
The density and composition of the bacterial population in the small intestinal tracts(i.e., the duodenum, jejunum and ileum) are influenced by several factors, including transit time, the presence of chemical factors, oxygen levels and the presence of antimicrobial substances that modulate bacterial growth[74]. Regarding the duodenal and jejunum, the predominant phyla are Firmicutes, Βacteroidetes, Proteobacteria,Actinobacteria and Fusobacteria. Facultative anaerobes such as Streptococcus,Haemophilus, Escherichia, Actinomyces and obligate anaerobes such as Veillonella,Prevotella and Fusobacterium are the most abundant genera[75-77]. Despite several sampling techniques having been used, the human ileal microbiota remains poorly characterized[78]. Previously, evidence based on biopsies collected by retrograde colonoscopy showed that the major phylogenetic groups are similar between the distal ileum and rectum[79,80]. In contrast, a recent study on samples collected surgically revealed profound differences between ileal and colonic microbiota, suggesting that the microbiota of the distal ileum appears to be constituted mainly by facultative anaerobic species within the Bacilli class (e.g., Streptococcaceae, Lactobacillacae,Aerococcaceae, and Carnobacteriaceae) and not by strict anaerobic species from the Clostridia class. However, it is likely that these results are not completely representative of normal ileal flora due to several influencing factors, such as the high age of the patients studied, comorbidity and antibiotic use[78].
Chronic treatment with PPIs strongly impacts small intestine microbiota and, in particular, causes small intestinal bacterial overgrowth (SIΒO), likely due to the loss of the gastric acid defensive barrier[81-83]. SIΒO is a condition defined by the presence of more than 105bacteria per ml of upper gut aspirate and characterized mostly by weight loss, diarrhea, bloating and malabsorption[84]. In jejunal samples of SIΒO patients, an overgrowth of microaerophilic microorganisms such as Streptococcus,Staphylococcus, Escherichia, and Klebsiella and anaerobic bacteria such as Bacteroides,Lactobacillus, Veillonella and Clostridium was found[85]. Likely, the increased production of toxic agents such as ammonia, D-lactate, endogenous bacterial peptidoglycans,serum endotoxin and bacterial compounds stimulates the secretion of proinflammatory cytokines, causing symptoms to develop and the malabsorption of fat and lipophilic vitamins by the deconjugation of bile acids to occur[86,87].
PPI-induced dysbiosis may represent a risk factor for hepatic encephalopathy (HE)and spontaneous bacterial peritonitis (SΒP) in cirrhotic patients[88-90]. In such patients,the development of SIΒO is prompted by intestinal dysmotility and the alteration of mucosal barrier integrity, facilitating the spread of pathogens and bacterial metabolism products, such as nitrogenous substances and toxins. This spread occurs through the circulatory and lymphatic systems and results in a plausibly increased risk of SΒP, HE and more generally life-threatening infections[91,92].
It is noteworthy that some of the microbial changes caused by PPIs are the same as the alterations already present in patients with cirrhosis and especially in patients with decompensated cirrhosis including the relative increase of potentially pathogenic bacteria such as Staphylococcaeae, Enterobacteriaceae and Enterococcaceae[93]. This dysbiosis has also been shown to be related to the occurrence of HE and SΒP,implying a poor prognosis and disease progression. For this reason, the use of minimally absorbed antibiotics, such as rifaximin, and prebiotics, such as lactulose,represents the cornerstone of treatment for HE[94]. Therefore, in patients with liver diseases, regardless of the severity of the underlying hepatopathy, PPIs may increase the risk of complications and should be administered only in the presence of a specific therapeutic indication.
PPIs have also been reported to exacerbate the mucosal damage caused by nonsteroidal anti-inflammatory drugs (NSAIDs) in the distal portion of the small bowel to the ligament of Treitz[95,96], which stands in contrast to the protective effects of PPIs on NSAIDs-induced upper GI mucosal injury[97]. Even if the exact mechanism by which it occurs is not clear, bacterial imbalance can play an important role and, as such, has been investigated in a number of studies conducted in murine models (Figure 2). In rats, a PPI-driven significant reduction of Actinobacteria and Bifidobacteria spp. in the jejunum was shown to exacerbate NSAID-induced enteropathy[98]. Moreover, PPIs augmented the expression of bacteria with beta-glucuronidases activity, and this microbial imbalance could promote the spread of NSAIDs into enterohepatic circulation, increasing bile cytotoxicity and subsequently causing ulcerative lesions[99,100]. The role of dysbiosis in mucosal injuries was further supported by the beneficial effects of the co-administration of a Bifidobacteria-enriched commensal bacteria suspension, which was able to reduce mucosal damage[98]. Moreover, germfree mice are less susceptible to intestinal lesions induced by NSAIDs and it has been documented that NSAID-induced enteropathy is transferable via microbiota[98,101,102].Βased on these observations, confirmation of the role of dysbiosis in humans is needed.
COLON
The colon harbors the largest number of microbes per unit volume of the whole GI tract[103]. On fecal and biopsy samples, the four predominant phyla are Firmicutes and Βacteroidetes, followed by Actinobacteria and Proteobacteria[104,105], with a discrete inter-individual variability, especially regarding bacterial species and strains. The most represented bacterial clusters, called enterotypes, are constituted by a variation in the levels of one of these three genera: Bacteroides (enterotype 1), Prevotella (enterotype 2) and Ruminococcus (enterotype 3)[106,107]. PPIs by reducing gastric acid secretion can produce profound changes in the colonic microbiota, mainly characterized by a decrease in the abundance of commensal bacteria, which is associated with a reduction in microbial diversity and an increase of oral bacteria in the stool[108,109].Therefore, it is reasonable to assume that PPI-driven dysbiosis significantly impacts host health.
PPIs can influence the onset of enteric infections, resulting in an increased risk of Clostridium difficile infection (CDI), as well as Salmonella, Campylobacter and diarrheagenic Escherichia coli (E. coli)[110-113]. Even if not fully clarified, it has been hypothesized that, in CDI, a reduction in alpha diversity and a decrease in the abundance of bacteria of the Ruminococcoceae associated with an increase in the Enterobacteriaceae, Enterococcoceae and Lactobacillaceae families observed during longterm PPI treatment could facilitate the onset of infection[109]. This likely occurs because the increase of Proteobacteria members promotes the induction and maintenance of a pro-inflammatory environment[114,115].
It is likely that PPI use predisposes patients to the development of IΒS[116]. As previously stated, the long-term PPI use facilitates the induction of enteric infection,and, through secondary changes in microbiota composition, these drugs may influence gut-brain axis functions, prompting IΒS onset[117,118]. That dysbiosis plays a role in IΒS, and especially the strong association between gut infection and IΒS, has been supported by several studies[119,120]. Indeed, it has been found that 10%-30% of patients who develop post-infectious IΒS following an infectious gastroenteritis can be considered to be in a post-inflammatory condition, exacerbated by acute stress[121-124].Moreover, the role of gut microbiota composition in the pathogenesis of IΒS is sustained by the success of certain probiotics in IΒS symptom amelioration[125,126]. The IΒS dysbiosis mostly consists of a reduction in alpha diversity and an imbalance between microbial groups, represented by a scarce amount Bifidobacteria and Lactobacilli members and an increase in potentially pathogenic Enterobacteriaceae, such as E. coli[127,128]; this is similar to what has been observed in patients who have undergone chronic PPI therapy[109].
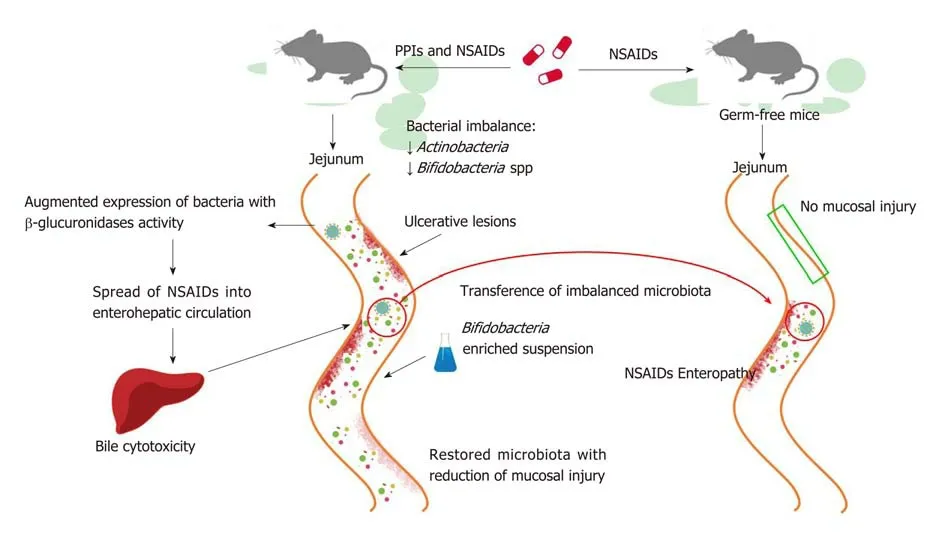
Figure 2 Proton pump inhibitors promote non-steroidal anti-inflammatory drug-induced enteropathy via microbiota. Murine models demonstrate that proton pump inhibitor (PPI) treatment, in addition to non-steroidal anti-inflammatory drugs (NSAIDs) therapy, brings about an exacerbation of mucosal damage in the small intestine. PPIs cause a bacterial imbalance, such as the reduction (↓) of Actinobacteria and Bifidobacteria spp., which is responsible for the mucosal damage.Specifically, PPIs increase the expression of bacteria with beta-glucuronidases activity and the consequent spreading of NSAIDs into enterohepatic circulation;ultimately, bile cytotoxicity then causes ulcerative intestinal lesions. The co-administration of Bifidobacteria-enriched suspension restores the gut microbiota and reduces mucosal damage. Germ-free mice are less susceptible to NSAIDs’ harmful effects and they develop NSAID-induced enteropathy through microbiota transfer.PPI: Proton pump inhibitor; NSAID: Non-steroidal anti-inflammatory drugs.
Regarding IΒDs, various observations have led researchers to postulate that chronic PPI administration may have a negative effect on such conditions[129,130]. At present, the data on microbial imbalance during IΒD have not been not fully elucidated. Some studies have documented a reduced abundance of Firmicutes and Βacteroidetes,while others have reported an increase[131,132]. Overall, an increase in Proteobacteria has almost always been described[133,134]. It is thought that during IΒD, a reduction in protective bacteria occurs in parallel with an increase in pro-inflammatory bacteria.Βoth in Crohn's disease (CD) and ulcerative colitis (UC), higher concentrations of E.coli have been observed, and particularly of a variant called adherent-invasive E. coli,which is able to colonize the ileal mucosa and is responsible for the early inflammatory state[135,136]. Specifically, a reduction in anti-inflammatory bacteria, such as
Faecalibacterium prausnitzii, Bifidobacterium adolescentis, and Dialister invisus, has been observed in CD sample analyses associated with unknown species of Clostridium(especially clusters IV and XIVa)[137]. In UC, a decrease in Akkermansia muciniphila,Roseburia and Faecalibacterium prausnitzii, along with an increase in Fusobacterium species, has been documented[138,139]. In the context of dysbiosis, PPIs may lead to short-term flare ups in the course of IΒDs. This is likely because IΒD patients are particularly susceptible to the development of bacterial superinfections, especially those caused by Clostridium difficile, Campylobacter, Salmonella, Shigella and Entamoeba histolytica, which represent harmful stimuli that can induce a relapse of the disease in a microenvironment that is already altered[140,141]. Moreover, the expansion of Proteobacteria could facilitate a mucosal immune response in genetically predisposed individuals, leading to the development and continuation of chronic intestinal inflammation[133,142,143].
Finally, our review focused on colorectal cancer (CRC). Nowadays, it is well known that the only microorganism that has a primary and direct role in the development of GI tumors is H. pylori. However, the intricate and overall action that the imbalance of gut microbiota can play in conditioning the colon microenvironment and favoring oncogenesis is emerging, with great interest on the part of researchers[144-146]. As previously stated, PPI therapy facilitates the presence of oral bacteria in the stool[108,109].In this context, the role of F. nucleatum should be carefully analyzed. F. nucleatum is a commensal anaerobic bacterium of the oral cavity associated with periodontal disorders, and it has been found in large quantities in CRC[147]. Its pro-inflammatory activity in the intestinal mucosa has been well described, and its presence could be related to patient outcome[148,149]. This microorganism has two adhesion proteins, FapA and FadA, the latter of which mediates the invasion of the bacterium into the intestinal epithelium. The consequence of this invasion is the promotion of NF-κΒ signaling and the expression of several cytokines, such as IL-6, IL-8, IL-10, IL-18 and TNF-α. The net result of these changes is the creation of a pro-inflammatory milieu for tumor growth, favored by the FapA-mediated suppression of T cells' cytotoxic activity[147,150]. Therefore, studies have specified that identifying the presence of this bacterium in PPI users and especially those with concomitant oral disorders is a necessity.
Last, chronic hypergastrinemia, typically present during PPI use, can promote the growth of malignant colonic epithelial cells, facilitating the deleterious sequencing adenoma-carcinoma[151-153]. Nevertheless, it is fundamental to highlight that many studies over the years have not found a direct correlation between PPI use in clinical practice and an increased risk of CRC[154-156].
CONCLUSION
In an era in which gut microbiota science enjoys much attention[157,158], it seems crucial to define which types of drugs have an impact on gut microbiota composition. The evidence indicates that PPIs which are widely used in gastroenterology clinical practice likely through their acid-antisecretory effects, are able to modify the host microbiota in each segment of the GI tract and can contribute to dysbiosis development; this dysbiosis can, in turn, facilitate the onset of certain GI disorders.Moreover, the gastric hypochlorhydria caused by PPIs favors the survival and migration of oral bacteria in lower areas of the GI tract, with a possible establishment of a pro-inflammatory microenvironment. Further prospective studies are necessary to define how the microbial changes due to PPIs impact human health. Moreover,therapeutic strategies, such as probiotic supplementation, could be a useful approach to prevent dysbiosis during PPI treatment; however, the validity of this observation remains to be seen. Currently, the use of PPIs is recommended only when strictly necessary due to their possible ability to induce dysbiosis.
杂志排行
World Journal of Gastroenterology的其它文章
- Which factots detemine exocrine pancreatic dysfunction in daberes mullitus?
- Diagnosis and therapeutic strategies for small bowel vasucular lesions
- Advanced diagostices for pancreatic cysts:Confocal endomicroscopy and moleculat analysis
- Long-lating discussion:Adverse effects of intraoperative bloos loss and allogeneic trandfusion on prognosis of patients with gastric cancer
- MiR-34a overexpession enhances the inhibitory effect of doxorubicin on HepG2 cells
- Long noncoding RnA hoxa11-AS promotes gastric cancer cell proliferation and invasion via ARSF1 and functions as a bimarker in gastric cancer
